Morphological and Phylogenetic Characterization of Five Novel Nematode-Trapping Fungi (Orbiliomycetes) from Yunnan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Fungal Isolation
2.3. Morphological Observation
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Phylogenetic Analysis
3. Results
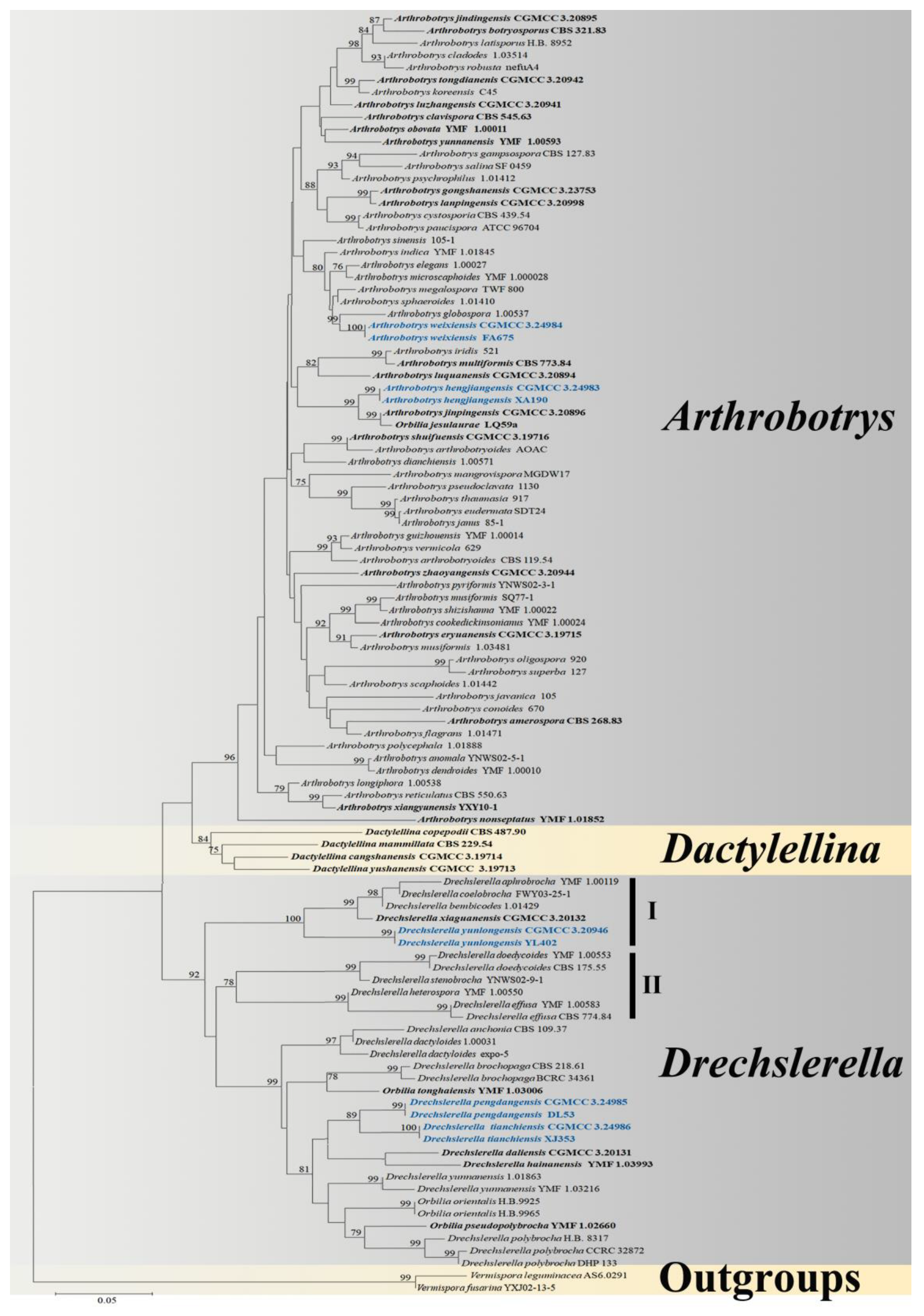
3.1. Phylogenetic Analysis
3.2. Taxonomy
3.3. Key to Known Species of Drechslerella
- 1. Conidia without super-cell…………………………………...………………………………2
- 1. Conidia with a super-cell………………………………………………………….…….……5
- 2. Conidia 1–3-septate…………………………………………………………………….……...3
- 2. Conidia 0–1-septate…………………………………………………………………….…...…4
- 3. 5–10 conidia cluster arrangement on a cluster of short denticles (5–10) at the apex of conidiophore, conidia 28.5–39.0 × 6.0–8.5 µm, microconidia cylindrical……………………………………………………………………………..O. tonghaiensis
- 3. Conidiophore produce 3–8 short denticles by repeated elongation, conidia are cylindrical, botuliform, 20–45 (30) × 5–12.5 (6) µm, and do not produce microconidia…………………………………………………………………………….....Dr. brochapaga
- 4. Conidia digitiform are mostly curved, 1-septate, 35–51.5 (42.1) × 6.5–8 (7.5) µm, with 3–13 conidia capitate arrangement at the apex of conidiophore…………..Dr. dactyleoids
- 4. Conidia are elongated and ellipsoidal, straight, 0-1-septate, 7.8–12.9 × 3.3–4.2 µm………………………………………………………………..………….Dr. yunnanensis
- 5. Conidia are sub-fusiform to fusiform……………………………………………………..…6
- 5. Conidia are ellipsoidal, elongate ellipsoidal, subellipsoidal, or obovate……………….12
- 6. Conidia are 1–2-septate…………………………………………………….....Dr. acrochaetum
- 6. Conidia are 1–5-septate………………………………………………………………….…....7
- 7. Conidia are 1–4-septate, mostly 3-septate……………………………………………….…..8
- 7. Conidia are 1–5-septate, mostly 4-septate…………………………………………………10
- 8. Conidia are smaller in size, 33–52 (42.5) × 9.5–28 (15.5) µm, swollen at both ends of cells………………….…………………………………………………..…..Dr. xiaguanensis
- 8. Conidia are bigger, sometimes more than 52 µm in length and usually greater than 15 µm in width; the cells at both ends are not enlarged……………………………….…..9
- 9. Conidia are wider, 40–57.5 (51) × 15.5–35 (24.6) µm, 2–4-septate, and conidiophore occasionally bear two conidia……………………………………....….…….Dr. aphrobrocha
- 9. Conidia are narrower, 42.5–62.5 (47) × 15–22.5 (16.9) µm, 1–4-septate, sub-fusiform, and conidiophore bear a single conidium……………………………………..…Dr. inquisitor
- 10. Conidia are 3–4-septate, smaller in size, 36–43.2 (40) × 16.8–21.6 (20.5) µm, producing obovoid, 1-septate microconidia………………………………………..….Dr. bembicodes
- 10. Conidia are bigger, do not produce microconidia………………………………………11
