Metabolic Plasticity and Virulence-Associated Factors of Sporothrix brasiliensis Strains Related to Familiar Outbreaks of Cat-to-Human Transmitted Sporotrichosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Paired Samples of Cats and Humans
2.2. Inoculum Preparation
2.3. Evaluation of Fungal Growth
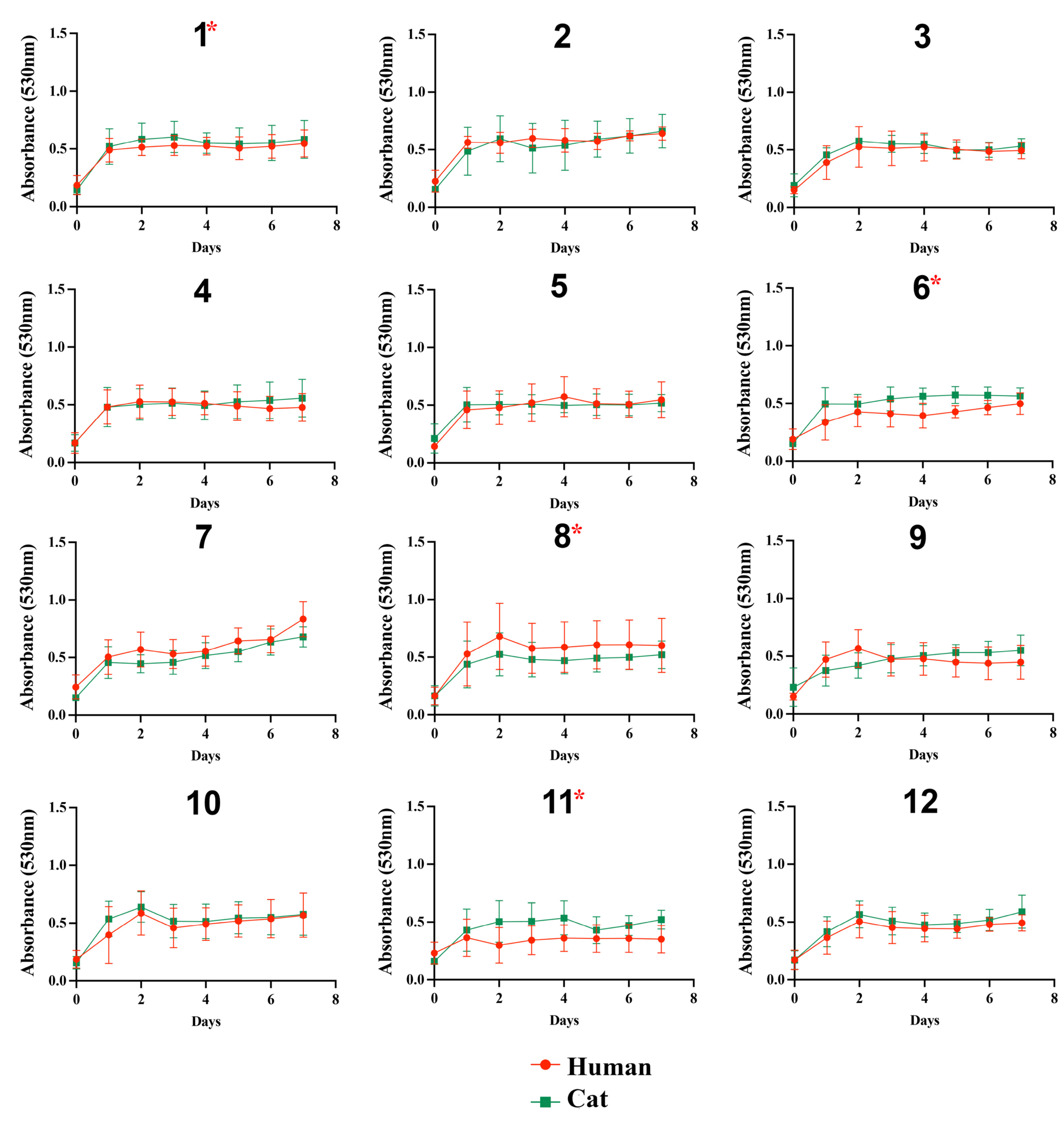
2.3.1. Growth Assay on Different Carbon Sources
2.3.2. Yeast Growth Assay in Powdered Claw Suspension
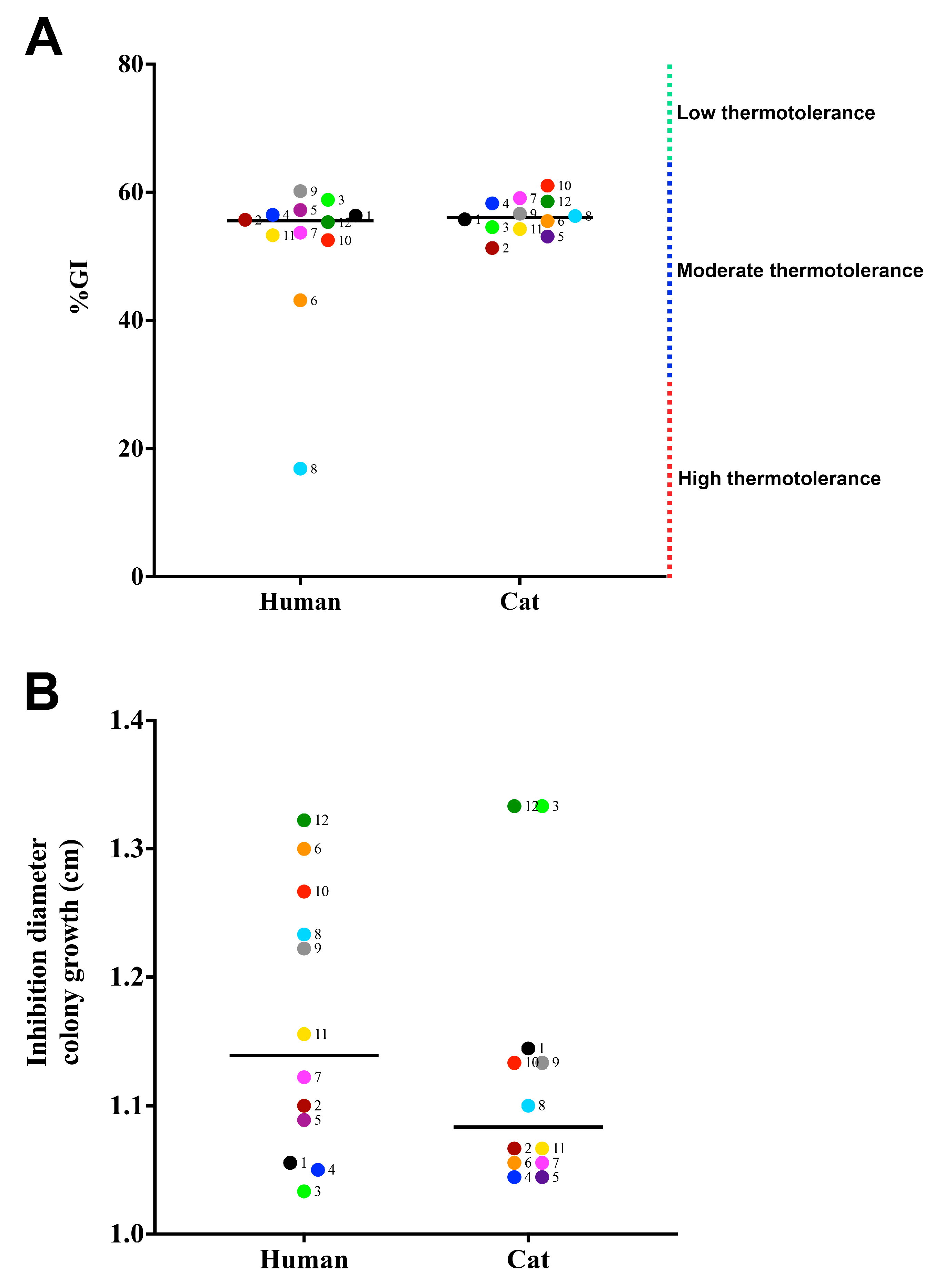
2.4. Thermotolerance Evaluation
2.5. Resistance to Oxidative Stress
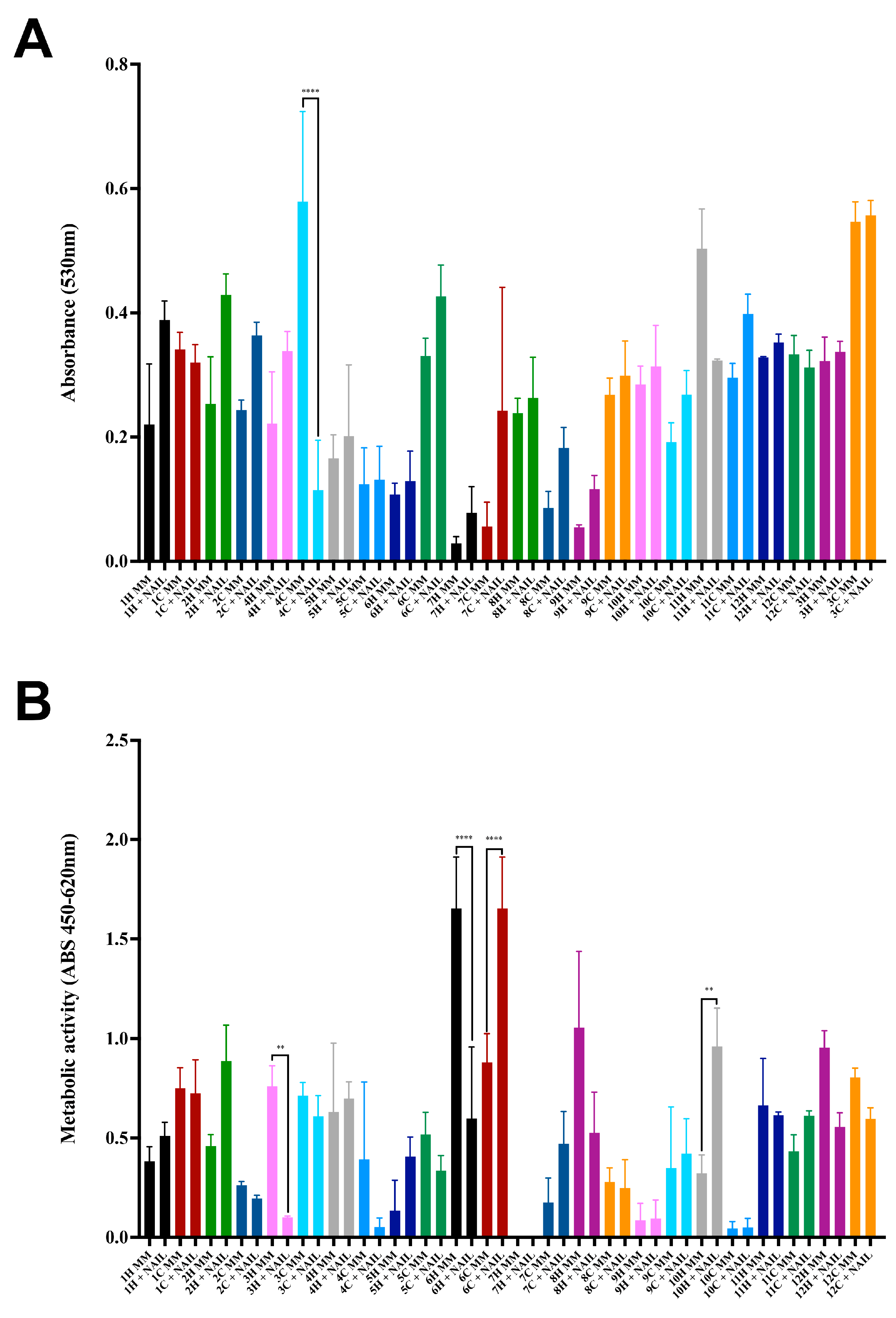
2.6. Production of Extracellular Enzymes
2.6.1. Urease Production
2.6.2. Hemolytic, Phospholipase, and Esterase Production
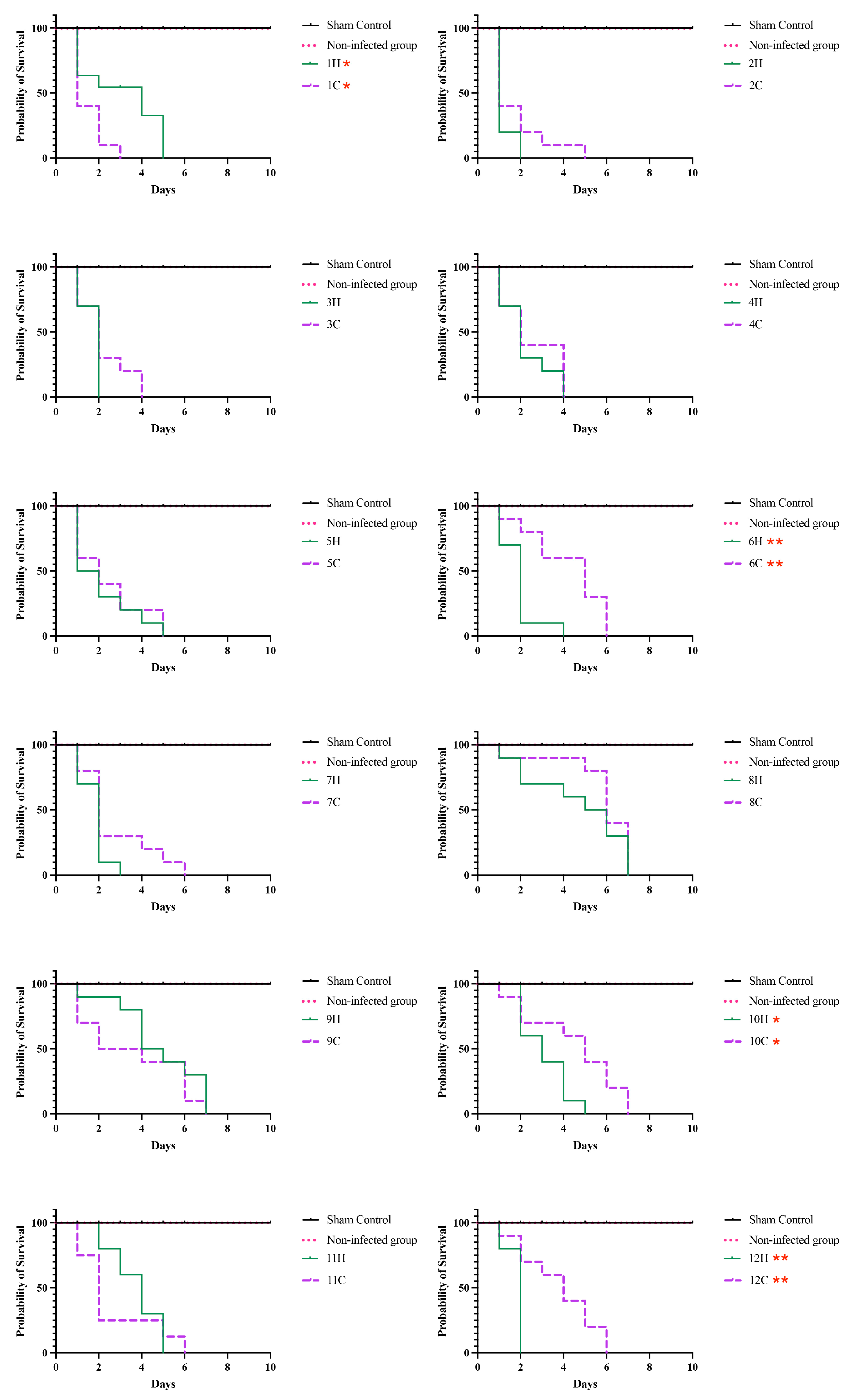
2.7. Survival Tests on Molitor
2.8. Statistical Analyses
3. Results
3.1. Fungal Growth on Different Substrates
3.2. Susceptibility to Physical and Chemical Stressors
3.3. Production of Extracellular Enzymes
3.4. Survival Tests on Tenebrio Molitor
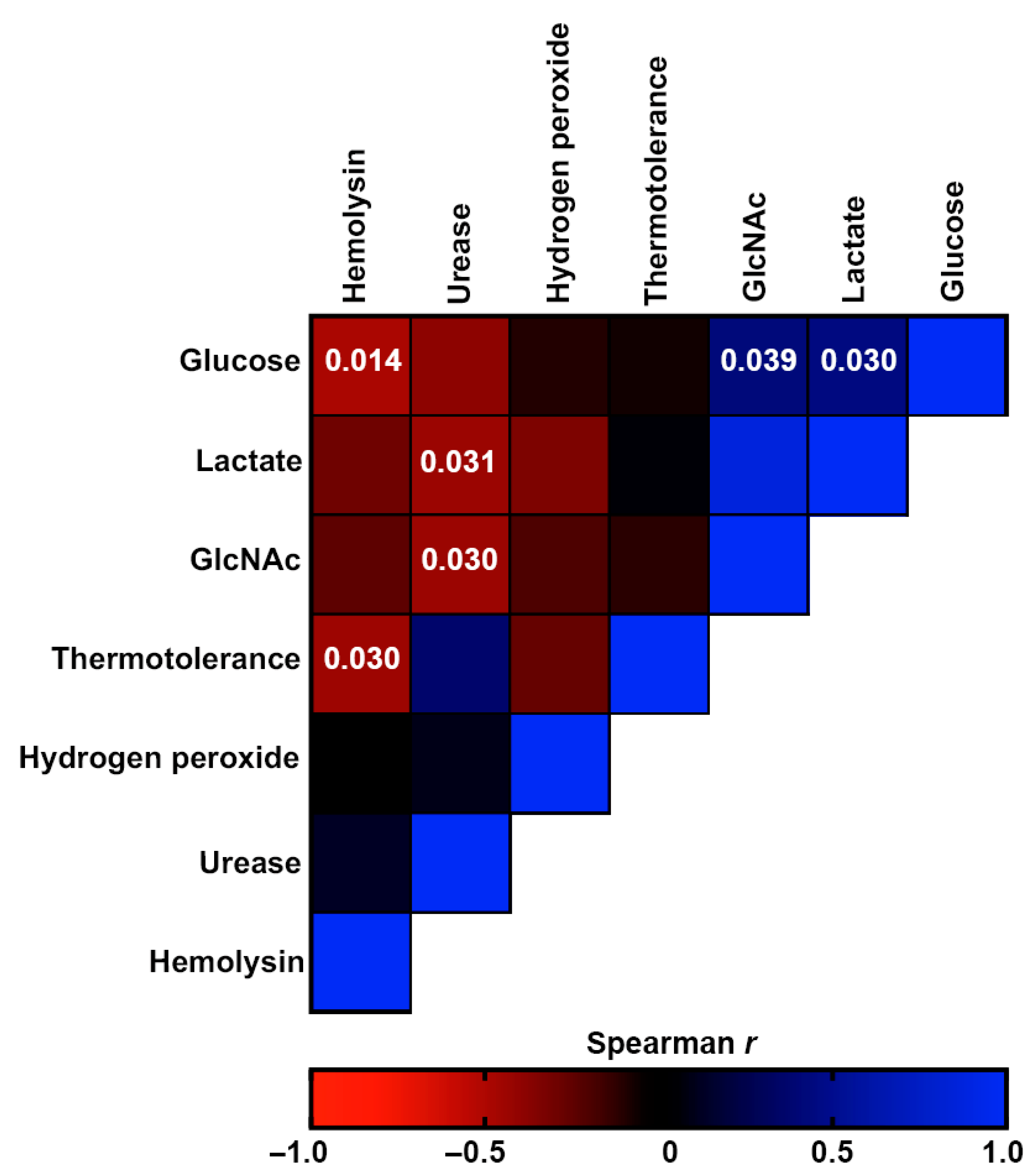
3.5. Comparative Analysis of Virulence-Related Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gremião, I.D.F.; Miranda, L.H.M.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic Epidemic of Sporotrichosis: Cat to Human Transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.M.; Della Terra, P.P.; Gremião, I.D.; Pereira, S.A.; Orofino-Costa, R.; de Camargo, Z.P. The Threat of Emerging and Re-Emerging Pathogenic Sporothrix Species. Mycopathologia 2020, 185, 813–842. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Guarro, J. Atlas of Clinical Fungi. Mycoses 1996, 39, 323. [Google Scholar] [CrossRef]
- Sigler, L.; Harris, J.L.; Dixon, D.M.; Flis, A.L.; Salkin, I.F.; Kemna, M.; Duncan, R.A. Microbiology and Potential Virulence of Sporothrix Cyanescens, a Fungus Rarely Isolated from Blood and Skin. J. Clin. Microbiol. 1990, 28, 1009–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamez-Castrellón, A.; Romeo, O.; García-Carnero, L.; Lozoya-Pérez, N.; Mora-Montes, H. Virulence Factors in Sporothrix Schenckii, One of the Causative Agents of Sporotrichosis. Curr. Protein Pept. Sci. 2020, 21, 295–312. [Google Scholar] [CrossRef]
- Hay, R.J.; Morris-Jones, R. Outbreaks of Sporotrichosis. Curr. Opin. Infect. Dis. 2008, 21, 119–121. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Bennett, J.E. Medical Mycology. Rev. Inst. Med. Trop. Sao Paulo 1992, 34, 504. [Google Scholar] [CrossRef] [Green Version]
- Read, S.I.; Sperling, L.C. Feline Sporotrichosis: Transmission to Man. Arch. Dermatol. 1982, 118, 429–431. [Google Scholar] [CrossRef]
