Germination of Microsporidian Spores: The Known and Unknown
Abstract
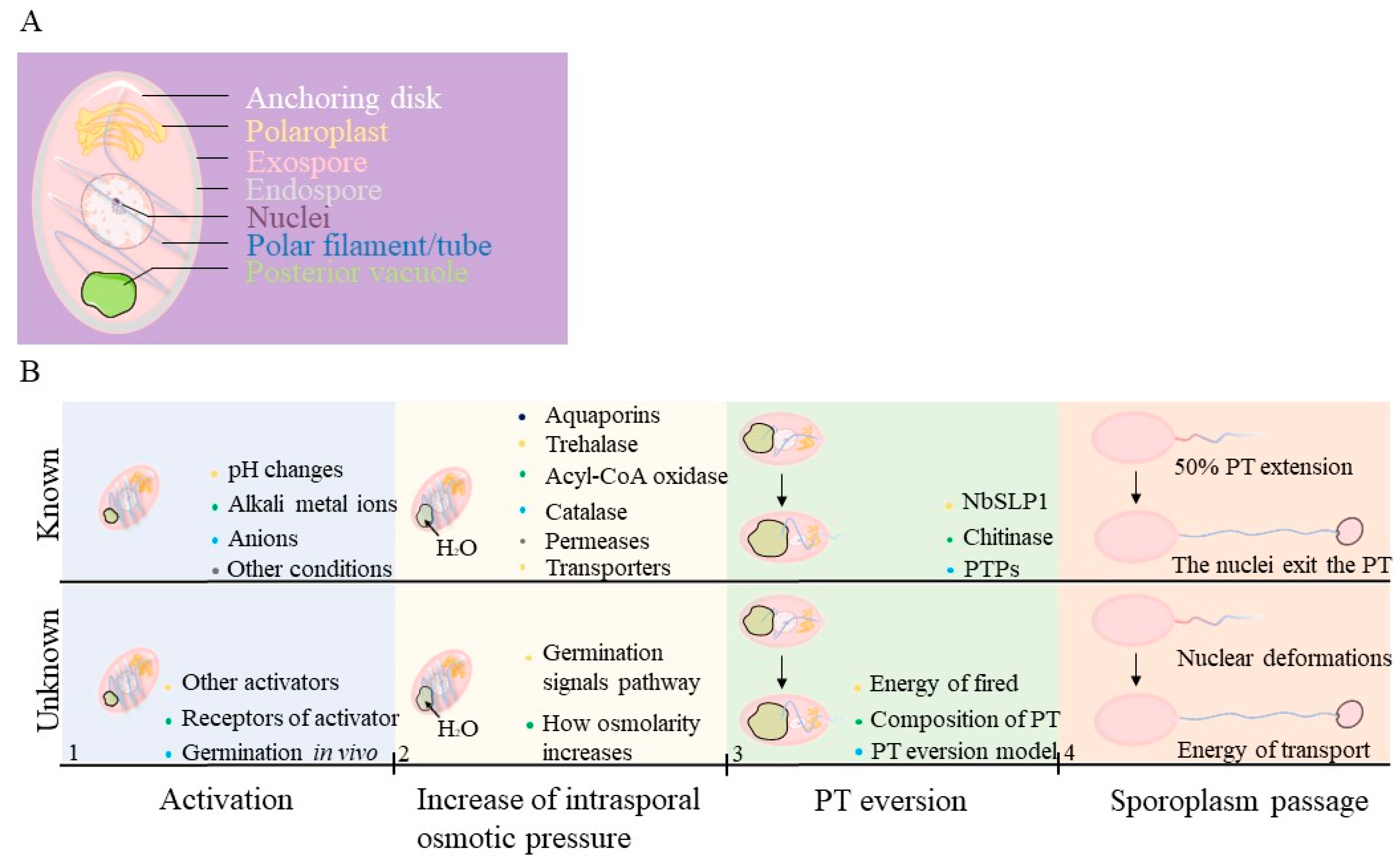
:1. Infectious Parasites: Microsporidia
2. Known about Microsporidian Spore Germination
2.1. Activation of Spore Germination
2.2. Energy of Germination
2.3. Polar Tube and Eversion Process during Germination
| Species | Protein/UniProtKB | Number of Amino Acids | Features | Mw(kDa) | References |
|---|---|---|---|---|---|
| Encephalitozoon cuniculi | EcPTP1/O76942 | 395 | Acidic proline-rich, hydrophobicity, presence of tandem repeats, mannosylated with O-glycosylation, signal peptide, interact with the Concanavalin A (ConA), localized on the whole PT | 37 | Xu et al., 2004 [113] Bouzahzah et al., 2010 [114] Delbac et al., 1998 [125] |
| EcPTP2/Q8SRT0 | 277 | Basic lysine-rich core, acidic tail, can form intermolecular disulfide bridges with PTP1, RGD motif and signal peptide, localized on the whole PT | 30 | Delbac et al., 2001 [111] Peuvel et al., 2002 [116] | |
| EcPTP3/Q8MTP3 | 1256 | Acidic core flanked by highly basic N- and C-termini, lacks cysteine residue, may assist in controlling PT extrusion, signal peptide, localized on the whole PT | 150 | Peuvel et al., 2002 [116] | |
| Encephalitozoon intestinalis | EiPTP1/Q5F2J0 | 371 | proline-rich, presence of tandem repeats, absent tryptophan and arginine, O-glycosylation and signal peptide, interact with the ConA, localized to polar filaments | 35 | Bouzahzah et al., 2010 [114] Peuvel et al., 2002 [116] |
| EiPTP2/P0CAT4 | 275 | lysine-rich, RGD motif, N-glycosylation and signal peptide | 30 | Peuvel et al., 2002 [116] | |
| Encephalitozoon hellem | EhPTP1/O76273 | 453 | proline-rich, presence of tandem repeats, mannosylated with N-, O-glycosylation, absent tryptophan and arginine, signal peptide, interact with the ConA, localized to polar filaments | 43 | Keohane et al., 1998 [110] Xu et al., 2004 [113] Peuvel et al., 2002 [116], |
| EhPTP2/P0CAT5 | 272 | lysine-rich, N-glycosylation, RGN motif and signal peptide, localized on the whole polar | 30 | Peuvel et al., 2002 [116] | |
| EhPTP4/I6UDI1 | 278 | signal peptide, located at the tip of the PT, N-, O-glycosylation | 36 | Han et al., 2017 [22] | |
| Nosema bombycis | NbPTP1/R0MQM8 | 409 | signal peptide, O-glycosylation, phosphorylation, localized on the whole PT | 55 | Wu et al., 2014 [126] |
| NbPTP2/R0KY97 | 277 | PI = 9.39, signal peptide, interacted with SWP5, presence in the whole polor tube, phosphorylation, localized on the whole PT | 39 | Li et al., 2012 [67] Wang et al., 2007 [127] Yi et al., 2019 [128] | |
| NbPTP3/J7EQ15 | 1370 | PI = 6.73, interacted with SWP5, localized on the whole PT | 150 | Li et al., 2012 [67] Wang et al., 2007 [127] | |
| NbPTP4/- | 222 | PI = 7.56, located at the front end of the PT and around the anchor disk, interact with Bmtubulin-α | 25.3 | Liu, 2019 [129] | |
| NbPTP5/R0KWI6 | 271 | PI = 8.68, located at the front end of the PT and around the anchor disk | 32.5 | Liu, 2019 [129] | |
| NbPTP6/R0MBR8 | 247 | rich in histidine (H) and serine (S), signal peptide, N-, O-glycosylation, cell-binding ability, localized on the whole PT | 28.3 | Lv et al., 2020 [117] | |
| NbSWP5/B3STN9 | 186 | PI = 4.39, localized to the exospore and the region of the PT, interacts with the PT proteins NbPTP2 and NbPTP3 | 20.3 | Li et al., 2012 [67,68] | |
| NbSWP7/B3STP1 | 287 | PI = 4.78, located in the PT and spore wall. | 32.8 | Yang et al., 2015 [69] | |
| NbSWP9/R0MLT0 | 367 | PI = 8.92, located in the spore wall, as well as anchoring disk and the front end of the PT after germination; interacts with NbPTP1 and NbPTP2 | 42.8 | Yang et al., 2015 [69] | |
| Enterocytozoon hepatopenaei | EhpPTP2/A0A1W0E7X7 | 284 | PI = 8.8, rich in lysine, super family domain (pfam17022), O-glycosylation | 32 | Wang et al., 2021 [130] |
| Antonospora locustae (formerly Nosema locustae) | AlPTP1/- | 355 | PI = 5.2, rich in proline and glycine, N-, O-glycosylation, interact with the ConA, localized on the whole PT | 34 | Polonais et al., 2005 [131] |
| AlPTP2/C8CG41 | 287 | PI = 9.1, signal peptide, O-glycosylation, rich in lysine, localized on the whole PT | 29 | Polonais et al., 2005 [131], | |
| AlPTP2b/C8CG42 | 568 | signal peptide, PI = 8.4, O-glycosylation, rich glycine and serine, b-turn structures, localized on the whole PT | 55 | Polonais et al., 2013 [132] | |
| AlPTP2c/C8CG43 | 599 | signal peptide, PI = 8.7, O-glycosylation, rich glycine and serine, b-turn structures | 56 | Polonais et al., 2013 [132] | |
| Paranosema grylli | PgPTP1/- | 351 | PI = 5.2, acidic and proline-rich, N-glycosylation, interact with the ConA | 33 | Polonais et al., 2005 [131] |
| PgPTP2/- | 287 | PI = 8.9, rich in lysine | 29 | Polonais et al., 2005 [131] | |
| Nosema pernyi | NpPTP1/A0A482G4U9 | 394 | PI = 5.82, signal peptide | 39.16 | Wang et al., 2019 [133] |
| NpPTP2/A0A482G3T3 | 277 | PI = 9.39, signal peptide | 30.8 | Wang et al., 2019 [133] | |
| NpPTP3/A0A0N7ABT9 | 1370 | PI = 6.52, localized on the whole polar | 148.56 | Wang et al., 2019 [133] |
3. Spore Germination: Major Unanswered Questions
3.1. What Is the Initial Germination Signal and Receptor of Microsporidia?
3.2. How Do the Germination Signals Stimulate Microsporidian Polar Filament to Eject?
3.3. How Do Polar Tube Ejections Happen and Where Does the Energy Come from?
