Human and Feline Sporotrichosis in a Reference Center of Southeastern Brazil: Genetic Differentiation, Diversity, and Antifungal Susceptibility of Sporothrix Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining and Characterizing Isolates
2.2. Data Collection from Patients Included in the Study
2.3. Morphological Identification of Sporothrix Isolates
2.4. Molecular Assays
2.4.1. Extraction of Genomic DNA
2.4.2. Species-Specific PCR Targeting the Calmodulin-Encoding Gene (CAL)
2.4.3. Duplex PCR for Identifying Mating-Type (MAT) Idiomorph
2.4.4. Dimensioning Analysis
2.5. In Vitro Antifungal Susceptibility Profile
3. Results
3.1. Species and Mating-Type Idiomorph Identification
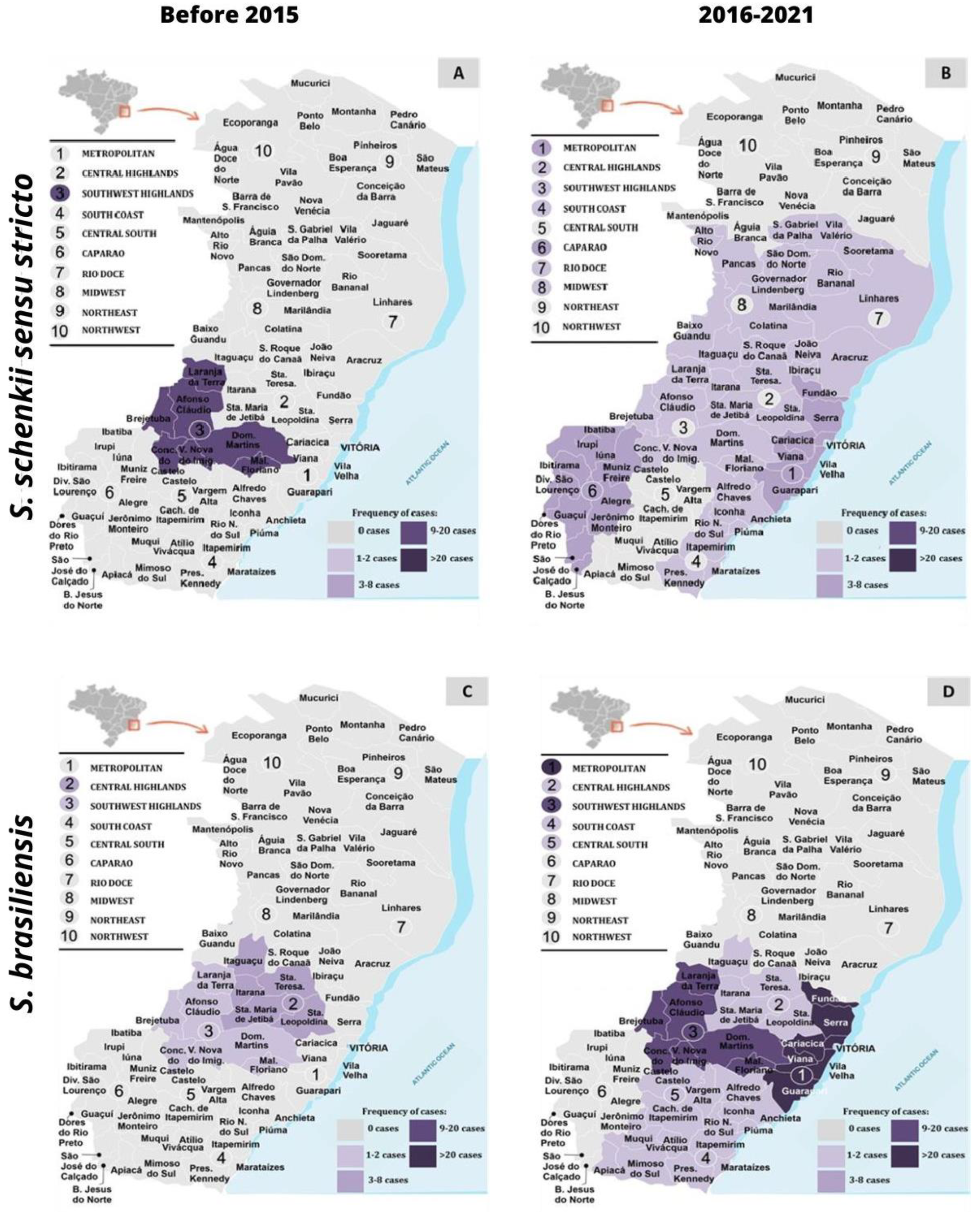
3.2. Distribution Geographical of Sporothrix spp. concerning Mating-Type Idiomorph and Genetic Diversity
3.3. Clinical and Epidemiological Characteristics of Human Sporotrichosis
4. In Vitro Antifungal Susceptibilities
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Carolis, E.; Posteraro, B.; Sanguinetti, M. Old and New Insights into Sporothrix Schenckii Complex Biology and Identification. Pathogens 2022, 11, 297. [Google Scholar] [CrossRef]
- Legabão, B.C.; Fernandes, J.A.; de Oliveira Barbosa, G.F.; Bonfim-Mendonça, P.S.; Svidzinski, T.I.E. The Zoonosis Sporotrichosis Can Be Successfully Treated by Photodynamic Therapy: A Scoping Review. Acta Trop. 2022, 228, 106341. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Hagen, F.; de Camargo, Z.P. A Spotlight on Sporothrix and Sporotrichosis. Mycopathologia 2022, 187, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.F.; Miranda, L.H.M.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic Epidemic of Sporotrichosis: Cat to Human Transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef] [PubMed]
- Ostafińska, A.; Jankowiak, R.; Bilański, P.; Solheim, H.; Wingfield, M.J. Six New Species of Sporothrix from Hardwood Trees in Poland. MycoKeys 2021, 82, 1–32. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Della Terra, P.P.; Gremião, I.D.; Pereira, S.A.; Orofino-Costa, R.; de Camargo, Z.P. The Threat of Emerging and Re-Emerging Pathogenic Sporothrix Species. Mycopathologia 2020, 185, 813–842. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix Species Causing Outbreaks in Animals and Humans Driven by Animal–Animal Transmission. PLoS Pathog. 2016, 12, e1005638. [Google Scholar] [CrossRef]
- Etchecopaz, A.N.; Lanza, N.; Toscanini, M.A.; Devoto, T.B.; Pola, S.J.; Daneri, G.L.; Iovannitti, C.A.; Cuestas, M.L. Sporotrichosis Caused by Sporothrix Brasiliensis in Argentina: Case Report, Molecular Identification and In Vitro Susceptibility Pattern to Antifungal Drugs. J. Mycol. Med. 2020, 30, 100908. [Google Scholar] [CrossRef]
- Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezú, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix Brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J. Fungi 2021, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Castro, R.; Pinto-Almazán, R.; Arenas, R.; Sánchez-Cárdenas, C.D.; Espinosa-Hernández, V.M.; Sierra-Maeda, K.Y.; Conde-Cuevas, E.; Juárez-Durán, E.R.; Xicohtencatl-Cortes, J.; Carrillo-Casas, E.M.; et al. Epidemiology of Clinical Sporotrichosis in the Americas in the Last Ten Years. J. Fungi 2022, 8, 588. [Google Scholar] [CrossRef]
- Thomson, P.; González, C.; Blank, O.; Ramírez, V.; Río, C.D.; Santibáñez, S.; Pena, P. Sporotrichosis Outbreak Due to Sporothrix Brasiliensis in Domestic Cats in Magallanes, Chile: A One-Health-Approach Study. J. Fungi 2023, 9, 226. [Google Scholar] [CrossRef]
- Caus, A.L.O.; Zanotti, R.L.; Faccini-Martínez, Á.A.; Paterlini, G.V.; Falqueto, A. Epidemiological and Clinical Aspects of Sporotrichosis in Espírito Santo State, Southeast Brazil: A Study of Three Decades (1982–2012). Am. J. Trop. Med. Hyg. 2019, 100, 706–713. [Google Scholar] [CrossRef]
- Da Cruz Bahiense Rocha, I.; Terra, P.P.D.; Cardoso de Oliveira, R.; Lubianca Zanotti, R.; Falqueto, A.; de Camargo, Z.P.; Rodrigues, A.M.; Gonçalves, S.S. Molecular-based Assessment of Diversity and Population Structure of Sporothrix spp. Clinical Isolates from Espírito Santo-Brazil. Mycoses 2021, 64, 420–427. [Google Scholar] [CrossRef]
- Rediguieri, B.C.; da Cruz Bahiense, I.; de Carvalho, J.A.; Leite, G.R.; Falqueto, A.; Rodrigues, A.M.; Gonçalves, S.S. Clinical, Epidemiological, and Epizootic Features of Sporothrix Brasiliensis in Espírito Santo, Brazil. Ecohealth 2022, 19, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Gonçalves, S.S.; de Carvalho, J.A.; Borba-Santos, L.P.; Rozental, S.; Camargo, Z.P.D. Current Progress on Epidemiology, Diagnosis, and Treatment of Sporotrichosis and Their Future Trends. J. Fungi 2022, 8, 776. [Google Scholar] [CrossRef]
- Della Terra, P.P.; Gonsales, F.F.; de Carvalho, J.A.; Hagen, F.; Kano, R.; Bonifaz, A.; Camargo, Z.P.D.; Rodrigues, A.M. Development and Evaluation of a Multiplex QPCR Assay for Rapid Diagnostics of Emerging Sporotrichosis. Transbound. Emerg. Dis. 2022, 69, e704–e716. [Google Scholar] [CrossRef]
- Losada, L.C.D.M.L.; Monteiro, R.C.; de Carvalho, J.A.; Hagen, F.; Fisher, M.C.; Spruijtenburg, B.; Meis, J.F.; de Groot, T.; Gonçalves, S.S.; Negroni, R.; et al. High-Throughput Microsatellite Markers Development for Genetic Characterization of Emerging Sporothrix Species. J. Fungi 2023, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.A.; Hagen, F.; Fisher, M.C.; de Camargo, Z.P.; Rodrigues, A.M. Genome-Wide Mapping Using New AFLP Markers to Explore Intraspecific Variation among Pathogenic Sporothrix Species. PLoS Negl. Trop. Dis. 2020, 14, e0008330. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.A.; Monteiro, R.C.; Hagen, F.; Camargo, Z.P.D.; Rodrigues, A.M. Trends in Molecular Diagnostics and Genotyping Tools Applied for Emerging Sporothrix Species. J. Fungi 2022, 8, 809. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.D.M.; Rodrigues, A.M.; Tsui, C.K.M.; de Almeida, L.G.P.; Van Diepeningen, A.D.; van den Ende, B.G.; Fernandes, G.F.; Kano, R.; Hamelin, R.C.; Lopes-Bezerra, L.M.; et al. Asexual Propagation of a Virulent Clone Complex in a Human and Feline Outbreak of Sporotrichosis. Eukaryot. Cell. 2015, 14, 158–169. [Google Scholar] [CrossRef]
- De Carvalho, J.A.; Pinheiro, B.G.; Hagen, F.; Gonçalves, S.S.; Negroni, R.; Kano, R.; Bonifaz, A.; de Camargo, Z.P.; Rodrigues, A.M. A New Duplex PCR Assay for the Rapid Screening of Mating-Type Idiomorphs of Pathogenic Sporothrix Species. Fungal Biol. 2021, 125, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Freitas, D.F.S.; Bernardes-Engemann, A.R.; Rodrigues, A.M.; Talhari, C.; Ferraz, C.E.; Veasey, J.V.; Quintella, L.; Sousa, M.S.L.A.D.; Vettorato, R.; et al. Human Sporotrichosis: Recommendations from the Brazilian Society of Dermatology for the Clinical, Diagnostic and Therapeutic Management. An. Bras. Dermatol. 2022, 97, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Macedo, P.M.D.; Rodrigues, A.M.; Bernardes-Engemann, A.R. Sporotrichosis: An Update on Epidemiology, Etiopathogenesis, Laboratory and Clinical Therapeutics. An. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef]
- Waller, S.B.; Dalla Lana, D.F.; Quatrin, P.M.; Ferreira, M.R.A.; Fuentefria, A.M.; Mezzari, A. Antifungal Resistance on Sporothrix Species: An Overview. Braz. J. Microbiol. 2021, 52, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Marimon, R.; Cano, J.; Gené, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. Globosa, and S. Mexicana, Three New Sporothrix Species of Clinical Interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular Diagnosis of Pathogenic Sporothrix Species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Lee, T.V.D.; Hornes, M.; Friters, A.; Pot, J.; Paleman, J.; Kuiper, M.; et al. AFLP: A New Technique for DNA Fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef]
- Vauterin, L.; Vauterin, P. Molecular Identification, Systematics, and Population Structure of Prokaryotes; Stackebrandt, E., Ed.; Springer: Berlin, Heidelberg, 2006; ISBN 978-3-540-23155-4. [Google Scholar]
- Kohonen, T. Self-Organizing Maps; Springer: Berlin, Heidelberg, 2001; Volume 30, ISBN 978-3-540-67921-9. [Google Scholar]
- Kohonen, T. The Self-Organizing Map. Proc. IEEE 1990, 78, 1464–1480. [Google Scholar] [CrossRef]
- M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard—Second Edition. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008.
