Sclerotinia sclerotiorum Agglutinin Modulates Sclerotial Development, Pathogenicity and Response to Abiotic and Biotic Stresses in Different Manners
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Cultural Media
2.2. Sequence Analysis of SSA
2.3. Extraction of DNA/RNA and cDNA Synthesis
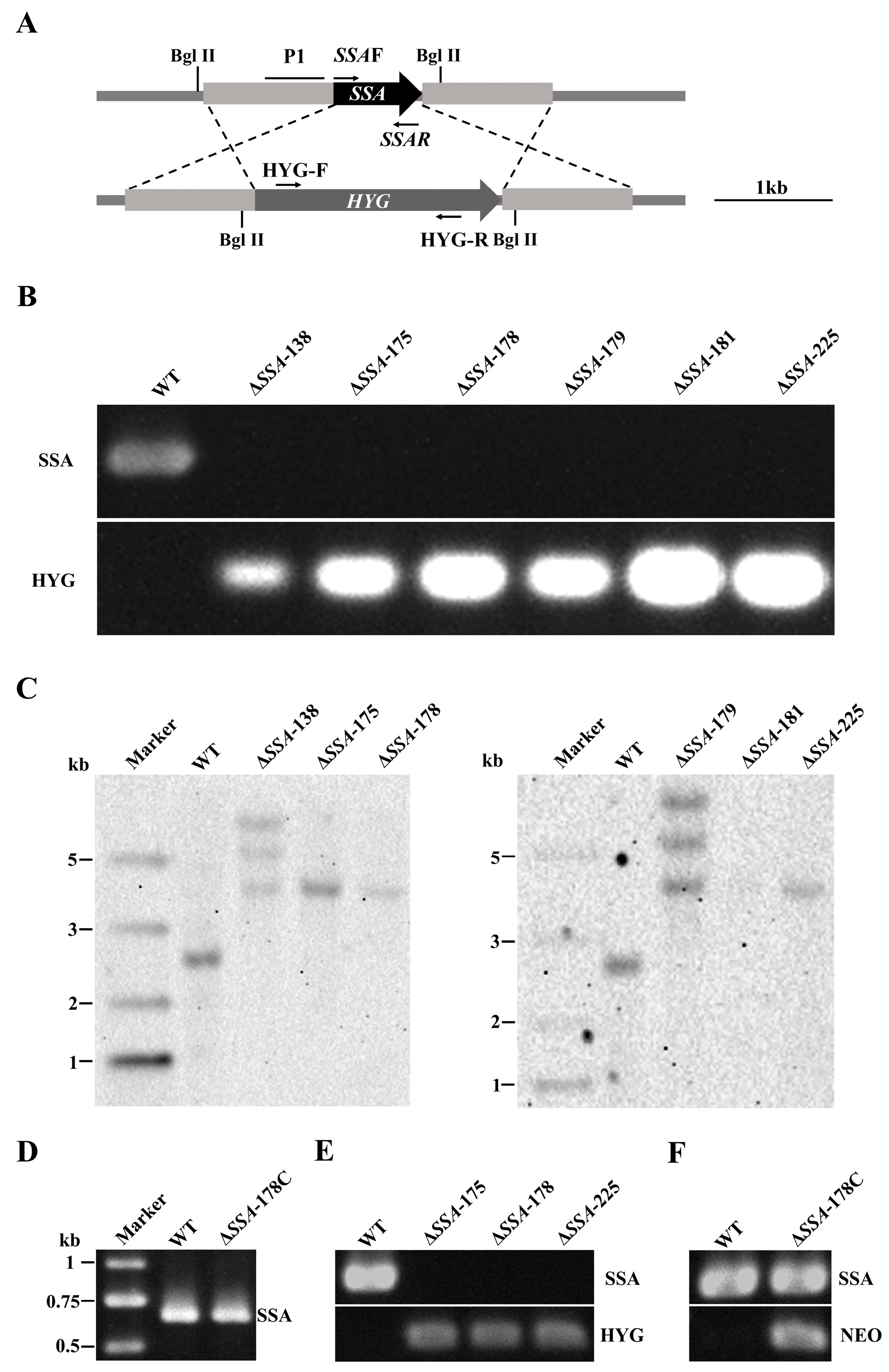
2.4. Disruption of SSA
2.5. Complementation of SSA
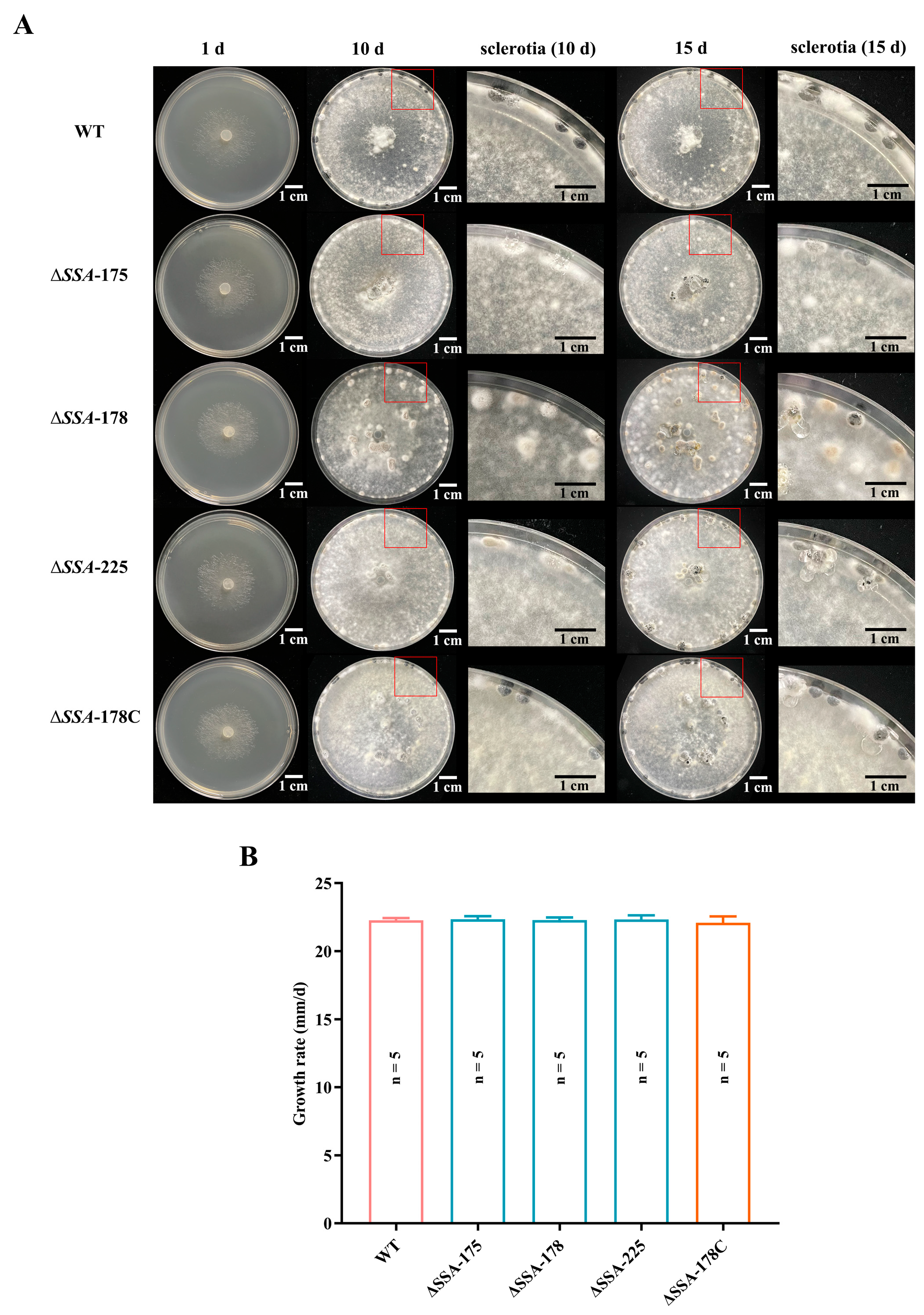
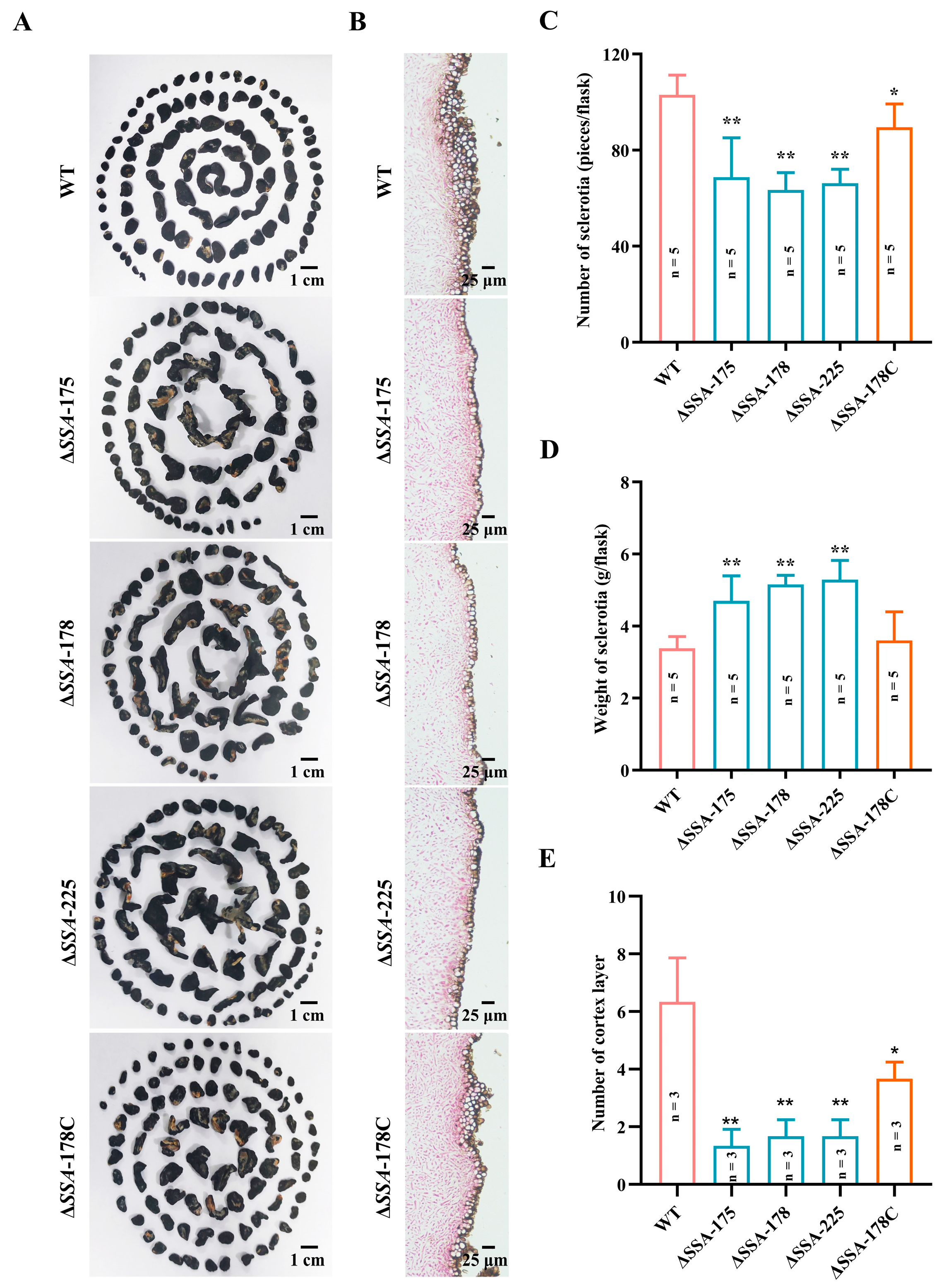
2.6. Determination of Mycelial Growth Rates and Sclerotial Formation
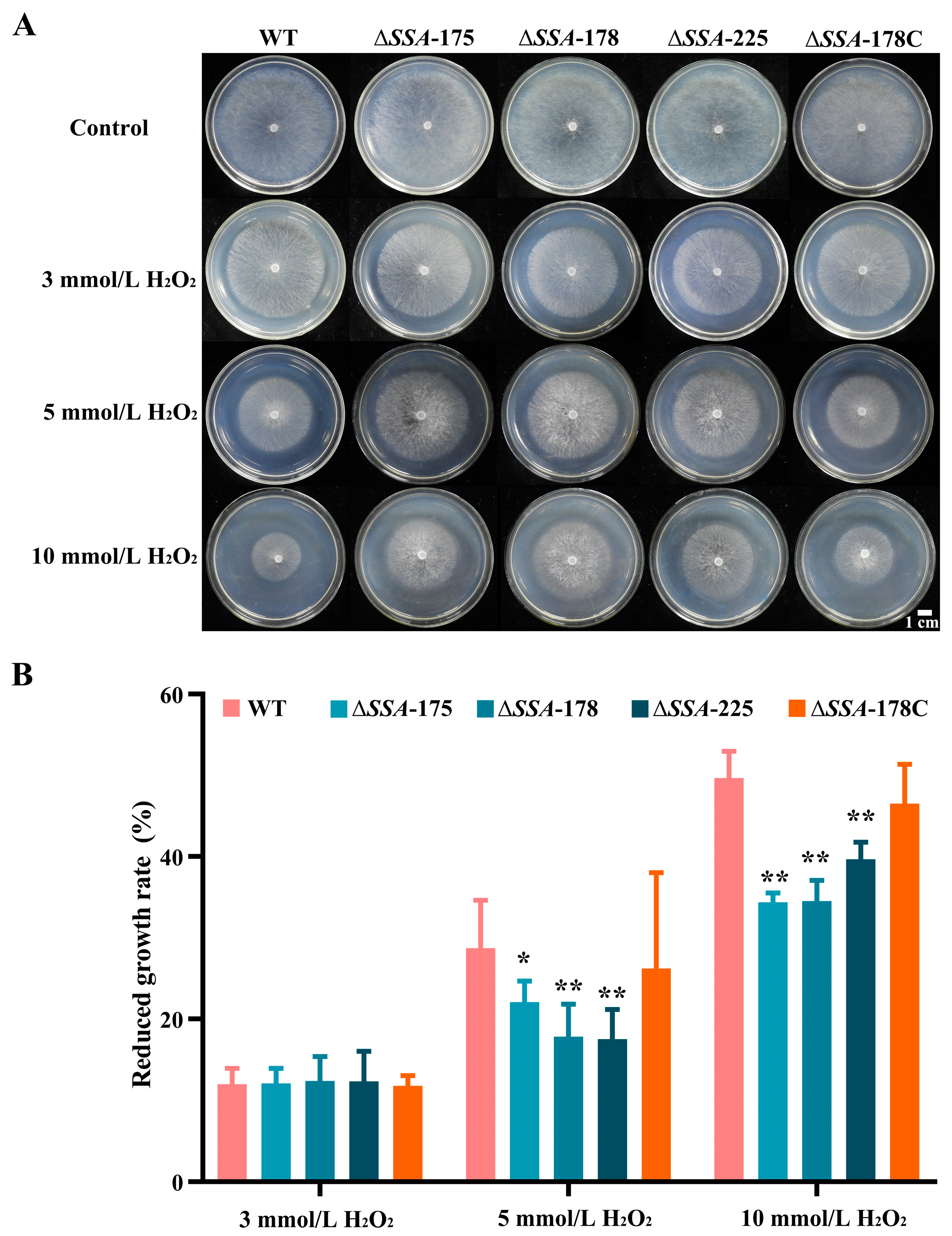
2.7. Assay for Response to Chemical Stresses
2.8. Pathogenicity Test
2.9. Dual Culturing
2.10. Data Analysis
3. Results
3.1. Identity of SSA
3.2. Disruption and Complementation of SSA
3.3. Effects of SSA Disruption on the Mycelial Growth Rate and Sclerotial Formation of S. sclerotiorum
3.4. Effect of SSA Disruption on the Response of S. sclerotiorum to Chemical Stresses
3.5. Effect of SSA Disruption on the Response of S. sclerotiorum to H2O2 Stresses
3.6. Effect of SSA Disruption on the Pathogenicity of S. sclerotiorum