3.4. How Do Nuclei Pass through the PT?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, L.M.; Edlind, T.D.; Vossbrinck, C.R.; Hashimoto, T. Microsporidian molecular phylogeny: The fungal connection. J. Eukaryot. Microbiol. 1999, 46, 17S–18S. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Marcet-Houben, M.; Gabaldón, T. Phylogenomics supports microsporidia as the earliest diverging clade of sequenced fungi. BMC Biol. 2012, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojko, J.; Reinke, A.W.; Stentiford, G.D.; Williams, B.; Rogers, M.S.; Bass, D. Microsporidia: A new taxonomic, evolutionary, and ecological synthesis. Trends Parasitol. 2022, 38, 642–659. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, E.; Sacchi, L. Cell biology and invasion of the microsporidia. Microbes Infect. 2001, 3, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.; Becnel, J.; Weiss, L.; Keeling, P.; Didier, E.; Bjornson, S.; Freeman, M.; Brown, M.; Roesel, K.; Sokolova, Y. Microsporidia–emergent pathogens in the global food chain. Trends Parasitol. 2016, 32, 336–348. [Google Scholar] [CrossRef] [Green Version]
- Becnel, J.J.; Weiss, L.M. Microsporidia: Pathogens of Opportunity; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Han, B.; Pan, G.; Weiss, L.M. Microsporidiosis in humans. Clin. Microbiol. Rev. 2021, 34, e00010-20. [Google Scholar] [CrossRef]
- Hasani, Z.; Aghdaei, H.A.; Balaii, H.; Azimirad, M.; Mirsamadi, E.; Mirjalali, H.; Zali, M. The first study on opportunistic intestinal microsporidiosis in IBD patients receiving immunosuppressive medications in Iran. Epidemiol. Infect. 2017, 145, 2095–2099. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Available online: http://www.moa.gov.cn/govpublic/xmsyj/202206/t20220629_6403635.htm (accessed on 15 July 2023).
- Dunn, A.M.; Terry, R.S.; Smith, J.E. Transovarial transmission in the microsporidia. Adv. Parasitol. 2001, 48, 57–100. [Google Scholar] [CrossRef]
- Didier, E.S.; Snowden, K.F.; Shadduck, J.A. Biology of microsporidian species infecting mammals. Adv. Parasitol. 1998, 40, 283–320. [Google Scholar] [CrossRef]
- Mathis, A.; Weber, R.; Deplazes, P. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 2005, 18, 423–445. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Bao, J.; Ma, Z.; Song, Y.; Han, B.; Ran, M.; Li, C.; Zhou, Z. Invertebrate host responses to microsporidia infections. Dev. Comp. Immunol. 2018, 83, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Pan, L.; Chen, Z.; Du, H.; Luo, B.; Luo, J.; Pan, G. The roles of microsporidia spore wall proteins in the spore wall formation and polar tube anchorage to spore wall during development and infection processes. Exp. Parasitol. 2018, 187, 93–100. [Google Scholar] [CrossRef]
- Cali, A.; Takvorian, P.M. Developmental morphology and life cycles of the microsporidia. In The Microsporidia and Microsporidiosis; American Society for Microbiology: Washington, DC, USA, 1999; pp. 85–128. [Google Scholar] [CrossRef]
- Jaroenlak, P.; Cammer, M.; Davydov, A.; Sall, J.; Usmani, M.; Liang, F.-X.; Ekiert, D.C.; Bhabha, G. 3-Dimensional organization and dynamics of the microsporidian polar tube invasion machinery. PLoS Pathog. 2020, 16, e1008738. [Google Scholar] [CrossRef]
- Thelohan, P. Sur la presence d’une capsule a filament dans les spores des microsporidies. CR Acad Sci 1894, 118, 1425–1427. [Google Scholar]
- Lom, J. On the structure of the extruded microsporidian polar filament. Z. Für Parasitenkd. 1972, 38, 200–213. [Google Scholar] [CrossRef]
- Lee, S.C.; Corradi, N.; Byrnes, E.J.; Torres-Martinez, S.; Dietrich, F.S.; Keeling, P.J.; Heitman, J. Microsporidia evolved from ancestral sexual fungi. Curr. Biol. 2008, 18, 1675–1679. [Google Scholar] [CrossRef] [Green Version]
- Undeen, A.H. A proposed mechanism for the germination of microsporidian (Protozoa: Microspora) spores. J. Theor. Biol. 1990, 142, 223–235. [Google Scholar] [CrossRef]
- James, T.Y.; Pelin, A.; Bonen, L.; Ahrendt, S.; Sain, D.; Corradi, N.; Stajich, J.E. Shared signatures of parasitism and phylogenomics unite Cryptomycota and microsporidia. Curr. Biol. 2013, 23, 1548–1553. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Polonais, V.; Sugi, T.; Yakubu, R.; Takvorian, P.M.; Cali, A.; Maier, K.; Long, M.; Levy, M.; Tanowitz, H.B. The role of microsporidian polar tube protein 4 (PTP4) in host cell infection. PLoS Pathog. 2017, 13, e1006341. [Google Scholar] [CrossRef]
- Franzen, C.; Müller, A.; Hartmann, P.; Salzberger, B. Cell invasion and intracellular fate of Encephalitozoon cuniculi (Microsporidia). Parasitology 2005, 130, 285–292. [Google Scholar] [CrossRef]
- Tamim El Jarkass, H.; Reinke, A.W. The ins and outs of host-microsporidia interactions during invasion, proliferation and exit. Cell. Microbiol. 2020, 22, e13247. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; He, X.; Cai, S.; He, X.; Lu, X. Transcriptome sequencing and characterization of ungerminated and germinated spores of Nosema bombycis. Acta Biochim. Biophys Sin 2016, 48, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, B.; Hu, S.; Liang, X.; Lu, X.; Shao, Y. Quantitative proteomic analysis of germination of Nosema bombycis spores under extremely alkaline conditions. Front. Microbiol. 2016, 7, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didier, E.S.; Maddry, J.A.; Brindley, P.J.; Stovall, M.E.; Didier, P.J. Therapeutic strategies for human microsporidia infections. Expert Rev. Anti-Infect. Ther. 2005, 3, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Conteas, C.; Berlin, O.; Ash, L.; Pruthi, J. Therapy for human gastrointestinal microsporidiosis. Am. J. Trop. Med. Hyg. 2000, 63, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Fei, Z.; Pan, G.; Weiss, L.M.; Zhou, Z. Current Therapy and Therapeutic Targets for Microsporidiosis. Front. Microbiol. 2022, 13, 835390. [Google Scholar] [CrossRef] [PubMed]
- Vávra, J.; Ronny Larsson, J. Structure of microsporidia. In Microsporidia: Pathogens of Opportunity; Wiley-Blackwell: Hoboken, N J, USA, 2014; pp. 1–70. [Google Scholar] [CrossRef]
- Weiss, L.M.; Delbac, F.; Russell Hayman, J.; Pan, G.; Dang, X.; Zhou, Z. The microsporidian polar tube and spore wall. In Microsporidia: Pathogens of Opportunity, 1st ed.; Weiss, L.M., Becnel, J.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 261–306. [Google Scholar] [CrossRef]
