Colletotrichum siamense, a Novel Causal Agent of Viburnum odoratissimum Leaf Blotch and Its Sensitivity to Fungicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation
2.2. DNA Extraction, PCR Amplification and Sequencing
2.3. Sequence Alignment and Phylogenetic Analyses
2.4. Morphological Characteristics and Biological Characteristics
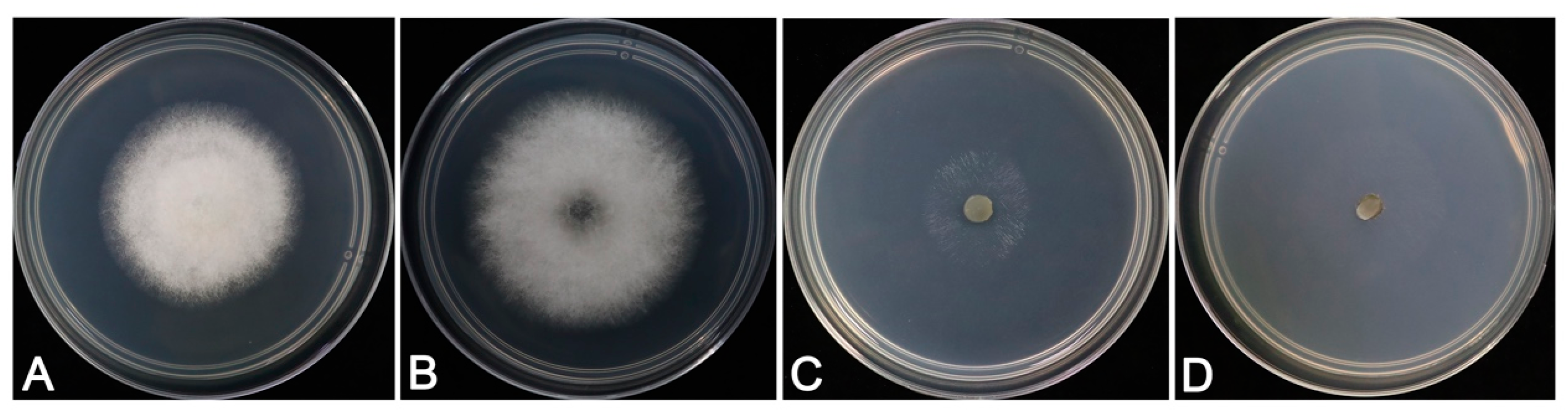
2.5. Pathogenicity Tests
2.6. Susceptibility of Colletotrichum Isolates to Fungicides
3. Results
3.1. Disease Symptoms and Fungal Isolation
3.2. Phylogenetic Analyses
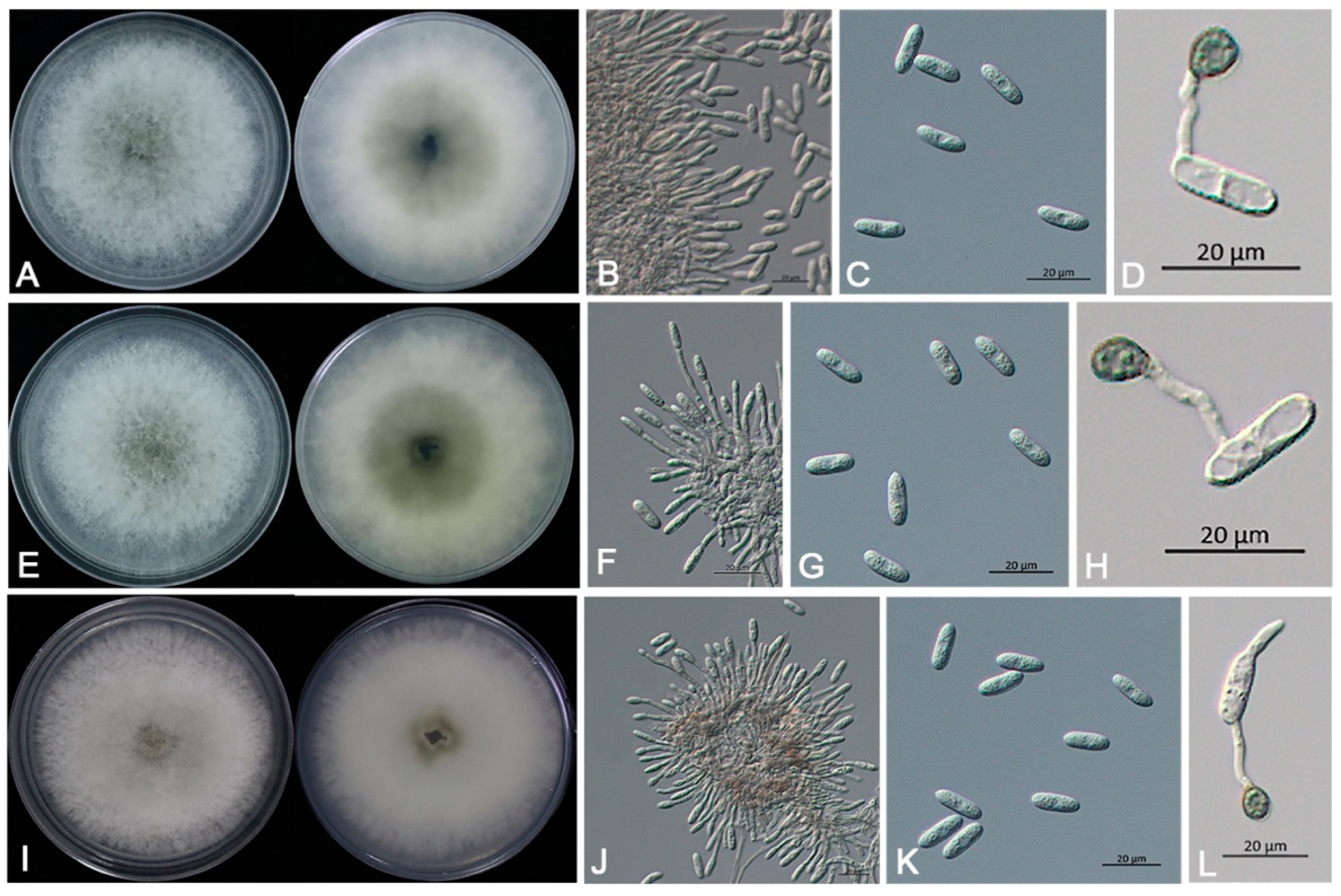
3.3. Morphological Identification and Biological Characteristics
3.4. Pathogenicity of Fungal Isolates
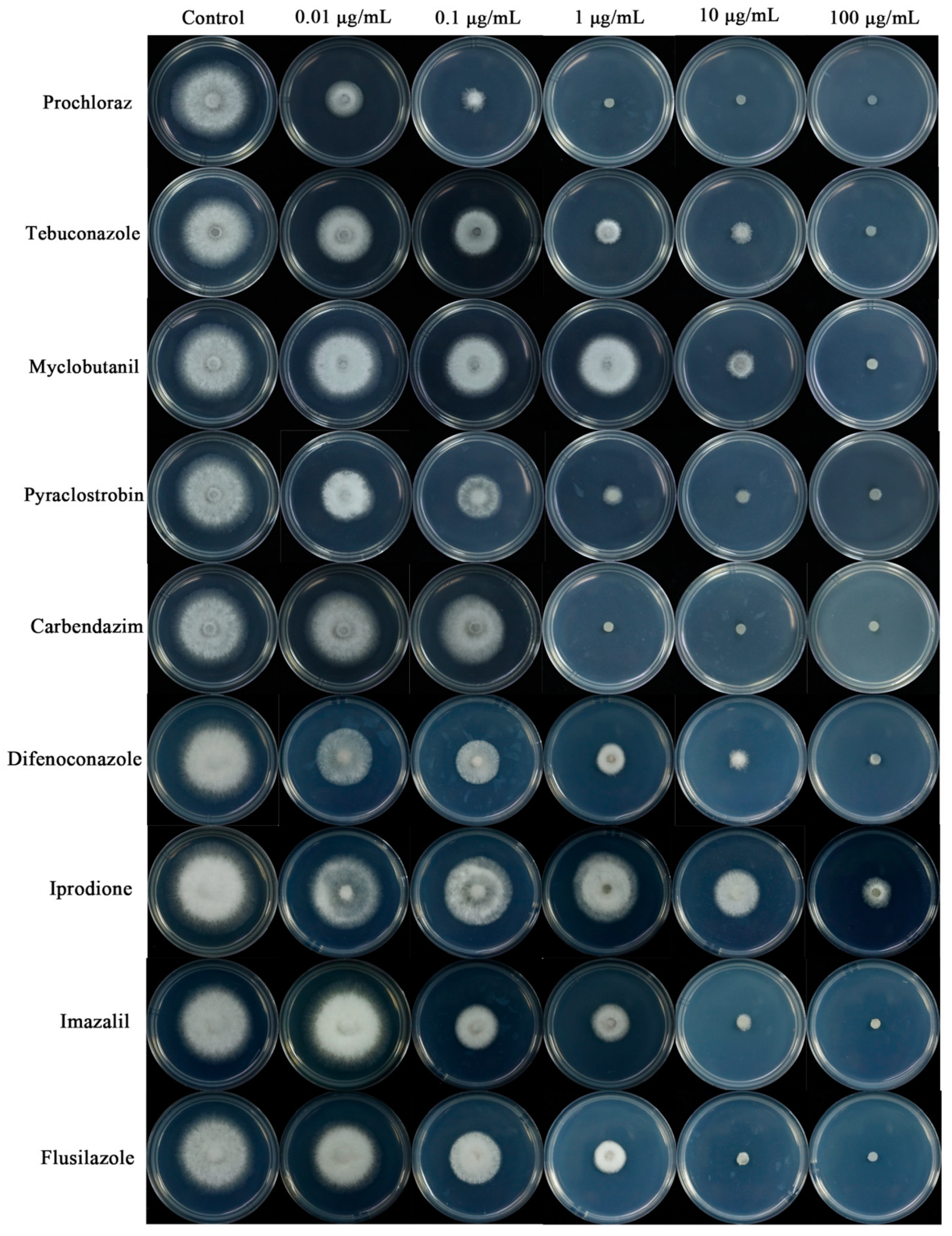
3.5. Susceptibility of Colletotrichum Isolates to Fungicides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plants of the World Online. 2023. Available online: http://www.plantsoftheworldonline.org/ (accessed on 6 February 2023).
- Yang, X.X.; Yue, S.S.; Gao, L.X.; Cheng, J.S.; Xie, J.T.; Fu, Y.P. First report of anthracnose caused by Colletotrichum gloeosporioides on Viburnum odoratissimum in China. Plant Dis. 2015, 99, 1647. [Google Scholar] [CrossRef]
- Cho, S.E.; Lee, S.H.; Lee, S.Y.; Lee, C.K.; Shin, H.D. First report of powdery mildew caused by Erysiphe hedwigii on Viburnum awabuki in Korea. Plant Dis. 2016, 100, 2533. [Google Scholar] [CrossRef]