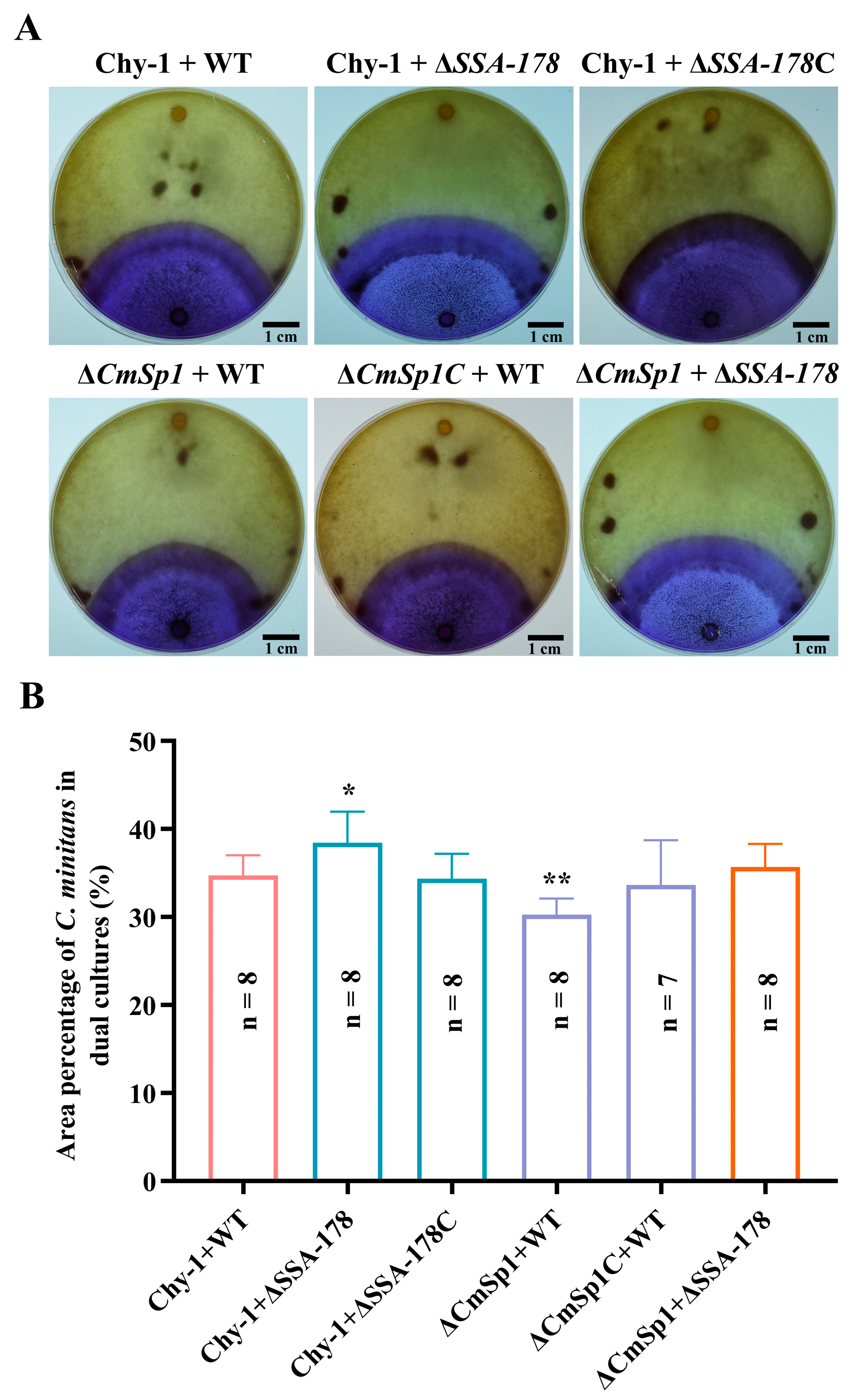
3.7. Effect of SSA Disruption on the Resistance of S. sclerotiorum to Mycoparasitism by C. minitans
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, X.F.; Rollins, J.A. Mechanisms of broad host range necrotrophic pathogenesis in Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1128–1140. [Google Scholar] [CrossRef]
- Xia, S.T.; Xu, Y.; Hoy, R.; Zhang, J.L.; Qin, L.; Li, X. The notorious soilborne pathogenic fungus Sclerotinia sclerotiorum: An update on genes studied with mutant analysis. Pathogens 2020, 9, 27. [Google Scholar] [CrossRef]
- Harper, G.E.; Frampton, C.M.; Stewart, A. Factors influencing survival of sclerotia of Sclerotium cepivorum in New Zealand soils. N. Z. J. Crop. Hortic. Sci. 2002, 30, 29–35. [Google Scholar] [CrossRef]
- Cubeta, M.A.; Cody, B.R.; Kohli, Y.; Kohn, L.M. Clonality of Sclerotinia sclerotiorum on infected cabbage in eastern North Carolina. Phytopathology 1997, 87, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Attanayake, R.N.; Xu, L.S.; Chen, W.D. Sclerotinia sclerotiorum populations: Clonal or recombining? Trop. Plant Pathol. 