- Lom, J.; Vavra, J. The mode of sporoplasm extrusion in microsporidian spores. Acta Protozool. 1963, 1, 1–13. [Google Scholar]
- Shadduck, J.A.; Greeley, E. Microsporidia and human infections. Clin. Microbiol. Rev. 1989, 2, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Weiss, L.M. The microsporidian polar tube: A highly specialised invasion organelle. Int. J. Parasitol. 2005, 35, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Keohane, E.M.; Weiss, L.M. Characterization and function of the microsporidian polar tube: A review. Folia Parasitol. 1998, 45, 117–127. [Google Scholar]
- Undeen, A.H.; Epsky, N.D. In vitro and in vivo germination of Nosema locustae (Microspora: Nosematidae) spores. J. Invertebr. Pathol. 1990, 56, 371–379. [Google Scholar] [CrossRef]
- Han, B.; Takvorian, P.M.; Weiss, L.M. Invasion of host cells by microsporidia. Front. Microbiol. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imura, Y.; Nakamura, H.; Arai, R.; Hatakeyama, Y. Comparison of the Germination Conditions of Two Large-Spore Microsporidia Using Potassium and Sodium Ion Solutions. Insects 2023, 14, 185. [Google Scholar] [CrossRef]
- Ishihara, R. Stimuli causing extrusion of polar filaments of Glugea fumiferanae spores. Can. J. Microbiol. 1967, 13, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Undeen, A.; Avery, S. Germination of experimentally nontransmissible microsporidia. J. Invertebr. Pathol. 1984, 43, 299–301. [Google Scholar] [CrossRef]
- Undeen, A. Spore-hatching processes in some Nosema species with particular reference to N. algerae Vavra and Undeen. In Selected Topics on the Genus Nosema (Microsporida); Entomological Society of America: Annapolis, MD, USA, 1978; pp. 29–49. [Google Scholar]
- Pleshinger, J.; Weidner, E. The microsporidian spore invasion tube. IV. Discharge activation begins with pH-triggered Ca2+ influx. J. Cell Biol. 1985, 100, 1834–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T. Identification of the Target Protein of Monoclonal Antibody G9 in Nosema bombycis. Master’s Thesis, Southwest University, Chongqing, China, 2013. [Google Scholar]
- Tan, X. Extraction of spore wall proteins and the expression patterns of main SWP of Nosema bombycis. Master’s Thesis, Southwest University, Chongqing, China, 2008. [Google Scholar]
- Wu, Z.; Li, Y.; Pan, G.; Tan, X.; Hu, J.; Zhou, Z.; Xiang, Z. Proteomic analysis of spore wall proteins and identification of two spore wall proteins from Nosema bombycis (Microsporidia). Proteomics 2008, 8, 2447–2461. [Google Scholar] [CrossRef]
- Bullajr, L.A.; Cheng, T.C. Biology of the Microsporidia; Springer: New York, NY, USA, 1976. [Google Scholar] [CrossRef]
- Frixione, E.; Ruiz, L.; Undeen, A.H. Monovalent cations induce microsporidian spore germination in vitro. J. Eukaryot. Microbiol. 1994, 41, 464–468. [Google Scholar] [CrossRef]
- Frixione, E.; Ruiz, L.; Santillán, M.; de Vargas, L.V.; Tejero, J.M.; Undeen, A.H. Dynamics of polar filament discharge and sporoplasm expulsion by microsporidian spores. Cell Motil. Cytoskelet. 1992, 22, 38–50. [Google Scholar] [CrossRef]
- Undeen, A.; Avery, S. Effect of anions on the germination of Nosema algerae (Microspora: Nosematidae) spores. J. Invertebr. Pathol. 1988, 52, 84–89. [Google Scholar] [CrossRef]
- Undeen, A.H.; Meer, R.K.V. The Effect of Ultraviolet Radiation on the Germination of Nosema algerae Vávra and Undeen (Microsporida: Nosematidae) Spores 1. J. Protozool. 1990, 37, 194–199. [Google Scholar] [CrossRef]
- Weidner, E.; Byrd, W. The microsporidian spore invasion tube. II. Role of calcium in the activation of invasion tube discharge. J. Cell Biol. 1982, 93, 970–975. [Google Scholar] [CrossRef]
- Undeen, A.H.; Frixione, E. The role of osmotic pressure in the germination of Nosema algerae spores 1. J. Protozool. 1990, 37, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K. Effect of potassium ion on filament evagination of spores of Nosema bombycis as studied by neutralization method. Annot. Zool. Jpn. 1964, 37, 102–103. [Google Scholar] [CrossRef]
- Ohshima, K. Stimulative or inhibitive substance to evaginate the filament of Nosema bombycis NÄGELI. I. The case of artificial buffer solution. Jpn. J. Zool 1964, 14, 209. [Google Scholar]
- Undeen, A.H. The Germination of Vavraia culicis Spores 1. J. Protozool. 1983, 30, 274–277. [Google Scholar] [CrossRef]
- Malone, L.A. Factors controlling in vitro hatching of Vairimorpha plodiae (Microspora) spores and their infectivity to Plodia interpunctella, Heliothis virescens, and Pieris brassicae. J. Invertebr. Pathol. 1984, 44, 192–197. [Google Scholar] [CrossRef]
- LEITCH, G.J.; HE, Q.; WALLACE, S.; VISVESVARA, G.S. Inhibition of the spore polar filament extrusion of the microsporidium, Encephalitozoon hellem, isolated from an AIDS patient 1. J. Eukaryot. Microbiol. 1993, 40, 711–717. [Google Scholar] [CrossRef]
- He, Q.; Leitch, G.J.; Visvesvara, G.S.; Wallace, S. Effects of nifedipine, metronidazole, and nitric oxide donors on spore germination and cell culture infection of the microsporidia Encephalitozoon hellem and Encephalitozoon intestinalis. Antimicrob. Agents Chemother. 1996, 40, 179–185. [Google Scholar] [CrossRef]
- Weidner, E. The microsporidian spore invasion tube. III. Tube extrusion and assembly. J. Cell Biol. 1982, 93, 976–979. [Google Scholar] [CrossRef]
- Bohne, W.; Ferguson, D.J.; Kohler, K.; Gross, U. Developmental expression of a tandemly repeated, glycine-and serine-rich spore wall protein in the microsporidian pathogen Encephalitozoon cuniculi. Infect. Immun. 2000, 68, 2268–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southern, T.R.; Jolly, C.E.; Lester, M.E.; Hayman, J.R. EnP1, a microsporidian spore wall protein that enables spores to adhere to and infect host cells in vitro. Eukaryot. Cell 2007, 6, 1354–1362. [Google Scholar] [CrossRef] [Green Version]