- Gremião, I.D.F.; Oliveira, M.M.E.; Monteiro de Miranda, L.H.; Saraiva Freitas, D.F.; Pereira, S.A. Geographic Expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef]
- Bastos de Lima Barros, M.; Schubach, A.D.O.; Francesconi do Valle, A.C.; Gutierrez Galhardo, M.C.; Conceicao-Silva, F.; Pacheco Schubach, T.M.; Santos Reis, R.; Wanke, B.; Feldman Marzochi, K.B.; Conceicao, M.J. Cat-Transmitted Sporotrichosis Epidemic in Rio de Janeiro, Brazil: Description of a Series of Cases. Clin. Infect. Dis. 2004, 38, 529–535. [Google Scholar] [CrossRef]
- Barros, M.B.L.; Schubach, A.O.; Schubach, T.M.P.; Wanke, B.; Lambert-Passos, S.R. An Epidemic of Sporotrichosis in Rio de Janeiro, Brazil: Epidemiological Aspects of a Series of Cases. Epidemiol. Infect. 2008, 136, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Bento, A.; de Sena Costa, A.S.; Lima, S.L.; do Monte Alves, M.; de Azevedo Melo, A.S.; Rodrigues, A.M.; da Silva-Rocha, W.P.; Milan, E.P.; Chaves, G.M. The Spread of Cat-Transmitted Sporotrichosis Due to Sporothrix Brasiliensis in Brazil towards the Northeast Region. PLoS Negl. Trop. Dis. 2021, 15, e0009693. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, S.; de Camargo, Z.P. Emergence of Pathogenicity in the Sporothrix Schenckii Complex. Med. Mycol. 2013, 51, 405–412. [Google Scholar] [CrossRef]
- Oliveira, M.M.E.; Almeida-Paes, R.; Corrêa-Moreira, D.; Borba, C.D.M.; Menezes, R.C.; Freitas, D.F.S.; do Valle, A.C.F.; Schubach, A.D.O.; Barros, M.B.d.L.; Nosanchuk, J.D.; et al. A Case of Sporotrichosis Caused by Different Sporothrix Brasiliensis Strains: Mycological, Molecular, and Virulence Analyses. Mem. Inst. Oswaldo Cruz 2019, 114, 633–654. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, M.L.; Rodrigues, A.M.; Fernandes, G.F.; de Camargo, Z.P.; de Hoog, G.S. Human Sporotrichosis beyond the Epidemic Front Reveals Classical Transmission Types in Espírito Santo, Brazil. Mycoses 2015, 58, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Falqueto, A.; Bravim Maifrede, S.; Araujo Ribeiro, M. Unusual Clinical Presentation of Sporotrichosis in Three Members of One Family. Int. J. Dermatol. 2012, 51, 434–438. [Google Scholar] [CrossRef]
- Alvarez, C.M.; Oliveira, M.M.E.; Pires, R.H. Sporotrichosis: A Review of a Neglected Disease in the Last 50 Years in Brazil. Microorganisms 2022, 10, 2152. [Google Scholar] [CrossRef]
- Roberto, T.N.; de Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Hahn, R.C.; de Camargo, Z.P.; Rodrigues, A.M. Exploring Genetic Diversity, Population Structure, and Phylogeography in Paracoccidioides Species Using AFLP Markers. Stud. Mycol. 2021, 100, 100131. [Google Scholar] [CrossRef]
- De Carvalho, J.A. Análise da Diversidade Genética em Agentes da Esporotricose Usando Marcadores Aflp (Amplified Fragment Length Polymorphism). Master’s Thesis, Universidade Federal de São Paulo, São Paulo, Brazil, 2020. [Google Scholar]
- Poester, V.R.; Basso, R.P.; Stevens, D.A.; Munhoz, L.S.; de Souza Rabello, V.B.; Almeida-Paes, R.; Zancopé-Oliveira, R.M.; Zanchi, M.; Benelli, J.L.; Xavier, M.O. Treatment of Human Sporotrichosis Caused by Sporothrix Brasiliensis. J. Fungi 2022, 8, 70. [Google Scholar] [CrossRef]