- 11. Conidia are 1–4-septate, 36–54 (47) × 17–27 (23.6) µm, producing cylindrical, globose, or ellipsoidal chlamydospore…………………………………………....Dr. yunlongensis
- 11. Conidia are 2–5-septate, 45.6–55.2 (49.5) × 16.8–21.6 (19.8) µm, both ends cells are slender, and do not produce chlamydospore……………………………..Dr. coelobrocha
- 12. Conidia are obovate and 1-septate…………………………………………………….….13
- 12. Conidia are ellipsoidal, elongate ellipsoidal, and 0–3-septate………………………....14
- 13. Conidia are obovate, 29–43 (35) × 15–19 (16.8) µm, base cells are pyramidal, with 3–8 conidia capitate arrangement at the apex of conidiophore….....................Dr. anchonia
- 13. Conidia are obovate or sub-ellipsoidal, 35 × 24 µm, single conidium bear at the apex of conidiophore……………………………………………………………..…...Dr. polybrocha
- 14. Conidiophore is branched or bears more than 1 conidium………………………….…15
- 14. Conidiophore is unbranched, bears a single conidium……………………………..…..16
- 15. Conidiophore is unbranched or produces 1–2 short branches near the apex, each branch bearing a single conidium, with conidia 30–41 (36.2) × 14.5–24 (18.7) µm, 1–2-septate………………………………………………………………...……Dr. tianchiensis
- 15. Conidiophore is unbranched, bearing a loose head consisting of 2–12 conidia, with conidia 32.5–45 (38.9) × 17.5–25 (21.4) µm, 1–2-septate……………………..….Dr. effusa
- 16. Conidiophore produces a swollen, knob-like apex………………………………..……17
- 16. Conidiophore produces a truncated, non-swelling apex……………………………....20
- 17. Produces cylindrical, clavate, or bottle-shaped, 1-septate microconidia……………...18
- 17. Does not produce microconidia…………………………………………………………..19
- 18. Macroconidia are bigger, 17.5–45 (34) × 17.5–25 (20.4) µm, 1–2-septate, mostly 1-septate, and microconidia are bigger, 23–40 (31.3)× 5–8 (6.8) µm……………………………………………………………………….....…Dr. heterospora
- 18. Macroconidia are smaller, 26–30 × 16–22.2 µm, 0–2-septate, mostly 2-septate, and microconidia smaller, 14.7–23 × 3.3–6 µm…………………….………...O. pseudopolybrocha
- 19. Conidia are bigger, 30–45 (38) × 17–27 (22.4) µm, 1–2-septate, and basal cells are tiny…………………………………………………………………...…...…Dr. pengdangensis
- 19. Conidia are 25–52.5 (33.2) × 12.5–29 (17.3) µm, and 1–3-septate………….Dr. doedycoides
- 20. Conidia are elongated and ellipsoidal, 1–3-septate, mostly 3-septate, 34–56.5 × 12.5–16.5 µm, and do not produce microconidia………………………………Dr. stenobrocha
- 20. Conidia are ellipsoidal, 0–2-septate, and produce clavate or bottle-shaped microconidia……………………………………………………………………………………...21
- 21. Macroconidia are thinner, 20–49.5 (38.5) × 8.5–15 (12) µm, 1–2-septate, mostly 2-septate, and microconidia wider, 6.5–22 (15.5) × 3.5–7 (5) µm……….……Dr. daliensis