- Peuvel-Fanget, I.; Polonais, V.; Brosson, D.; Texier, C.; Kuhn, L.; Peyret, P.; Vivarès, C.; Delbac, F. EnP1 and EnP2, two proteins associated with the Encephalitozoon cuniculi endospore, the chitin-rich inner layer of the microsporidian spore wall. Int. J. Parasitol. 2006, 36, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Takvorian, P.; Cali, A.; Wang, F.; Zhang, H.; Orr, G.; Weiss, L.M. Identification of a new spore wall protein from Encephalitozoon cuniculi. Infect. Immun. 2006, 74, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosson, D.; Kuhn, L.; Prensier, G.; Vivarès, C.P.; Texier, C. The putative chitin deacetylase of Encephalitozoon cuniculi: A surface protein implicated in microsporidian spore-wall formation. FEMS Microbiol. Lett. 2005, 247, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Hayman, J.R.; Hayes, S.F.; Amon, J.; Nash, T.E. Developmental expression of two spore wall proteins during maturation of the microsporidian Encephalitozoon intestinalis. Infect. Immun. 2001, 69, 7057–7066. [Google Scholar] [CrossRef] [Green Version]
- Polonais, V.; Mazet, M.; Wawrzyniak, I.; Texier, C.; Blot, N.; El Alaoui, H.; Delbac, F. The human microsporidian Encephalitozoon hellem synthesizes two spore wall polymorphic proteins useful for epidemiological studies. Infect. Immun. 2010, 78, 2221–2230. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Pan, G.; Li, T.; Huang, W.; Chen, J.; Geng, L.; Yang, D.; Wang, L.; Zhou, Z. SWP5, a spore wall protein, interacts with polar tube proteins in the parasitic microsporidian Nosema bombycis. Eukaryot. Cell 2012, 11, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Lu, X.; Qiu, H.; Li, M.; Feng, Z. Identification of a Nosema bombycis (Microsporidia) spore wall protein corresponding to spore phagocytosis. Parasitology 2011, 138, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Pan, G.; Dang, X.; Shi, Y.; Li, C.; Peng, P.; Luo, B.; Bian, M.; Song, Y.; Ma, C. Interaction and assembly of two novel proteins in the spore wall of the microsporidian species Nosema bombycis and their roles in adherence to and infection of host cells. Infect. Immun. 2015, 83, 1715–1731. [Google Scholar] [CrossRef]
- Yang, D.; Dang, X.; Peng, P.; Long, M.; Ma, C.; Qin, J.J.G.; Wu, H.; Liu, T.; Zhou, X.; Pan, G. NbHSWP11, a microsporidia Nosema bombycis protein, localizing in the spore wall and membranes, reduces spore adherence to host cell BME. J. Parasitol. 2014, 100, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Geng, L.; Long, M.; Li, T.; Li, Z.; Yang, D.; Ma, C.; Wu, H.; Ma, Z.; Li, C. Identification of a novel chitin-binding spore wall protein (NbSWP12) with a BAR-2 domain from Nosema bombycis (microsporidia). Parasitology 2013, 140, 1394–1402. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Sheng, X.; Huang, J.; Sun, Q.; Huang, Y.; Wang, R.; Wu, Y.; Long, M.; Bao, J. Heterologous Expressed NbSWP12 from Microsporidia Nosema bombycis Can Bind with Phosphatidylinositol 3-Phosphate and Affect Vesicle Genesis. J. Fungi 2022, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, J.; Sun, B.; Zheng, R.; Li, B.; Li, Z.; Tan, Y.; Wei, J.; Pan, G.; Li, C. Engineered resistance to Nosema bombycis by in vitro expression of a single-chain antibody in Sf9-III cells. PLoS ONE 2018, 13, e0193065. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dang, X.; Ma, Q.; Liu, F.; Pan, G.; Li, T.; Zhou, Z. Characterization of a novel spore wall protein NbSWP16 with proline-rich tandem repeats from Nosema bombycis (microsporidia). Parasitology 2015, 142, 534–542. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Pan, G.; He, W.; Zhang, R.; Hu, J.; Zhou, Z. Identification of a novel spore wall protein (SWP26) from microsporidia Nosema bombycis. Int. J. Parasitol. 2009, 39, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, L.; Xu, J.; Jiang, P.; An, Q.; Pu, Y.; Jiang, Y.; He, S.; Tao, X.; Luo, J. Expression and identification of a novel spore wall protein in microsporidian Nosema bombycis. J. Eukaryot. Microbiol. 2020, 67, 671–677. [Google Scholar] [CrossRef]
- Liang, X.; Tang-Yan, A.; Zhen, Z.; Zhen-Gang, M.; Ze-Yang, Z. Gene cloning, expression and subcellular localization of a novel spore wall protein of Nosema ceranae (Microsporidia). Acta Entomol. Sin. 2021, 64, 1070–1079. [Google Scholar] [CrossRef]
- He, N.; Zhang, Y.; Duan, X.L.; Li, J.H.; Huang, W.-F.; Evans, J.D.; DeGrandi-Hoffman, G.; Chen, Y.P.; Huang, S.K. RNA interference-mediated knockdown of genes encoding spore wall proteins confers protection against Nosema ceranae infection in the european honey bee, Apis mellifera. Microorganisms 2021, 9, 505. [Google Scholar] [CrossRef]
- Jaroenlak, P.; Boakye, D.W.; Vanichviriyakit, R.; Williams, B.; Sritunyalucksana, K.; Itsathitphaisarn, O. Identification, characterization and heparin binding capacity of a spore-wall, virulence protein from the shrimp microsporidian, Enterocytozoon hepatopenaei (EHP). Parasites Vectors 2018, 11, 177. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, Y.; Fang, W.; Zhou, J.; Li, X. Identification, sequence characteristics and expression analyses of four spore wall protien genes of Enterocytozoon hepatopenaei (EHP) in Litopenaeus vannamei. Mar. Fish 2021, 43, 81–92. [Google Scholar] [CrossRef]
- Fan, X.; Wei, C.; Yang, X.; Xiao, A.; Tan, N.; Chen, J.; Long, M.; Pan, G.; Wan, Y.; Zhou, Z. Proteomic Analysis of Spore Surface Proteins and Characteristics of a Novel Spore Wall Protein and Biomarker, EhSWP3, from the Shrimp Microsporidium Enterocytozoon hepatopenaei (EHP). Microorganisms 2022, 10, 367. [Google Scholar] [CrossRef]
- Chen, L.; Li, R.; You, Y.; Zhang, K.; Zhang, L. A novel spore wall protein from Antonospora locustae (Microsporidia: Nosematidae) contributes to sporulation. J. Eukaryot. Microbiol. 2017, 64, 779–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Ye, H.; Shang, Z.; Sun, L.; Guo, Y.; Li, N.; Xiao, L.; Feng, Y. Identification and Characterization of Three Spore Wall Proteins of Enterocytozoon Bieneusi. Front. Cell. Infect. Microbiol. 2022, 12, 808986. [Google Scholar] [CrossRef]