- Orofino-Costa, R.; Rodrigues, A.M.; de Macedo, P.M.; Bernardes-Engemann, A.R. Sporotrichosis: An Update on Epidemiology, Etiopathogenesis, Laboratory and Clinical Therapeutics. An. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef] [Green Version]
- Falcão, E.M.M.; Pires, M.C.D.S.; Andrade, H.B.; Gonçalves, M.L.C.; Almeida-Paes, R.; Do Valle, A.C.F.; Bastos, F.I.; Gutierrez-Galhardo, M.C.; Freitas, D.F.S. Zoonotic Sporotrichosis with Greater Severity in Rio de Janeiro, Brazil: 118 Hospitalizations and 11 Deaths in the Last 2 Decades in a Reference Institution. Med. Mycol. 2020, 58, 141–143. [Google Scholar] [CrossRef]
- Gremião, I.D.F.; Oliveira, M.M.E.; Monteiro de Miranda, L.H.; Saraiva Freitas, D.F.; Pereira, S.A. Geographic Expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezú, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix Brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J. Fungi 2021, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; González, C.; Blank, O.; Ramírez, V.; del Río, C.; Santibáñez, S.; Pena, P. Sporotrichosis Outbreak Due to Sporothrix Brasiliensis in Domestic Cats in Magallanes, Chile: A One-Health-Approach Study. J. Fungi 2023, 9, 226. [Google Scholar] [CrossRef]
- Rachman, R.; Ligaj, M.; Chinthapalli, S.; Wani, R.S. Zoonotic Acquisition of Cutaneous Sporothrix Braziliensis Infection in the UK. BMJ Case Rep. 2022, 15, e248418. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Freitas, D.F.S.; Bernardes-Engemann, A.R.; Rodrigues, A.M.; Talhari, C.; Ferraz, C.E.; Veasey, J.V.; Quintella, L.; de Sousa, M.S.L.A.; Vettorato, R.; et al. Human Sporotrichosis: Recommendations from the Brazilian Society of Dermatology for the Clinical, Diagnostic and Therapeutic Management. Bras Dermatol. 2022, 97, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Silva-Bailão, M.G.; de Lima, P.S.; Oliveira, M.M.E.; Oliveira, L.C.; Almeida-Paes, R.; Borges, C.L.; Bailão, A.M.; Coelho, A.S.G.; de Soares, C.M.A.; Zancopé-Oliveira, R.M. Comparative Proteomics in the Three Major Human Pathogenic Species of the Genus Sporothrix. Microbes Infect. 2021, 23, 104762. [Google Scholar] [CrossRef]
- Boechat, J.S.; Oliveira, M.M.E.; Almeida-Paes, R.; Gremião, I.D.F.; de Machado, A.C.S.; de Oliveira, R.V.C.; Figueiredo, A.B.F.; de Rabello, V.B.S.; Silva, K.B.D.L.; Zancopé-Oliveira, R.M.; et al. Feline Sporotrichosis: Associations between Clinical-Epidemiological Profiles and Phenotypic-Genotypic Characteristics of the Etiological Agents in the Rio de Janeiro Epizootic Area. Mem. Inst. Oswaldo