2019, 44, 23–31. [Google Scholar] [CrossRef]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 8, e1002230. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, M.C.; Denton-Giles, M. The control of sclerotinia stem rot on oilseed rape (Brassica napus): Current practices and future opportunities. Plant Pathol. 2016, 65, 859–877. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Cell wall structure and biogenesis in Aspergillus species. Biosci. Biotechnol. Biochem. 2016, 80, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Rodicio, R.; Heinisch, J.J. Together we are strong—Cell wall integrity sensors in yeasts. Yeast 2010, 27, 531–540. [Google Scholar] [CrossRef]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef]
- Jung, U.S.; Levin, D.E. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 1999, 34, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.S.; Sobering, A.K.; Romeo, M.J.; Levin, D.E. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 2002, 46, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Tiwary, A.K.; Kennedy, J.F. Lectins: Sources, activities, and applications. Crit. Rev. Biotechnol. 1999, 19, 145–178. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K. Microbial lectins and their prospective mitogenic potential. Crit. Rev. Microbiol. 2014, 40, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Bhari, R.; Kaur, H.P. Characteristics of yeast lectins and their role in cell-cell interactions. Biotechnol. Adv. 2011, 29, 726–731. [Google Scholar] [CrossRef]
- Varrot, A.; Basheer, S.M.; Imberty, A. Fungal lectins: Structure, function and potential applications. Curr. Opin. Struct. Biol. 2013, 23, 678–685. [Google Scholar] [CrossRef]
- Stratford, M.; Carter, A.T. Yeast flocculation: Lectin synthesis and activation. Yeast 1993, 9, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahmood, S.; Giummely, P.; Bonaly, R.; Delmotte, F.; Monsigny, M. Kluyveromyces bulgaricus yeast lectins. Isolation of N-acetylglucosamine and galactose-specific lectins: Their relation with flocculation. J. Biol. Chem. 1988, 263, 3930–3934. [Google Scholar] [CrossRef]
- Kellens, J.T.C.; Goldstein, I.J.; Peumans, W.J. Lectins in different members of the Sclerotiniaceae. Mycol. Res. 1992, 96, 495–502. [Google Scholar] [CrossRef]