- 21. Macroconidia are 32.5–43 × 17–25 µm, 0–2-septate, mostly 1 or 2-septate, and microconidia are 18.2–22.8 × 4.2–5.3 µm…………………………..…..…………Dr. hainanensis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swe, A.; Li, J.; Zhang, K.Q.; Pointing, S.B.; Jeewon, R.; Hyde, K.D. Nematode-trapping fungi. Curr. Res. Environ. Appl. Mycol. 2011, 1, 1–26. [Google Scholar]
- Zhang, K.Q.; Mo, M.H. Flora Fungorum Sinicorum: Arthrobotrys et Gengra Cetera Cognata; Science Press: Beijing, China, 2006; Volume 33. [Google Scholar]
- Zhang, K.Q.; Hyde, K.D. Nematode-Trapping Fungi; Springer Science & Business: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Corda, A.K.J. Pracht-Flora Europaeischer Schimmelbildungen; G. Fleischer: Leipzig, Germany, 1839; p. 43. [Google Scholar]
- Drechsler, C. Morphological features of some more fungi that capture and kill nematode. J. Wash. Acad. Sci. 1933, 23, 267–270. [Google Scholar]
- Wang, X.; Li, G.H.; Zou, C.G.; Ji, X.L.; Liu, T.; Zhao, P.J.; Liang, L.M.; Xu, J.P.; An, Z.Q.; Zheng, X.; et al. Bacteria can mobilize nematode-trapping fungi to kill nematodes. Nat. Commun. 2014, 5, 5776. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.Q.; Zhang, F.; Li, Z.Q.; Zhou, F.P.; Yang, X.Y.; Xiao, W. Morphological and multigene phylogenetic analyses reveal two new nematode-trapping fungi (Arthrobotrys, Orbiliaceae) from Yunnan, China. Phytotaxa 2023, 591, 263–272. [Google Scholar] [CrossRef]
- Zopf, W.F. Zur Kenntniss der Infections-Krankheiten niederer Thiere und Pflanzen. Acad. Nat. 1888, 52, 314–376. [Google Scholar]
- Li, T.F.; Zhang, K.Q.; Liu, X.Z. Taxonomy of Nematophagous Fungi; Chinese Scientific and Technological Publications: Beijing, China, 2000. [Google Scholar]
- Yang, E.; Xu, L.; Yang, Y.; Zhang, X.; Xiang, M.; Wang, C.; An, Z.Q.; Liu, X.Z. Origin and evolution of carnivorism in the Ascomycota (fungi). Proc. Natl. Acad. Sci. USA 2012, 109, 10960–10965. [Google Scholar] [CrossRef]
- Ahrén, D.; Ursing, B.M.; Tunlid, A. Phylogeny of nematode-trapping fungi based on 18S rDNA sequences. FEMS Microbiol. Lett. 1998, 158, 179–184. [Google Scholar] [CrossRef]
- Pfister, D.H. Castor, Pollux and life histories of fungi. Mycologia 1997, 89, 1–23. [Google Scholar] [CrossRef]
- Scholler, M.; Hagedorn, G.; Rubner, A. A reevaluation of predatory orbiliaceous fungi. II. A new generic concept. Sydowia 1999, 51, 89–113. [Google Scholar]
- Yang, Y.; Yang, E.; An, Z.; Liu, X.Z. Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on evidence from rRNA-encoding DNA and multiprotein sequences. Proc. Natl. Acad. Sci. USA 2007, 104, 8379–8384. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.L.; Liu, B.; Liu, X.Z. Taxonomy of Dactylella complex and Vermispora. I. Generic concepts based on morphology and ITS sequences data. Fungal Divers. 2007, 26, 73–83. [Google Scholar]