Cruz 2018, 113, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Cruz, I.L.R.; Freitas, D.F.S.; de Macedo, P.M.; Gutierrez-Galhardo, M.C.; do Valle, A.C.F.; de Almeida, M.A.; Coelho, R.A.; Brito-Santos, F.; Figueiredo-Carvalho, M.H.G.; Zancopé-Oliveira, R.M.; et al. Evolution of Virulence-Related Phenotypes of Sporothrix Brasiliensis Isolates from Patients with Chronic Sporotrichosis and Acquired Immunodeficiency Syndrome. Braz. J. Microbiol. 2020, 52, 5. [Google Scholar] [CrossRef]
- Schubach, T.M.P.; de Schubach, A.O.; Okamoto, T.; Figueiredo, F.B.; Pereira, S.A.; Leme, L.R.P.; dos Santos, I.B.; dos Reis, R.S.; de Paes, R.A.; de Perez, M.A.; et al. Utilidade Do Coágulo Sangüíneo Para o Isolamento de Sporothrix Schenckii de Gatos Naturalmente Infectados. Braz. J. Vet. Res. Anim. Sci. 2004, 41, 404–408. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Host-Pathogen Interactions: Redefining the Basic Concepts of Virulence and Pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef] [Green Version]
- Casadevall, A. Fungi and the Rise of Mammals. PLoS Pathog. 2012, 8, e1002808. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Carvalho, M.H.G.; Ramos, L.D.S.; Barbedo, L.S.; De Oliveira, J.C.A.; Dos Santos, A.L.S.; Almeida-Paes, R.; Zancopé-Oliveira, R.M. Relationship between the Antifungal Susceptibility Profile and the Production of Virulence-Related Hydrolytic Enzymes in Brazilian Clinical Strains of Candida Glabrata. Mediat. Inflamm. 2017, 2017, 8952878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, M.S.; Villavicencio, L.L.F.; Arredondo, K.S.; Sabanero, G.B.; Villagómez-Castro, J.C.; Jiménez, G.C.; Bernal, G.S.; Guerrero, H.T. Proteases of Sporothrix Schenckii: Cytopathological Effects on a Host-Cell Model. Rev. Iberoam. De Micol. 2018, 35, 32–38. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; Brito-Santos, F.; Oliveira, M.M.E.; Bailão, A.M.; Borges, C.L.; de Araújo, G.R.S.; Frases, S.; de Soares, C.M.A.; Zancopé-Oliveira, R.M. Interaction with Pantoea Agglomerans Modulates Growth and Melanization of Sporothrix Brasiliensis and Sporothrix Schenckii. Mycopathologia 2019, 184, 367–381. [Google Scholar] [CrossRef]
- Carnero, L.C.G.; Pérez, N.E.L.; Hernández, S.E.G.; Álvarez, J.A.M. Immunity and Treatment of Sporotrichosis. J. Fungi 2018, 4, 100. [Google Scholar] [CrossRef] [Green Version]
- Brilhante, R.S.N.; Fernandes, M.R.; Pereira, V.S.; da Costa, A.C.; de Oliveira, J.S.; de Aguiar, L.; Rodrigues, A.M.; de Camargo, Z.P.; Pereira-Neto, W.A.; Sidrim, J.J.C.; et al. Biofilm Formation on Cat Claws by Sporothrix Species: An Ex Vivo Model. Microb. Pathog. 2021, 150, 104670. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular Diagnosis of Pathogenic Sporothrix Species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef] [Green Version]