- Rabello, V.B.S.; Almeida, M.A.; Bernardes-Engemann, A.R.; Almeida-Paes, R.; de Macedo, P.M.; Zancopé-Oliveira, R.M. The Historical Burden of Sporotrichosis in Brazil: A Systematic Review of Cases Reported from 1907 to 2020. Braz. J. Microbiol. 2022, 53, 231–244. [Google Scholar] [CrossRef]
- Veasey, J.V.; Carvalho, G.d.S.M.; Ruiz, L.R.B.; Neves Neto, M.F.; Zaitz, C. Epidemiological and Geographical Distribution Profile of Urban Sporotrichosis in the City of São Paulo. An. Bras. Dermatol. 2022, 97, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, E.; Arrillaga, A.; Dalcín, L.; Carbia, M.; Arteta, Z.; Perera, P. Clinical and Epidemiological Characteristics of Sporotrichosis in a Reference Center of Uruguay. J. Fungi 2022, 8, 322. [Google Scholar] [CrossRef]
- Xue, S.-L.; Li, L. Oral Potassium Iodide for the Treatment of Sporotrichosis. Mycopathologia 2009, 167, 355–356. [Google Scholar] [CrossRef]
- Belda, W.; Domingues Passero, L.F.; Stradioto Casolato, A.T. Lymphocutaneous Sporotrichosis Refractory to First-Line Treatment. Case Rep. Dermatol. Med. 2021, 2021, 9453701. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.; Gupta, S. Potassium Iodide Remains the Most Effective Therapy for Cutaneous Sporotrichosis. J. Dermatol. Treat. 2003, 14, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Abreu, D.P.B.; Almeida-Paes, R.; Brilhante, R.S.N.; Chakrabarti, A.; Chowdhary, A.; Hagen, F.; Córdoba, S.; Gonzalez, G.M.; Govender, N.P.; et al. Multicenter, International Study of MIC/MEC Distributions for Definition of Epidemiological Cutoff Values for Sporothrix Species Identified by Molecular Methods. Antimicrob. Agents Chemother. 2017, 61, e01057-17. [Google Scholar] [CrossRef]
- Vettorato, R.; Heidrich, D.; Fraga, F.; Ribeiro, A.C.; Pagani, D.M.; Timotheo, C.; Amaro, T.G.; Vettorato, G.; Scroferneker, M.L. Sporotrichosis by Sporothrix Schenckii Senso Stricto with Itraconazole Resistance and Terbinafine Sensitivity Observed In Vitro and In Vivo: Case Report. Med. Mycol. Case Rep. 2018, 19, 18–20. [Google Scholar] [CrossRef]
- Fischman Gompertz, O.; Rodrigues, A.M.; Fernandes, G.F.; Bentubo, H.D.L.; de Camargo, Z.P.; Petri, V. Atypical Clinical Presentation of Sporotrichosis Caused by Sporothrix Globosa Resistant to Itraconazole. Am. J. Trop. Med. Hyg. 2016, 94, 1218–1222. [Google Scholar] [CrossRef]
- Boechat, J.S.; Pereira, S.A.; de Sá Machado, A.C.; Viana, P.G.; Almeida-Paes, R.; Zancopé-Oliveira, R.M.; Gremião, I.D.F.; de Oliveira, M.M.E. Canine Sporotrichosis: Polyphasic Taxonomy and Antifungal Susceptibility Profiles of Sporothrix Species in an Endemic Area in Brazil. Braz. J. Microbiol. 2021, 52, 135–143. [Google Scholar] [CrossRef]
- Borba-Santos, L.P.; Rodrigues, A.M.; Gagini, T.B.; Fernandes, G.F.; Castro, R.; de Camargo, Z.P.; Nucci, M.; Lopes-Bezerra, L.M.; Ishida, K.; Rozental, S. Susceptibility of Sporothrix Brasiliensis Isolates to Amphotericin B, Azoles, and Terbinafine. Med. Mycol. 2015, 53, 178–188. [Google Scholar] [CrossRef]