- Michael, J.B.; Uwe, B.; Donald, H.P. Phylogeny and taxonomy of the genera of Erysiphaceae, part 1: Golovinomyces. Mycologia 2022, 114, 964–993. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, Y.; Liu, Z. First Report of Alternaria spp. causing leaf spot on sweet Viburnum in China. Plant Dis. 2021, 105, 2253. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wu, S.; Si, Y.Z.; Li, D.W.; Zhu, L.H. First report of Diaporthe eres causing leaf spot of Viburnum odoratissimum var. awabuki in China. Plant Dis. 2023, 107, 954. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.W.; Ye, L.; Dong, S.Y.; Huai, B.Y.; Tan, G.J. First report of Neofusicoccum parvum causing leaf spot on coral trees (Viburnum odoratissimum) in China. Plant Dis. 2022, 106, 3000. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Song, Q.; Liu, J.; Yang, Q.; Luan, F.; Li, D. First report of leaf spot caused by Corynespora cassiicola on Viburnum odoratissimum var. awabuki (sweet viburnum) in China. Plant Dis. 2022, 106, 1062. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Camporesi, E.; Elgorban, A.M.; Bahkali, A.H.; Yan, J.Y.; Hyde, K.D. A new species of Colletotrichum from Sonchus sp. in Italy. Phytotaxa 2017, 314, 55–63. [Google Scholar] [CrossRef]
- Damm, U.; Sato, T.; Alizadeh, A.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud. Mycol. 2019, 92, 1–46. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 2009, 39, 45–87. [Google Scholar]
- Liu, F.; Cai, L.; Crous, P.W.; Damm, U. The Colletotrichum gigasporum species complex. Persoonia 2014, 33, 83–97. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Slippers, B.; Wingfield, M.J. Global food and fibre security threatened by current inefficiencies in fungal identification. Philos. Trans. R. Soc. B-Biol. Sci. 2016, 371, 20160024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y.P.; Zhou, D.; Yang, T.; Dai, L.; Li, L.; Zhao, H.; Liu, X.; Cai, Z.Y. Characterization of Colletotrichum causing anthracnose on rubber trees in Yunnan: Two new records and two new species from China. Plant Dis. 2023, in press. [CrossRef]
- Buchta, V.; Nekolova, J.; Jiraskova, N.; Bolehovska, R.; Wipler, J.; Hubka, V. Fungal keratitis caused by Colletotrichum dematium: Case study and review. Mycopathologia 2019, 184, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, J.; Vilaplana, F.; Nadal, J.; García-Barberán, I.; Barraquer, R.I. Treatment resistant fungal keratitis caused by Colletotrichum gloeosporioides. Arch. Soc. Esp. Oftalmol. 2016, 91, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, J.; Giordano, R.; Gouli, S.; Gouli, V.; Parker, B.L.; Skinner, M.; TeBeest, D.; Cesnik, R. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 2008, 100, 353–374. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, D.W.; Zhu, Y.N.; Si, Y.Z.; Huang, J.H.; Zhu, L.H.; Ye, J.R.; Huang, L. Diversity and pathogenicity of Colletotrichum species causing anthracnose on Cunninghamia lanceolata. Plant Pathol. 2022, 71, 1757–1773. [Google Scholar] [CrossRef]
- Zhang, W.; Damm, U.; Crous, P.W.; Groenewald, J.Z.; Niu, X.; Lin, J.; Li, Y. Anthracnose disease of carpetgrass (Axonopus compressus) caused by Colletotrichum hainanense sp. nov. Plant Dis. 2020, 104, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Phoulivong, S.; Cai, L.; Chen, H.; McKenzie, E.H.; Abdelsalam, K.; Chukeatirote, E.; Hyde, K.D. Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers. 2010, 44, 33–43. [Google Scholar] [CrossRef]