- Reis, R.S.; Almeida-Paes, R.; de Muniz, M.M.; Tavares, P.M.e.S.; Monteiro, P.C.F.; Schubach, T.M.P.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M.; Santos Reis, R.; Almeida-Paes, R.; et al. Molecular Characterisation of Sporothrix Schenckii Isolates from Humans and Cats Involved in the Sporotrichosis Epidemic in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2009, 104, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, G.M.P.; Borba-Santos, L.P.; Vila, T.; Gremião, I.D.F.; Pereira, S.A.; De Souza, W.; Rozental, S. Sporothrix Spp. Biofilms Impact in the Zoonotic Transmission Route: Feline Claws Associated Biofilms, Itraconazole Tolerance, and Potential Repurposing for Miltefosine. Pathogens 2022, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.F.S.F.F.S.; Santos, S.S.; Almeida-Paes, R.; De Oliveira, M.M.E.; Do Valle, A.C.F.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M.; Nosanchuk, J.D.; Me De Oliveira, M.; Cf Do Valle, A.; et al. Increase in Virulence of Sporothrix Brasiliensis over Five Years in a Patient with Chronic Disseminated Sporotrichosis. Virulence 2015, 6, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida-Paes, R.; De Oliveira, L.C.; Oliveira, M.M.E.; Gutierrez-Galhardo, M.C.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Phenotypic Characteristics Associated with Virulence of Clinical Isolates from the Sporothrix Complex. Biomed Res. Int. 2015, 2015, 212308. [Google Scholar] [CrossRef] [Green Version]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate Method for Detection of Phospholipase Activity in Candida Albicans. Sabouraudia 1982, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Aktaz, E.; Yigit, N.; Ayyildiz, A. Esterase Activity in Various Candida Species. J. Int. Med. Res. 2002, 30, 322–324. [Google Scholar] [CrossRef]
- Lozoya-Pérez, N.E.; García-Carnero, L.C.; Martínez-Álvarez, J.A.; Martínez-Duncker, I.; Mora-Montes, H.M. Tenebrio Molitor as an Alternative Model to Analyze the Sporothrix Species Virulence. Infect. Drug Resist. 2021, 14, 2059–2072. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-Enabled Heat Mapping for All. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Kaerger, K.; Schwartze, V.U.; Dolatabadi, S.; Nyilasi, I.; Kovács, S.A.; Binder, U.; Papp, T.; de Hoog, S.; Jacobsen, I.D.; Voigt, K. Adaptation to Thermotolerance in Rhizopus Coincides with Virulence as Revealed by Avian and Invertebrate Infection Models, Phylogeny, Physiological and Metabolic Flexibility. Virulence 2015, 6, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamayo, D.; Muñoz, J.F.; Almeida, A.J.; Puerta, J.D.; Restrepo, Á.; Cuomo, C.A.; McEwen, J.G.; Hernández, O. Paracoccidioides Spp. Catalases and Their Role in Antioxidant Defense against Host Defense Responses. Fungal Genet Biol. 2017, 100, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mroczyńska, M.; Brillowska-Dabrowska, A. Virulence of Clinical Candida Isolates. Pathogens 2021, 10, 466. [Google Scholar] [CrossRef]
- de Andrade, I.B.; Figueiredo-Carvalho, M.H.G.; da Silva Chaves, A.L.; Coelho, R.A.; Almeida-Silva, F.; Zancopé-Oliveira, R.M.; Frases, S.; Brito-Santos, F.; Almeida-Paes, R. Metabolic and Phenotypic Plasticity May Contribute for the Higher Virulence of Trichosporon Asahii over Other Trichosporonaceae Members. Mycoses 2022, 66, 430–440. [Google Scholar] [CrossRef]
- Miao, J.; Regan, J.; Cai, C.; Palmer, G.E.; Williams, D.L.; Kruppa, M.D.; Peters, B.M. Glycogen Metabolism in Candida Albicans Impacts Fitness and Virulence during Vulvovaginal and Invasive Candidiasis. mBio 2023, 14. [Google Scholar] [CrossRef]
- Cabañes, F.J. Sporotrichosis in Brazil: Animals + humans = one Health. Rev. Iberoam. Micol. 2020, 37, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Araújo, G.R.D.S.; De Souza, W.; Frases, S. The Hidden Pathogenic Potential of Environmental Fungi. Future Microbiol. 2017, 12, 1533–1540. [Google Scholar] [CrossRef]
- Ballard, E.; Melchers, W.J.G.; Zoll, J.; Brown, A.J.P.; Verweij, P.E.; Warris, A. In-Host Microevolution of Aspergillus Fumigatus: A Phenotypic and Genotypic Analysis. Fungal Genet. Biol. 2018, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Chen, M.; Xu, J. Molecular Markers Reveal Epidemiological Patterns and Evolutionary Histories of the Human Pathogenic Cryptococcus. Front. Cell Infect. Microbiol. 2021, 11, 683670. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, N.; Harris, S.D.; Everhart, S.E. Evolutionary Significance of Fungal Hypermutators: Lessons Learned from Clinical Strains and Implications for Fungal Plant Pathogens. mSphere 2022, 7, e0008722. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, Y.; Xu, J. Phenotypic Plasticity in the Productions of Virulence Factors Within and Among Serotypes in the Cryptococcus Neoformans Species Complex. Mycopathologia 2022, 187, 65–83. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, Y.; Tan, J.; Peng, J.; Hu, N.; Liu, Q.; Cao, Y.L.; Zhang, Y.; Chen, J.; Huang, X. QCR7 Affects the Virulence of Candida Albicans and the Uptake of Multiple Carbon Sources Present in Different Host Niches. Front. Cell Infect. Microbiol. 2023, 13, 1136698. [Google Scholar] [CrossRef]