- Maschio-Lima, T.; Marques, M.D.R.; Lemes, T.H.; Brizzotti-Mazuchi, N.S.; Caetano, M.H.; de Almeida, B.G.; Bianco, L.M.; Monteiro, R.C.; Rodrigues, A.M.; de Camargo, Z.P.; et al. Clinical and Epidemiological Aspects of Feline Sporotrichosis Caused by Sporothrix Brasiliensis and In Vitro Antifungal Susceptibility. Vet. Res. Commun. 2021, 45, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Sanchotene, K.O.; Brandolt, T.M.; Klafke, G.B.; Poester, V.R.; Xavier, M.O. In Vitro Susceptibility of Sporothrix Brasiliensis: Comparison of Yeast and Mycelial Phases. Med. Mycol. 2017, 55, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.C. Susceptibilidade a Antifúngicos de Isolados de Sporothrix Brasiliensis Provenientes de Gatos Do Rio de Janeiro. Ph.D. Thesis, Instituto Nacional de Infectologia Evandro Chagas, Rio de Janeiro, Brazil, 2015. [Google Scholar]






| Isolate Code | Other Code | Species | MAT | Source | Geographic Origin |
|---|---|---|---|---|---|
| Ss43 | - | S. brasiliensis | 1-1 | Human | Ceara-BR |
| Ss54 | CBS 132990 | S. brasiliensis | 1-1 | Cat | Rio Grande do Sul-BR |
| Ss62 | CBS 132991 | S. brasiliensis | 1-1 | Human | Espírito Santo-BR |
| Ss95 | - | S. brasiliensis | 1-2 | Human | Rio de Janeiro-BR |
| Ss128 | - | S. brasiliensis | 1-1 | Human | São Paulo-BR |
| Ss149 | - | S. brasiliensis | 1-1 | Human | Rio Grande do Sul-BR |
| Ss151 | CBS 132994 | S. brasiliensis | 1-1 | Dog | Rio Grande do Sul-BR |
| Ss177 | FMR 8309 | S. brasiliensis | 1-2 | Human | Rio de Janeiro-BR |
| Ss178 | CBS 120339 | S. brasiliensis | 1-1 | Human | Rio de Janeiro-BR |
| Ss237 | - | S. brasiliensis | 1-2 | Human | Espírito Santo-BR |
| Ss252 | CBS 133011 | S. brasiliensis | 1-2 | Cat | Rio de Janeiro-BR |
| Ss292 | - | S. brasiliensis | 1-2 | Cat | São Paulo-BR |
| Ss295 | - | S. brasiliensis | 1-1 | Cat | São Paulo-BR |
| Ss607 | - | S. brasiliensis | 1-2 | Human | Pernambuco-BR |
| Ss609 | - | S. brasiliensis | 1-2 | Human | Pernambuco-BR |
| Ss03 | CBS 132963 | S. schenckii | 1-2 | Human | Rio Grande do Sul-BR |
| Ss17 | - | S. schenckii | 1-2 | Human | Paraná-BR |
| Ss58 | - | S. schenckii | 1-2 | Human | São Paulo-BR |
| Ss63 | CBS 132968 | S. schenckii | 1-2 | Human | Espírito Santo-BR |
| Ss110 | - | S. schenckii | 1-2 | Human | Minas Gerais-BR |
| Ss185 | CBS 359.36 | S. schenckii | 1-1 | Human | USA |
| Ss06 | CBS 132922 | S. globosa | 1-1 | Human | Minas Gerais-BR |
| Ss179 | CBS 120340 | S. globosa | 1-2 | Human | Spain |
| Ss236 | CBS 132925 | S. globosa | 1-2 | Human | Minas Gerais-BR |
| Ss376 | - | S. globosa | 1-1 | - | Espírito Santo-BR |
| Ss521 | - | S. globosa | 1-1 | Human | Rio Grande do Sul-BR |
| Ss545 | - | S. globosa | 1-1 | - | Mexico |
| Patients | ||
|---|---|---|
| Variables | N | Percentage (%) |
| Gender | ||
| Female | 79 | 59.9 |
| Male | 53 | 40.1 |
| Clinical presentation | ||
| Lymphocutaneous | 92 | 69.7 |
| Cutaneous-Fixed | 37 | 28 |
| Disseminated | 3 | 2.3 |
| Source of infection | ||
| Zoonosis | 112 | 84.9 |
| Environmental | 20 | 15.1 |
| Therapy of Choice before Diagnosis | * Number of Patients/% | Treatment of Choice after Diagnosis Definitive | Number of Patients/% |