- Zhu, W.; Yi-Ren, J.; Bin-He, W.; Ying, S.; Xi-Sheng, L.; Yong1, W.; Li1, Q. Cloning, Sequence Analysis and Prokaryotic Expression of Nosema pernyi Spore Wall Protein Gene NpSWP1. Sci. Seric. 2014, 40, 688–693. [Google Scholar] [CrossRef]
- Ma, Y.; Lei, P.; Wang, D.; Zhong, W.; Wang, Y.; Qin, L. A New Spore Wall Protein 9 Gene Cloned from Nosema pernyi (Microsporidia) Isolated from Chinese Oak Silkworm, Antheraea pernyi. J. Adv. Microbiol 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Feng, Z.; Yan, X.S.; Yong-hong, Z.; Fen-fen, T.; Yulan, S.; Shi-liang, C.; Xing-rong, B. Cloning, analysis, and prokarvotic expression of a gene coding for the spore wall protein NpSWP12 of Nosema philosamiae. J. Pathog. Biol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Xi, Z.; JinShan, X.; Xao-Yan, Z.; Ze-Yang, Z. Extraction and Identification pf Partial Spore Wall Proteins of Nosema antheraeae and Sequence Analysis of Spore Wall Protein 8. Sci. Seric. 2010, 36, 949–956. [Google Scholar] [CrossRef]
- Zhu, F.; Shen, Z.; Guo, X.; Xu, X.; Tao, H.; Tang, X.; Xu, L. A new isolate of Nosema sp. (Microsporidia, Nosematidae) from Phyllobrotica armata Baly (Coleoptera, Chrysomelidae) from China. J. Invertebr. Pathol. 2011, 106, 339–342. [Google Scholar] [CrossRef]
- Corradi, N.; Keeling, P.J. Microsporidia: A journey through radical taxonomical revisions. Fungal Biol. Rev. 2009, 23, 1–8. [Google Scholar] [CrossRef]
- Dall, D. A theory for the mechanism of polar filament extrusion in the Microspora. J. Theor. Biol. 1983, 105, 647–659. [Google Scholar] [CrossRef]
- Undeen, A.H.; Meer, R.K.V. Conversion of intrasporal trehalose into reducing sugars during germination of Nosema algerae (Protista: Microspora) spores: A quantitative study. J. Eukaryot. Microbiol. 1994, 41, 129–132. [Google Scholar] [CrossRef]
- De Graaf, D.C.; Masschelein, G.; Vandergeynst, F.; De Brabander, H.F.; Jacobs, F.J. In vitro germination of Nosema apis (Microspora: Nosematidae) spores and its effect on their αα-trehalose/D-glucose ratio. J. Invertebr. Pathol. 1993, 62, 220–225. [Google Scholar] [CrossRef]
- Undeen, A.H.; Vander Meer, R.K. Microsporidian intrasporal sugars and their role in germination. J. Invertebr. Pathol. 1999, 73, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Findley, A.M.; Weidner, E.H.; Carman, K.R.; Xu, Z.; Godbar, J.S. Role of the posterior vacuole in Spraguea lophii (Microsporidia) spore hatching. Folia Parasitol. 2005, 52, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Weidner, E.; Findley, A. Peroxisomal catalase in extrusion apparatus posterior vacuole of microsporidian spores. Biol. Bull. 2002, 203, 212. [Google Scholar] [CrossRef]
- Weidner, E.; Findley, A. Catalase in microsporidian spores before and during discharge. Biol. Bull. 2003, 205, 236–237. [Google Scholar] [CrossRef]
- Frixione, E.; Ruiz, L.; Cerbón, J.; Undeen, A.H. Germination of Nosema algerae (Microspora) spores: Conditional inhibition by D2O, ethanol and Hg2+ suggests dependence of water influx upon membrane hydration and specific transmembrane pathways. J. Eukaryot. Microbiol. 1997, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Weidner, E.; Findley, A.M.; Dolgikh, V.; Sokolova, J. Microsporidian biochemistry and physiology. In The Microsporidia and Microsporidiosis; American Society for Microbiology: Washington, DC, USA, 1999; pp. 172–195. [Google Scholar] [CrossRef]
- Hoch, G.; Schafellner, C.; Henn, M.W.; Schopf, A. Alterations in carbohydrate and fatty acid levels of Lymantria dispar larvae caused by a microsporidian infection and potential adverse effects on a co-occurring endoparasitoid, Glyptapanteles liparidis. Arch. Insect Biochem. Physiol. 2002, 50, 109–120. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Z.; Shang, R.; Qi, J.; Zhang, Y.; Tang, S.; Shen, Z. In vitro expression and functional characterization of NPA motifs in aquaporins of Nosema bombycis. Parasitol. Res. 2018, 117, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Takvorian, P.M.; Han, B.; Cali, A.; Rice, W.J.; Gunther, L.; Macaluso, F.; Weiss, L.M. An ultrastructural study of the extruded polar tube of Anncaliia algerae (Microsporidia). J. Eukaryot. Microbiol. 2020, 67, 28–44. [Google Scholar] [CrossRef]
- Cali, A.; Weiss, L.M.; Takvorian, P.M. Brachiola algerae spore membrane systems, their activity during extrusion, and a new structural entity, the multilayered interlaced network, associated with the polar tube and the sporoplasm. J. Eukaryot. Microbiol. 2002, 49, 164–174. [Google Scholar] [CrossRef]
- Kudo, R.; Daniels, E. An electron microscope study of the spore of a microsporidian, Thelohania californica. J. Protozool. 1963, 10, 112–120. [Google Scholar] [CrossRef]
- Weidner, E. Ultrastructural study of microsporidian invasion into cells. Z. Für Parasitenkd. 1972, 40, 227–242. [Google Scholar] [CrossRef]
- Han, B.; Weiss, L.M. Microsporidia: Obligate intracellular pathogens within the fungal kingdom. Microbiol. Spectr. 2017, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, R. On the structure of some microsporidian spores. J. Parasitol. 1920, 6, 178–182. [Google Scholar] [CrossRef]
- Lv, Q.; Zhou, B.; Liao, H.; He, X.; Chen, Y.; Pan, G.; Long, M.; Zhou, Z. Proteomic profile of polar filament and polar tube from fungal pathogen microsporidium Nosema bombycis provides new insights into its unique invasion organelle. J. Proteom. 2022, 263, 104617. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Takvorian, P.M.; Weiss, L.M. The Function and Structure of the Microsporidia Polar Tube. Exp Suppl. 2022, 114, 179–213. [Google Scholar] [CrossRef]