- Al-Attas, S.A.; Amro, S.O. Candidal Colonization, Strain Diversity, and Antifungal Susceptibility among Adult Diabetic Patients. Ann. Saudi Med. 2010, 30, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Ballou, E.R.; Avelar, G.M.; Childers, D.S.; Mackie, J.; Bain, J.M.; Wagener, J.; Kastora, S.L.; Panea, M.D.; Hardison, S.E.; Walker, L.A.; et al. Lactate Signalling Regulates Fungal β-Glucan Masking and Immune Evasion. Nat. Microbiol. 2016, 2, 16238. [Google Scholar] [CrossRef] [Green Version]
- Barelle, C.J.; Priest, C.L.; MacCallum, D.M.; Gow, N.A.R.; Odds, F.C.; Brown, A.J.P. Niche-Specific Regulation of Central Metabolic Pathways in a Fungal Pathogen. Cell Microbiol. 2006, 8, 961–971. [Google Scholar] [CrossRef] [Green Version]
- CHINNAPUN, D. Virulence Factors Involved in Pathogenicity of Dermatophytes. J. Sci. Tech. 2015, 12, 573–580. [Google Scholar]
- Hansberg, W.; Salas-Lizana, R.; Domínguez, L. Fungal Catalases: Function, Phylogenetic Origin and Structure. Arch. Biochem. Biophys. 2012, 525, 170–180. [Google Scholar] [CrossRef]
- Román, E.; Prieto, D.; Martin, R.; Correia, I.; Mesa Arango, A.C.; Alonso-Monge, R.; Zaragoza, O.; Pla, J. Role of Catalase Overproduction in Drug Resistance and Virulence in Candida Albicans. Future Microbiol. 2016, 11, 1279–1297. [Google Scholar] [CrossRef]
- Shakesheff, K.M.; Evora, C.; Soriano, I.; Langer, R. The Adsorption of Poly(Vinyl Alcohol) to Biodegradable Microparticles Studied by X-Ray Photoelectron Spectroscopy (XPS). J. Colloid Interface Sci. 1997, 185, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.D.; Del Giudice, G.; Rappuoli, R. Helicobacter Pylori Interactions with Host Serum and Extracellular Matrix Proteins: Potential Role in the Infectious Process. Microbiol. Mol. Biol. Rev. 2002, 66, 617–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olszewski, M.A.; Noverr, M.C.; Chen, G.-H.; Toews, G.B.; Cox, G.M.; Perfect, J.R.; Huffnagle, G.B. Urease Expression by Cryptococcus Neoformans Promotes Microvascular Sequestration, Thereby Enhancing Central Nervous System Invasion. Am. J. Pathol. 2004, 164, 1761. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.A.; Freitas, D.F.S.; Oliveira, R.V.C.; Fichman, V.; Varon, A.G.; Freitas, A.D.; Lamas, C.C.; Andrade, H.B.; Veloso, V.G.; Almeida-Paes, R.; et al. Meningeal Sporotrichosis Due to Sporothrix Brasiliensis: A 21-Year Cohort Study from a Brazilian Reference Center. J. Fungi 2022, 9, 17. [Google Scholar] [CrossRef]