- Candy, L.; Van Damme, E.J.M.; Peumans, W.J.; Menu-Bouaouiche, L.; Erard, M.; Rouge, P. Structural and functional characterization of the GalNAc/Gal-specific lectin from the phytopathogenic ascomycete Sclerotinia sclerotiorum (Lib.) de Bary. Biochem. Biophys. Res. Commun. 2003, 308, 396–402. [Google Scholar] [CrossRef]
- Van Damme, E.J.M.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Rouge, P.; Peumans, W.J. The Sclerotinia sclerotiorum agglutinin represents a novel family of fungal lectins remotely related to the Clostridium botulinum non-toxin haemagglutinin HA33/A. Glycoconj. J. 2007, 24, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Sulzenbacher, G.; Roig-Zamboni, V.; Peumans, W.J.; Rouge, P.; Van Damme, E.J.M.; Bourne, Y. Crystal structure of the GalNAc/Gal-specific agglutinin from the phytopathogenic ascomycete Sclerotinia sclerotiorum reveals novel adaptation of a beta-trefoil domain. J. Mol. Biol. 2010, 400, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, Z.; Zibaee, A.; Sendi, J.J.; Ramzi, S. Effect of Sclerotinia sclerotiorum agglutinin on digestive α-amylase of Pieris brassicae L. (Lepidoptera: Pieridae). Rom. J. Biochem. 