- Keohane, E.M.; Orr, G.A.; Takvorian, P.M.; Cali, A.; Tanowitz, H.B.; Wittner, M.; Weiss, L.M. Purification and characterization of a microsporidian polar tube protein. Mol. Biochem. Parasitol. 1996, 79, 255–259. [Google Scholar] [CrossRef]
- Keohane, E.M.; Orr, G.A.; Zhang, H.S.; Takvorian, P.M.; Cali, A.; Tanowitz, H.B.; Wittner, M.; Weiss, L.M. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol. Biochem. Parasitol. 1998, 94, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Delbac, F.; Peuvel, I.; Metenier, G.; Peyretaillade, E.; Vivares, C.P. Microsporidian invasion apparatus: Identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species. Infect. Immun. 2001, 69, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Takvorian, P.; Cali, A.; Weiss, L.M. Lectin binding of the major polar tube protein (PTP1) and its role in invasion. J. Eukaryot. Microbiol. 2003, 50, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Takvorian, P.M.; Cali, A.; Orr, G.; Weiss, L.M. Glycosylation of the major polar tube protein of Encephalitozoon hellem, a microsporidian parasite that infects humans. Infect. Immun. 2004, 72, 6341–6350. [Google Scholar] [CrossRef] [Green Version]
- Bouzahzah, B.; Weiss, L.M. Glycosylation of the major polar tube protein of Encephalitozoon cuniculi. Parasitol. Res. 2010, 107, 761–764. [Google Scholar] [CrossRef]
- Bouzahzah, B.; Nagajyothi, F.; Ghosh, K.; Takvorian, P.M.; Cali, A.; Tanowitz, H.B.; Weiss, L.M. Interactions of Encephalitozoon cuniculi polar tube proteins. Infect. Immun. 2010, 78, 2745–2753. [Google Scholar] [CrossRef] [Green Version]
- Peuvel, I.; Peyret, P.; Méténier, G.; Vivarès, C.P.; Delbac, F. The microsporidian polar tube: Evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi. Mol. Biochem. Parasitol. 2002, 122, 69–80. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, L.; Fan, Y.; Meng, X.; Liu, K.; Zhou, B.; Chen, J.; Pan, G.; Long, M.; Zhou, Z. Identification and characterization a novel polar tube protein (NbPTP6) from the microsporidian Nosema bombycis. Parasites Vectors 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Keohane, E.M.; Weiss, L.M. The structure, function, and composition of the microsporidian polar tube. In The Microsporidia and Microsporidiosis; American Society for Microbiology: Washington, DC, USA, 1999; pp. 196–224. [Google Scholar] [CrossRef]
- Dang, X.; Pan, G.; Li, T.; Lin, L.; Ma, Q.; Geng, L.; He, Y.; Zhou, Z. Characterization of a subtilisin-like protease with apical localization from microsporidian Nosema bombycis. J. Invertebr. Pathol. 2013, 112, 166–174. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Liu, F.; Dang, X.; Sun, Q.; Sheng, X.; Hu, M.; Chen, J.; Pan, G.; Zhou, Z. Maturation of Subtilisin-like Protease NbSLP1 from Microsporidia Nosema bombycis. Front. Cell. Infect. Microbiol. 2022, 12, 897509. [Google Scholar] [CrossRef] [PubMed]
- Power, S.D.; Adams, R.M.; Wells, J.A. Secretion and autoproteolytic maturation of subtilisin. Proc. Natl. Acad. Sci. USA 1986, 83, 3096–3100. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Dumitrescu, D.G.; Blankenship, L.R.; Herkert, D.; Hatzios, S.K. Functional characterization of a subtilisin-like serine protease from Vibrio cholerae. J. Biol. Chem. 2019, 294, 9888–9900. [Google Scholar] [CrossRef]
- Han, B.; Zhou, K.; Li, Z.; Sun, B.; Ni, Q.; Meng, X.; Pan, G.; Li, C.; Long, M.; Li, T. Characterization of the first fungal glycosyl hydrolase family 19 chitinase (NbchiA) from Nosema bombycis (Nb). J. Eukaryot. Microbiol. 2016, 63, 37–45. [Google Scholar] [CrossRef]
- Leiro, J.; Ortega, M.; Siso, M.; Sanmartin, M.; Ubeira, F. Effects of chitinolytic and proteolytic enzymes on in vitro phagocytosis of microsporidians by spleen macrophages of turbot, Scophthalmus maximus L. Vet. Immunol. Immunopathol. 1997, 59, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Delbac, F.; Peyret, P.; Méténier, G.; David, D.; Danchin, A.; Vivarès, C.P. On proteins of the microsporidian invasive apparatus: Complete sequence of a polar tube protein of Encephalitozoon cuniculi. Mol. Microbiol. 1998, 29, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Long, M.; Chen, J.; Zhi, L.; Li, Z.; Pan, G.; Li, T.; Zhou, Z. Cloning and prokaryotic expression of Nosema bombycis polar tube protein 1 (PTP1). Sci. Seric. 2014, 2, 22. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chambon, C.; Lu, C.D.; Huang, K.W.; Vivarès, C.P.; Texier, C. A proteomic-based approach for the characterization of some major structural proteins involved in host-parasite relationships from the silkworm parasite Nosema bombycis (Microsporidia). Proteomics 2007, 7, 1461–1472. [Google Scholar] [CrossRef]
- Yi, M.; Lü, Q.; Liu, K.; Wang, L.; Wu, Y.; Zhou, Z.; Long, M. Expression, purification and localization analysis of polar tube protein 2 (NbPTP2) from Nosema bombycis. Sci. Agric. Sin. 2019, 52, 1830–1838. [Google Scholar]
- Liu, K. Identification of Polar Tube Protein 4(NbPTP4) and 5(NbPTP5) from Nosema bombycis and the Role of NbPTP4 in Host Cell Infection. Master’s Thesis, Southwest University, Chongqing, China, 2019. [Google Scholar]
- Wang, L.; Lv, Q.; Meng, X.; Chen, J.; Wang, Y.; Pan, G.; Long, M.; Zhou, Z. Identification and characterization polar tube protein 2 (PTP2) from Enterocytozoon hepatopenaei and its potential effect on shrimp microsporidian germination activity evaluation. Aquaculture 2021, 544, 737062. [Google Scholar] [CrossRef]
- Polonais, V.; Prensier, G.; Méténier, G.; Vivarès, C.P.; Delbac, F. Microsporidian polar tube proteins: Highly divergent but closely linked genes encode PTP1 and PTP2 in members of the evolutionarily distant Antonospora and Encephalitozoon groups. Fungal Genet. Biol. 2005, 42, 791–803. [Google Scholar] [CrossRef]