- Nayak, A.P.; Green, B.J.; Beezhold, D.H. Fungal Hemolysins. Med. Mycol. 2013, 51, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lloret, A.; Hartmann, K.; Pennisi, M.G.; Ferrer, L.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Sporotrichosis in Cats: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2013, 15, 619–623. [Google Scholar] [CrossRef]
- Nevitt, T.; Thiele, D.J. Host Iron Withholding Demands Siderophore Utilization for Candida Glabrata to Survive Macrophage Killing. PLoS Pathog. 2011, 7, e1001322. [Google Scholar] [CrossRef]
- De Lima Barros, M.B.; Pacheco Schubach, T.M.; Gutierrez Galhardo, M.C.; De Oliveira Schubach, A.; Fialho Monteiro, P.C.; Santos Reis, R.; Zancopé-Oliveira, R.M.; Dos Santos Lazéra, M.; Cuzzi-Maya, T.; Moita Blanco, T.C.; et al. Sporotrichosis: An Emergent Zoonosis in Rio de Janeiro. Mem. Inst. Oswaldo Cruz 2001, 96, 777–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, P.C.; Morey, A.T.; Castanheira, G.M.; Bocate, K.P.; Panagio, L.A.; Ito, F.A.; Furlaneto, M.C.; Yamada-Ogatta, S.F.; Costa, I.N.; Mora-Montes, H.M.; et al. Tenebrio Molitor (Coleoptera: Tenebrionidae) as an Alternative Host to Study Fungal Infections. J. Microbiol. Methods 2015, 118, 182–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rantala, M.J.; Dubovskiy, I.M.; Pölkki, M.; Krama, T.; Contreras-Garduño, J.; Krams, I.A. Effect of Juvenile Hormone on Resistance against Entomopathogenic Fungus Metharizium Robertsii Differs between Sexes. J. Fungi 2020, 6, 298. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Stafford, K.C. Potential of Tenebrio Molitor (Coleoptera: Tenebrionidae) as a Bioassay Probe for Metarhizium Brunneum (Hypocreales: Clavicipitaceae) Activity against Ixodes Scapularis (Acari: Ixodidae). J. Econ. Entomol. 2011, 104, 2095–2098. [Google Scholar] [CrossRef]
- Jirakkakul, J.; Roytrakul, S.; Srisuksam, C.; Swangmaneecharern, P.; Kittisenachai, S.; Jaresitthikunchai, J.; Punya, J.; Prommeenate, P.; Senachak, J.; So, L.; et al. Culture Degeneration in Conidia of Beauveria Bassiana and Virulence Determinants by Proteomics. Fungal Biol. 2018, 122, 156–171. [Google Scholar] [CrossRef]
- Fornari, G.; Gomes, R.R.; Degenhardt-Goldbach, J.; Do sSantos, S.S.; De Almeida, S.R.; Dos Santos, G.D.; Muro, M.D.; Bona, C.; Scola, R.H.; Trindade, E.S.; et al. A Model for Trans-Kingdom Pathogenicity in Fonsecaea Agents of Human Chromoblastomycosis. Front. Microbiol. 2018, 9, 2211. [Google Scholar] [CrossRef] [Green Version]