|---|---|---|---|
| Cephalexin | 43/32.6 | Itraconazole | 98/74.2 |
| Amoxicillin + Clavulanate | 19/14.4 | Potassium iodide | 21/15.9 |
| Ceftriaxone | 14/10.6 | Amphotericin B | 1/0.8 |
| Penicillin G | 13/9.9 | Itraconazole + Potassium Iodide | 10/7.6 |
| Sulfamethoxazole + trimethoprim | 13/9.9 | Amphotericin B + Itraconazole | 2/1.5 |
| Amoxicillin | 11/8.3 | ||
| Itraconazole | 11/8.3 | ||
| Clindamycin | 8/6.1 | ||
| Azithromycin | 7/5.3 | ||
| Levofloxacin | 7/5.3 | ||
| Ciprofloxacin | 6/4.6 | ||
| Mupirocin | 3/2.3 | ||
| Terbinafine | 3/2.3 | ||
| Fluconazole | 2/1.5 | ||
| Acyclovir | 1/0.8 | ||
| Doxycycline | 1/0.8 | ||
| Ivermectin | 1/0.8 |
| Specie (n) | Antifungal | MIC Range µg/mL | MIC50 | MIC90 |
|---|---|---|---|---|
| S. brasiliensis (84) | Amphotericin B | 0.5–2.0 | 2.0 | 2.0 |
| Itraconazole | 0.5–8.0 | 2.0 | 4.0 | |
| Posaconazole | 1.0–4.0 | 2.0 | 4.0 | |
| Terbinafine | 0.03–0.25 | 0.06 | 0.125 | |
| S. schenckii (12) | Amphotericin B | 1.0–2.0 | 2.0 | 2.0 |
| Itraconazole | 1.0–8.0 | 2.0 | 4.0 | |
| Posaconazole | 1.0–2.0 | 2.0 | 2.0 | |
| Terbinafine | 0.03–0.125 | 0.06 | 0.125 |
| Specie (n) | Antifungal | MIC Range µg/mL | MIC50 | MIC90 |
|---|---|---|---|---|
| S. brasiliensis (20) | Amphotericin B | 0.5−2.0 | 1.0 | 2.0 |
| Itraconazole | 1.0−4.0 | 2.0 | 4.0 | |
| Posaconazole | 0.5−2.0 | 2.0 | 2.0 | |
| Terbinafine | 0.03−0.125 | 0.06 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, S.S.; da Cruz Bahiense Rocha, I.; Rediguieri, B.C.; de Carvalho, J.A.; Maifrede, S.B.; Kruschewsky, W.L.L.; Falqueto, A.; Rodrigues, A.M. Human and Feline Sporotrichosis in a Reference Center of Southeastern Brazil: Genetic Differentiation, Diversity, and Antifungal Susceptibility of Sporothrix Species. J. Fungi 2023, 9, 831. https://doi.org/10.3390/jof9080831
Gonçalves SS, da Cruz Bahiense Rocha I, Rediguieri BC, de Carvalho JA, Maifrede SB, Kruschewsky WLL, Falqueto A, Rodrigues AM. Human and Feline Sporotrichosis in a Reference Center of Southeastern Brazil: Genetic Differentiation, Diversity, and Antifungal Susceptibility of Sporothrix Species. Journal of Fungi. 2023; 9(8):831. https://doi.org/10.3390/jof9080831
Chicago/Turabian StyleGonçalves, Sarah Santos, Isabela da Cruz Bahiense Rocha, Bruno Carneiro Rediguieri, Jamile Ambrósio de Carvalho, Simone Bravim Maifrede, Wdson Luis Lima Kruschewsky, Aloísio Falqueto, and Anderson Messias Rodrigues. 2023. "Human and Feline Sporotrichosis in a Reference Center of Southeastern Brazil: Genetic Differentiation, Diversity, and Antifungal Susceptibility of Sporothrix Species" Journal of Fungi 9, no. 8: 831. https://doi.org/10.3390/jof9080831
APA StyleGonçalves, S. S., da Cruz Bahiense Rocha, I., Rediguieri, B. C., de Carvalho, J. A., Maifrede, S. B., Kruschewsky, W. L. L., Falqueto, A., & Rodrigues, A. M. (2023). Human and Feline Sporotrichosis in a Reference Center of Southeastern Brazil: Genetic Differentiation, Diversity, and Antifungal Susceptibility of Sporothrix Species. Journal of Fungi, 9(8), 831. https://doi.org/10.3390/jof9080831