- Polonais, V.; Belkorchia, A.; Roussel, M.; Peyretaillade, E.; Peyret, P.; Diogon, M.; Delbac, F. Identification of two new polar tube proteins related to polar tube protein 2 in the microsporidian Antonospora locustae. FEMS Microbiol. Lett. 2013, 346, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, Y.; Wang, D.; Liu, W.; Chen, J.; Jiang, Y.; Yang, R.; Qin, L. Polar tube structure and three polar tube proteins identified from Nosema pernyi. J. Invertebr. Pathol. 2019, 168, 107272. [Google Scholar] [CrossRef]
- Kung, C.; Martinac, B.; Sukharev, S. Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 2010, 64, 313–329. [Google Scholar] [CrossRef] [Green Version]
- Nakjang, S.; Williams, T.A.; Heinz, E.; Watson, A.K.; Foster, P.G.; Sendra, K.M.; Heaps, S.E.; Hirt, R.P.; Martin Embley, T. Reduction and expansion in microsporidian genome evolution: New insights from comparative genomics. Genome Biol. Evol. 2013, 5, 2285–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docampo, R.; Moreno, S. The role of Ca2+ in the process of cell invasion by intracellular parasites. Parasitol. Today 1996, 12, 61–65. [Google Scholar] [CrossRef]
- Dean, P.; Sendra, K.M.; Williams, T.; Watson, A.K.; Major, P.; Nakjang, S.; Kozhevnikova, E.; Goldberg, A.V.; Kunji, E.R.; Hirt, R.P. Transporter gene acquisition and innovation in the evolution of Microsporidia intracellular parasites. Nat. Commun. 2018, 9, 1709. [Google Scholar] [CrossRef] [Green Version]
- Tsaousis, A.D.; Kunji, E.R.; Goldberg, A.V.; Lucocq, J.M.; Hirt, R.P.; Embley, T.M. A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature 2008, 453, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Hayman, J.R.; Southern, T.R.; Nash, T.E. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect. Immun. 2005, 73, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Southern, T.R.; Jolly, C.E.; Russell Hayman, J. Augmentation of microsporidia adherence and host cell infection by divalent cations. FEMS Microbiol. Lett. 2006, 260, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Luo, J.; Xu, J.-Z.; Wang, C.-X.; Meng, X.-Z.; Pan, G.-Q.; Li, T.; Zhou, Z.-Y. Morphology and transcriptome analysis of Nosema bombycis sporoplasm and insights into the initial infection of microsporidia. Msphere 2020, 5, e00958-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, Y.; Zhang, L.; Shen, H.; Zhang, S.; Cao, X.; Qiao, Y.; Jiang, G.; Cheng, J.; Wan, X.; Fan, X. Comparative transcriptome analysis of non-germinated and germinated spores of Enterocytozoon hepatopenaei (EHP) in vitro. J. Invertebr. Pathol. 2023, 197, 107900. [Google Scholar] [CrossRef]
- Chioralia, G.; Trammer, T.; Maier, W.A.; Seitz, H.M. Morphologic changes in Nosema algerae (Microspora) during extrusion. Parasitol. Res. 1997, 84, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Toguebaye, B.S.; Marchand, B. Intracellular emergence of the microsporidian sporoplasm as revealed by electron microscopy in Nosema couilloudi (Microspora, Nosematidae). Arch. Für Protistenkd. 1987, 134, 397–407. [Google Scholar] [CrossRef]
- Weidner, E.; Manale, S.; Halonen, S.; Lynn, J. Microsporidian spore invasion tubes as revealed by fluorescent probes. Biol. Bull. 1994, 187, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Dissanaike, A.; Canning, E.U. The mode of emergence of the sporoplasm in Microsporidia and its relation to the structure of the spore. Parasitology 1957, 47, 92–99. [Google Scholar] [CrossRef]
- Dissanaike, A. Emergence of the sporoplasm in Nosema helminthorum. Nature 1955, 175, 1002–1003. [Google Scholar] [CrossRef]
- Troemel, E.R.; Becnel, J.J. Genome analysis and polar tube firing dynamics of mosquito-infecting microsporidia. Fungal Genet. Biol. 2015, 83, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Purrini, K. Light and electron microscopic studies on the microsporidian Pleistophora schubergi neustriae n. subsp. (Microsporida: Phylum Microspora) parasitizing the larvae of Malacosoma neustriae L. (Lymantriidae, Lepidoptera). Arch. Für Protistenkd. 1982, 125, 345–355. [Google Scholar] [CrossRef]
- De Leeuw, R.; Gruenbaum, Y.; Medalia, O. Nuclear lamins: Thin filaments with major functions. Trends Cell Biol. 2018, 28, 34–45. [Google Scholar] [CrossRef]
- Bone, C.R.; Starr, D.A. Nuclear migration events throughout development. J. Cell Sci. 2016, 129, 1951–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, A.; Hieda, M.; Yokoyama, Y.; Nishioka, Y.; Yoshidome, K.; Tsujimoto, M.; Matsuura, N. Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN 1, SUN 2, and nesprin-2 in breast cancer. Cancer Med. 2015, 4, 1547–1557. [Google Scholar] [CrossRef]
- Venables, R.; McLean, S.; Luny, D.; Moteleb, E.; Morley, S.; Quinlan, R.; Lane, E.; Hutchison, C. Expression of individual lamins in basal cell carcinomas of the skin. Br. J. Cancer 2001, 84, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, T.A.; Misteli, T. The lamin protein family. Genome Biol. 2011, 12, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aurrecoechea, C.; Barreto, A.; Brestelli, J.; Brunk, B.P.; Caler, E.V.; Fischer, S.; Gajria, B.; Gao, X.; Gingle, A.; Grant, G. AmoebaDB and MicrosporidiaDB: Functional genomic resources for Amoebozoa and Microsporidia species. Nucleic Acids Res. 2010, 39, D612–D619. [Google Scholar] [CrossRef] [Green Version]
- Corradi, N.; Pombert, J.-F.; Farinelli, L.; Didier, E.S.; Keeling, P.J. The complete sequence of the smallest known nuclear genome from the microsporidian Encephalitozoon intestinalis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denais, C.M.; Gilbert, R.M.; Isermann, P.; McGregor, A.L.; Te Lindert, M.; Weigelin, B.; Davidson, P.M.; Friedl, P.; Wolf, K.; Lammerding, J. Nuclear envelope rupture and repair during cancer cell migration. Science 2016, 352, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Species | Protein/UniProtKB | Number of Amino Acids | PI | Mainly Location | Features | Mw (kDa) | References |
|---|---|---|---|---|---|---|---|