| Case | Original Number | Host | Strain Identification |
|---|---|---|---|
| 1 | 15485-1 | Human | 1H |
| 34-1 | Cat | 1C | |
| 2 | 15647-1 | Human | 2H |
| 47 | Cat | 2C | |
| 3 | 19536 | Human | 3H |
| 856 | Cat | 3C | |
| 4 | 16393-2 | Human | 4H |
| 92-2 | Cat | 4C | |
| 5 | 16415 | Human | 5H |
| 99-2 | Cat | 5C | |
| 6 | 16459 | Human | 6H |
| 75-1 | Cat | 6C | |
| 7 | 16672 | Human | 7H |
| 142-1 | Cat | 7C | |
| 8 | 17878 | Human | 8H |
| 98-2 | Cat | 8C | |
| 9 | 16910-1 | Human | 9H |
| 195-1 | Cat | 9C | |
| 10 | 17500 | Human | 10H |
| 165-1 | Cat | 10C | |
| 11 | 19182 | Human | 11H |
| 721-1 | Cat | 11C | |
| 12 | 19481 | Human | 12H |
| 792-1 | Cat | 12C |
| Virulence Factor(s) | Correlation with Time to Death (r) | R2 Change | df | F Value | p Value |
|---|---|---|---|---|---|
| Glucose | 4765 | 0.3199 | 1 | 4765 | 0.0443 |
| Lactate | −7434 | 0.2641 | 1 | 4202 | 0.0571 |
| NAG | −3996 | 0.2695 | 1 | 1879 | 0.1894 |
| Thermotolerance | 1144 | 0.4754 | 1 | 0.05934 | 0.8106 |
| Hydrogen peroxide | −0.5222 | 0.1866 | 1 | 0.01536 | 0.9029 |
| Urease | −8150 | 0.4145 | 1 | 1220 | 0.2858 |
| Hemolysin | −3454 | 0.3832 | 1 | 1480 | 0.2399 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa-Junior, D.; de Andrade, I.B.; Alves, V.; Avellar-Moura, I.; Rodrigues-Alves, T.; de Souza Rabello, V.B.; de S. Araújo, G.R.; Borba-Santos, L.P.; Zancopé-Oliveira, R.M.; Almeida-Paes, R.; et al. Metabolic Plasticity and Virulence-Associated Factors of Sporothrix brasiliensis Strains Related to Familiar Outbreaks of Cat-to-Human Transmitted Sporotrichosis. J. Fungi 2023, 9, 724. https://doi.org/10.3390/jof9070724
Corrêa-Junior D, de Andrade IB, Alves V, Avellar-Moura I, Rodrigues-Alves T, de Souza Rabello VB, de S. Araújo GR, Borba-Santos LP, Zancopé-Oliveira RM, Almeida-Paes R, et al. Metabolic Plasticity and Virulence-Associated Factors of Sporothrix brasiliensis Strains Related to Familiar Outbreaks of Cat-to-Human Transmitted Sporotrichosis. Journal of Fungi. 2023; 9(7):724. https://doi.org/10.3390/jof9070724
Chicago/Turabian StyleCorrêa-Junior, Dario, Iara Bastos de Andrade, Vinicius Alves, Igor Avellar-Moura, Tânia Rodrigues-Alves, Vanessa Brito de Souza Rabello, Glauber R. de S. Araújo, Luana Pereira Borba-Santos, Rosely Maria Zancopé-Oliveira, Rodrigo Almeida-Paes, and et al. 2023. "Metabolic Plasticity and Virulence-Associated Factors of Sporothrix brasiliensis Strains Related to Familiar Outbreaks of Cat-to-Human Transmitted Sporotrichosis" Journal of Fungi 9, no. 7: 724. https://doi.org/10.3390/jof9070724