- Cooke, R.C.; Dickinson, C.H. Nematode-trapping species of Dactylella and Monacrosporium. Trans. Br. Mycol. Soc. 1965, 48, 621–629. [Google Scholar] [CrossRef]
- Schenck, S.; Kendrick, W.B.; Pramer, D. A new nematode-trapping hyphomycete and a reevaluation of Dactylaria and Arthrobotrys. Can. J. Bot. 1977, 55, 977–985. [Google Scholar] [CrossRef]
- Júnior, A.D.; Ferreira, V.M.; de Carvalho, L.M.; Álvares, F.B.V.; Vilela, V.L.R.; Ferraz, C.M.; Veloso, F.B.R.; Lima, T.F.; Braga, F.R.; de Araújo, J.V. Association of the nematophagous fungi Arthrobotrys musiformis and Monacrosporium sinense in vitro and in vivo for biological control of equine cyathostomins. Braz. J. Vet. Med. 2021, 43, e003021. [Google Scholar] [CrossRef]
- Soliman, M.S.; El-Deriny, M.M.; Ibrahim, D.S.S.; Zakaria, H.; Ahmed, Y. Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J. Appl. Microbiol. 2021, 131, 2402–2415. [Google Scholar] [CrossRef]
- Subramanian, C.V. Dactylella, Monacrosporium and Dactylina. J. Indian Bot. Soc. 1963, 42, 291–300. [Google Scholar]
- Liu, X.Z.; Zhang, K.Q. Nematode-trapping species of Monacrosporium with special reference to two new species. Mycol. Res. 1994, 98, 862–868. [Google Scholar] [CrossRef]
- Zhang, F.; Boonmee, S.; Bhat, J.D.; Xiao, W.; Yang, X.Y. New Arthrobotrys Nematode-Trapping Species (Orbiliaceae) from Terrestrial Soils and Freshwater Sediments in China. J. Fungi 2022, 8, 671. [Google Scholar] [CrossRef]
- Drechsler, C. Predacious fungi. Biol. Rev. Camb. Philos. Soc. 1941, 16, 265–290. [Google Scholar] [CrossRef]
- Duddington, C.L. Notes on the technique of handling predacious fungi. Trans. Brit. Mycol. Soc. 1955, 38, 97–103. [Google Scholar] [CrossRef]
- Eren, J.; Pramer, D. The most probable number of nematode-trapping fungi in soil. Soil Sci. 1965, 99, 285. [Google Scholar] [CrossRef]
- Gao, R.H.; Lei, L.P.; Liu, X.Z. A simple method for inducing and observing predacious devices of nematode-trapping fungi. Acta Mycol. Sin. 1996, 4, 304–305+326. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [Green Version]
- Swindell, S.R.; Plasterer, T.N. Seqman. In Sequence Data Analysis Guidebook; Swindell, S.R., Ed.; Springer: Totowa, NJ, USA, 1997; pp. 75–89. [Google Scholar] [CrossRef]