| Encephalitozoon cuniculi | EcSWP1/Q9XZV1 | 450 | 4.96 | Exospore | glycine- and serine-rich repeats | 51 | Bohne et al., 2000 [60] |
| EcEnP1/Q8SWL3 | 357 | 9.07 | Endospore | HBM | 40.5 | Southern et al., 2007 [61] Peuvel et al., 2006 [62] | |
| EcEnP2/EcSWP3/Q8SWI4 | 221 | 8.42 | Endospore | glycosylphosphatidylinositol (GPI) anchored and O-glycosylation sites | 22 | Peuvel et al., 2007 [62] Xu et al., 2006 [63] | |
| EcCDA/Q8SU65 | 254 | 4.43 | Endospore,plasma membrane | Glycoside hydrolase and deacetylase | 28.1 | Brosson et al., 2005 [64] | |
| Encephalitozoon intestinalis | EiSWP1/Q95WA3 | 388 | 4.78 | Exospore | - | 41 | Hayman et al., 2001 [65] |
| EiSWP2/Q95WA4 | 1002 | 3.68 | Exospore | Repeating of amino-acid units | 107 | Hayman et al., 2001 [65] | |
| EiEnP1/A7TZU4 | 348 | 8.84 | Exospore,endospore and polar membrane layer | HBM | 39.1 | Southern et al., 2007 [61] | |
| Encephalitozoon hellem | EhSWP1a/C3VJR1 | 509 | 4.30 | Exospore | - | 55 | Polonais et al., 2010 [66] |
| EhSWP1b/C3VJR2 | 533 | 4.64 | Exospore | - | 60 | Polonais et al., 2010 [66] | |
| Nosema bombycis | NbSWP1/B3STN5 | 278 | 7.95 | Endospore | - | 30.4 | Wu et al., 2008 [45] |
| NbSWP2/B3STN6 | 268 | 8.45 | Endospore | HBM | 25.3 | Wu et al., 2008 [45] | |
| NbSWP3/B3STN7 | 316 | 7.29 | Exospore | - | 32.7 | Wu et al., 2008 [45] | |
| NbSWP5/G0Z414 | 186 | 4.39 | Endospore and PT | Interacts with PTP2 and PTP3 | 20.3 | Li et al., 2012 [67,68] | |
| NbSWP7/B3STP1 | 287 | 4.78 | Exospore and endospore | Interacts with NbSWP9 | 32.8 | Yang et al., 2015 [69] | |
| NbSWP9/R0MLT0 | 367 | 8.32 | Exospore, endospore and PT | Interacts with PTP1, PTP2 and NbSWP9 | 42.8 | Yang et al., 2015 [69] | |
| NbSWP11/B3STP5 | 446 | 9.27 | Exospore and endospore | DnaJ domain and HBM | 52.3 | Yang et al., 2014 [70] | |
| NbSWP12/B3STP6 | 228 | 6.78 | Exospore, endospore, membrane of meront | BAR-2 domain and HBM | 26.6 | Chen et al., 2013 [71,72,73] | |
| NbSWP16/R0MN98 | 211 | 8.42 | Exospore | HBM and proline-rich tandem repeats | 44 | Wang et al., 2015 [74] | |
| NbSWP26/B9UJ97 | 223 | 5.09 | Exospore | HBM and N-glycosylation sites | 25.7 | Li et al., 2009 [75] | |
| Unnamed/EOB13250 | 244 | 10.24 | Endospore | Transmembrane domain | 28 | Wang et al., 2020 [76] | |
| Nosema ceranae | Unnamed/A0A0F9WE74 | 226 | 6.84 | Endospore | - | 26.19 | Liang et al., 2021 [77] |
| NcSWP8/A0A0F9WIV3 | 172 | 4.00 | - | Promote proliferation | 19.5 | He et al., 2021 [78] | |
| NcSWP12/A0A0F9WTX8 | 229 | 7.88 | - | Promote proliferation | 26.7 | He et al., 2021 [78] | |
| Enterocytozoon hepatopenaei | EhSWP1/A0A1W0E3P7 | 228 | 8.45 | Exospore and endospore | Exospore and endospore | 27 | Jaroenlak et al., 2018 [79] |
| EhSWP2/A0A1W0E3S3 | 228 | 5.12 | - | MICSWaP domains | 25.7 | Li et al., 2021 [80] | |
| EhSWP3/A0A1W0E914 | 249 | Exospore and endospore | transmembrane domains | 27.1 | Fan et al., 2022 [81] | ||
| EhSWP7/A0A1W0E705 | 250 | 5.04 | - | - | 25.3 | Li et al., 2021 [80] | |
| EhEnp1/A0A1W0E696 | 333 | 8.86 | - | - | 38.3 | Li et al., 2021 [80] | |
| Antonospora locustae (formerly Nosema locustae) | AlocSWP2/A0A1W0E3S3 | 222 | 5.16 | Exospore and endospore | HBM | 25 | Chen et al., 2017 [82] |
| EbSWP1/B7XHM5 | 228 | 7.06 | - | O-linked glycosylation site | 26.8 | Meng et al., 2022 [83] | |
| Enterocytozoon bieneusi | EbSWP2/B7XJH4 | 247 | 9.46 | - | N-linked glycosylation sites | 29.2 | Meng et al., 2022 [83] |
| EbSWP3/B7XHL8 | 229 | 5.15 | - | N-linked glycosylation sites | 25.9 | Meng et al., 2022 [83] | |
| NpSWP1/A0A060A4C2 | 278 | 7.02 | Endospore | Transmembrane | 32 | Zhu et al., 2014 [84] | |
| Nosema pernyi | NpSWP9/A0A0N7AC01 | 317 | 5.75 | - | - | 37.16 | Ma et al., 2017 [85] |
| NpSWP12/A0A0S2EGT8 | 228 | 5.96 | - | BAR domain | 26.6 | Feng et al., 2015 [86] | |
| Nosema antheraeae | NaSWP8/G3CU65 | 161 | 4.802 | - | HBM | 18.4 | Xi et al., 2010 [87] |
| Germination Stages | Known Information | Information Gaps |
|---|---|---|
| Activation | Spores may be triggered by pH changes, alkali metal ions (e. g. Na+, K+, Ca2+), anions (e. g. Cl−, CO32−, Br−), and other stimuli | The mechanisms by which receptors are triggered in vivo and the path of signaling in activation. |
| Increase of intrasporal osmotic pressure | An increased intrasporal osmotic pressure may be caused by trehalase, acyl-CoA oxidase, catalase, permeases, and some transporters. As soon as the spores absorb water through functional aquaporins, swelling of the polaroplasts and posterior vacuoles occurs. | The main substances that cause changes of intrasporal osmotic pressure. A challenge in understanding osmolarity increases is identifying the signaling pathway. |
| PT eversion | The subtilisin-like protease or chitinase may play a key role during the PT firing occurs. | The details of the PT structure, the energy source, and the ejection of the PT are still unclear. |
| Sporoplasm passage | As soon as the PT extend a half, the nuclei deform to fit into the PT and exit from the spore coat. Vesicles can be observed in the extruded PT. Sporoplasm returns to a circular shape in the tip of PT. | The sources of energy, regulation of the nuclear phases and deformation of sporoplasm are difficult to understand. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Chen, J.; Lv, Q.; Long, M.; Pan, G.; Zhou, Z. Germination of Microsporidian Spores: The Known and Unknown. J. Fungi 2023, 9, 774. https://doi.org/10.3390/jof9070774
Huang Q, Chen J, Lv Q, Long M, Pan G, Zhou Z. Germination of Microsporidian Spores: The Known and Unknown. Journal of Fungi. 2023; 9(7):774. https://doi.org/10.3390/jof9070774
Chicago/Turabian StyleHuang, Qingyuan, Jie Chen, Qing Lv, Mengxian Long, Guoqing Pan, and Zeyang Zhou. 2023. "Germination of Microsporidian Spores: The Known and Unknown" Journal of Fungi 9, no. 7: 774. https://doi.org/10.3390/jof9070774