2015, 52, 3–17. [Google Scholar]
- Hamshou, M.; Smagghe, G.; Shahidi-Noghabi, S.; De Geyter, E.; Lannoo, N.; van Damme, E.J.M. Insecticidal properties of Sclerotinia sclerotiorum agglutinin and its interaction with insect tissues and cells. Insect Biochem. Mol. Biol. 2010, 40, 883–890. [Google Scholar] [CrossRef]
- Shen, Y.; De Schutter, K.; Walski, T.; Van Damme, E.J.M.; Smagghe, G. Toxicity, membrane binding and uptake of the Sclerotinia sclerotiorum agglutinin (SSA) in different insect cell lines. Vitr. Cell. Dev. Biol. 2017, 53, 691–698. [Google Scholar] [CrossRef]
- Van Toor, R.F.; Jaspers, M.V.; Stewart, A. Effect of soil microorganisms on viability of sclerotia of Ciborinia camelliae, the causal agent of camellia flower blight. N. Z. J. Crop. Hortic. Sci. 2005, 33, 149–160. [Google Scholar] [CrossRef]
- Brown, A.J.P.; Brown, G.D.; Netea, M.G.; Gow, N.A.R. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014, 22, 614–622. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, FUNK-0035-2016. [Google Scholar] [CrossRef]