- Li, J.; Qian, W.; Qiao, M.; Bai, Y.; Yu, Z.F. A new Drechslerella species from Hainan, China. Mycotaxon 2013, 125, 183–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, M.; Baral, H.O.; Xu, J.; Zhang, K.Q.; Yu, Z.F. Morphological and molecular characterization of Orbilia pseudopolybrocha and O. tonghaiensis, two new species of Orbiliaceae from China. Int. J. Syst. Evol. Microbiol. 2020, 70, 2664–2676. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Jiang, L.; Yang, Y.Q.; Yang, X.Y.; Xiao, W. Two new nematode-trapping fungi (Arthrobotrys, Orbiliaceae) from Yunnan, China. Phytotaxa 2022, 568, 255–266. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. Available online: http://mafft.cbrc.jp/alignment/server (accessed on 15 March 2023). [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A. FigTree v1. 3.1. 2010. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 7 May 2020).
- tropical nematode-trapping fungus from Puerto Rico, supported by morphology and molecular phylogenetics. Willdenowia 2020, 50, 241–251. [CrossRef]
- Zhang, F.; Boonmee, S.; Monkai, J.; Yang, X.Y.; Xiao, W. Drechslerella daliensis and D. xiaguanensis (Orbiliales, Orbiliaceae), two new nematode-trapping fungi from Yunnan, China. Biodivers. Data J. 2022, 10, e96642. [Google Scholar] [CrossRef]
- Li, Y.; Hyde, K.D.; Jeewon, R.; Cai, L.; Vijaykrishna, D.; Zhang, K. Phylogenetics and evolution of nematode-trapping fungi (Orbiliales) estimated from nuclear and protein coding genes. Mycologia 2005, 97, 1034–1046. [Google Scholar] [CrossRef]
- Mo, M.; Huang, X.; Zhou, W.; Huang, Y.; Hao, Y.E.; Zhang, K.Q. Arthrobotrys yunnanensis sp. nov., the fourth anamorph of Orbilia auricolor. Fungal Divers. 2005, 18, 107–115. [Google Scholar]
- Norvell, L.L. Fungal nomenclature. 1. Melbourne approves a new Code. Mycotaxon 2011, 116, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Wingfield, M.J.; De Beer, Z.W.; Slippers, B.; Wingfield, B.D.; Groenewald, J.Z.; Lombard, L.; Crous, P.W. One fungus, one name promotes progressive plant pathology. Mol. Plant Pathol. 2012, 13, 604–613. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Yang, Y.-Q.; Zhou, F.-P.; Xiao, W.; Boonmee, S.; Yang, X.-Y. Morphological and Phylogenetic Characterization of Five Novel Nematode-Trapping Fungi (Orbiliomycetes) from Yunnan, China. J. Fungi 2023, 9, 735. https://doi.org/10.3390/jof9070735
Zhang F, Yang Y-Q, Zhou F-P, Xiao W, Boonmee S, Yang X-Y. Morphological and Phylogenetic Characterization of Five Novel Nematode-Trapping Fungi (Orbiliomycetes) from Yunnan, China. Journal of Fungi. 2023; 9(7):735. https://doi.org/10.3390/jof9070735
Chicago/Turabian StyleZhang, Fa, Yao-Quan Yang, Fa-Ping Zhou, Wen Xiao, Saranyaphat Boonmee, and Xiao-Yan Yang. 2023. "Morphological and Phylogenetic Characterization of Five Novel Nematode-Trapping Fungi (Orbiliomycetes) from Yunnan, China" Journal of Fungi 9, no. 7: 735. https://doi.org/10.3390/jof9070735