- Ren, L.; Li, G.Q.; Han, Y.C.; Jiang, D.H.; Huang, H.C. Degradation of oxalic acid by Coniothyrium minitans and its effects on production and activity of β-1, 3-glucanase of this mycoparasite. Biol. Control 2007, 43, 1–11. [Google Scholar] [CrossRef]
- Hu, Y.M.; Yang, L.; Li, G.Q. Optimization of culture conditions for production of chitinase by the mycoparasite Coniothyrium minitans. Chin. J. Biol. Control 2009, 26, 167–173. [Google Scholar]
- Xie, X.L.; Yang, L.; Wu, M.D.; Zhang, J.; Li, G.Q. Culture condition and characterization of factors affecting activity of the extracellular proteases produced by mycoparasite Coniothyrium minitans. Chin. J. Biol. Control 2016, 32, 406–413. [Google Scholar]
- Wang, Y.C.; Yu, H.; Xu, Y.P.; Wu, M.D.; Zhang, J.; Tsuda, K.; Liu, S.L.; Jiang, D.H.; Chen, W.D.; Wei, Y.D.; et al. Expression of a Mycoparasite Protease in Plant Petals Suppresses the Petal-Mediated Infection by Necrotrophic Pathogens. 2023. Available online: http://ssrn.com/abstract=4423202 (accessed on 19 April 2023).
- Zeng, L.M.; Zhang, J.; Han, Y.C.; Yang, L.; Wu, M.D.; Jiang, D.H.; Chen, W.D.; Li, G.Q. Degradation of oxalic acid by the mycoparasite Coniothyrium minitans plays an important role in interacting with Sclerotinia sclerotiorum. Environ. Microbiol. 2014, 16, 2591–2610. [Google Scholar] [CrossRef] [PubMed]
- Yu, H. Cloning and Functional Analysis of Extracellular Serine Protease Gene in the Mycoparasite Coniothyrium minitans; Huazhong Agricultural University Library: Wuhan, China, 2016. [Google Scholar]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geige, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Shang, Q.N.; Mo, C.M.; Xiao, Y.N.; Wang, G.F.; Xie, J.T.; Jiang, D.H.; Xiao, X.Q. Early secretory pathway-associated proteins SsEmp24 and SsErv25 are involved in morphogenesis and pathogenicity in a filamentous phytopathogenic fungus. mBio 2022, 12, e03172-21. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Song, J.J.; Wang, Y.C.; Yang, L.; Wu, M.D.; Li, G.Q.; Zhang, J. Biological characterization of the melanin biosynthesis gene Bcscd1 in the plant pathogenic fungus Botrytis cinerea. Fungal Genet. Biol. 2022, 161, 1087–1845. [Google Scholar] [CrossRef]
- Manning, J.C.; Romero, A.; Habermann, F.A.; Caballero, G.G.; Kaltner, H.; Gabius, H.J. Lectins: A primer for histochemists and cell biologists. Histochem. Cell Biol. 2017, 147, 199–222. [Google Scholar] [CrossRef]
- Willaert, R.G. Adhesins of yeasts: Protein structure and interactions. J. Fungi 2018, 4, 119. [Google Scholar] [CrossRef]
- Erental, A.; Dickman, M.B.; Yarden, O. Sclerotial development in Sclerotinia sclerotiorum: Awakening molecular analysis of a “Dormant” structure. Fungal Biol. Rev. 2008, 22, 6–16. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–6. [Google Scholar] [CrossRef]
- Marciano, P.; di Lenna, P.; Margo, P. Oxalic acid, cell-wall degrading enzymes and pH in pathogenesis and their significance in the virulence of two Sclerotinia sclerotiorum isolates on sunflower. Physiol. Plant Pathol. 1983, 22, 339–345. [Google Scholar] [CrossRef]
- Huang, H.C.; Kokko, E.G. Ultrastructure of hyperparasitism of Coniothyrium minitans on sclerotia of Sclerotinia sclerotiorum. Can. J. Bot. 1987, 65, 2483–2489. [Google Scholar] [CrossRef]
- Huang, H.C.; Kokko, E.G. Penetration of hyphae of Sclerotinia sclerotiorum by Coniothyrium minitans without the formation of appressoria. J. Phytopathol. 1988, 123, 133–139. [Google Scholar] [CrossRef]
- Giczey, G.; Kerényi, Z.; Fülöp, L.; Hornok, L. Expression of cmg1, an exo-β-1,3-glucanase gene from Coniothyrium minitans, increases during sclerotial parasitism. Appl. Environ. Microbiol. 2001, 67, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Xie, X.L.; Yang, L.; Zhang, J.; Li, G.Q.; Jiang, D.H. Susceptibility of Sclerotinia sclerotiorum strains different in oxalate production to infection by the mycoparasite Coniothyrium minitans. World J. Microbiol. Biotechnol. 2011, 27, 2799–2805. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, Y.; Wei, J.; Zhang, J.; Wu, M.; Li, G.; Yang, L. Sclerotinia sclerotiorum Agglutinin Modulates Sclerotial Development, Pathogenicity and Response to Abiotic and Biotic Stresses in Different Manners. J. Fungi 2023, 9, 737. https://doi.org/10.3390/jof9070737
Wang Y, Xu Y, Wei J, Zhang J, Wu M, Li G, Yang L. Sclerotinia sclerotiorum Agglutinin Modulates Sclerotial Development, Pathogenicity and Response to Abiotic and Biotic Stresses in Different Manners. Journal of Fungi. 2023; 9(7):737. https://doi.org/10.3390/jof9070737
Chicago/Turabian StyleWang, Yongchun, Yuping Xu, Jinfeng Wei, Jing Zhang, Mingde Wu, Guoqing Li, and Long Yang. 2023. "Sclerotinia sclerotiorum Agglutinin Modulates Sclerotial Development, Pathogenicity and Response to Abiotic and Biotic Stresses in Different Manners" Journal of Fungi 9, no. 7: 737. https://doi.org/10.3390/jof9070737
APA StyleWang, Y., Xu, Y., Wei, J., Zhang, J., Wu, M., Li, G., & Yang, L. (2023). Sclerotinia sclerotiorum Agglutinin Modulates Sclerotial Development, Pathogenicity and Response to Abiotic and Biotic Stresses in Different Manners. Journal of Fungi, 9(7), 737. https://doi.org/10.3390/jof9070737