- De Silva, D.D.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Colletotrichum species associated with chili anthracnose in Australia. Plant Pathol. 2017, 66, 254–267. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Martino, I.; Gilardi, G.; Garibaldi, A.; Gullino, M.L. Colletotrichum spp. causing anthracnose on ornamental plants in northern Italy. J. Plant Pathol. 2021, 103, 127–137. [Google Scholar] [CrossRef]
- Backman, P.A.; Williams, J.C.; Crawford, M.A. Yield losses in soybeans from anthracnose caused by Colletotrichum truncatum. Plant Dis. 1982, 66, 1032–1034. [Google Scholar] [CrossRef]
- Freeman, S.; Katan, T.; Shabi, T. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Dis. 1998, 82, 596–605. [Google Scholar] [CrossRef]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.S.; Waller, J.M.; Abang, M.M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Simmonds, J.H. A study of the species of Colletotrichum causing ripe fruit rots in Queensland. Qld. J. Agric. Anim. Sci. 1965, 22, 437–459. [Google Scholar]
- Sutton, B.C. The genus Glomerella and its anamorph Colletotrichum. In Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CABI: Wallingford, UK, 1992; pp. 1–26. [Google Scholar]
- Than, P.P.; Jeewon, R.; Hyde, K.D.; Pongsupasamit, S.; Mongkolporn, O.; Taylor, P.W.J. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathol. 2008, 57, 562–572. [Google Scholar] [CrossRef]
- Thaung, M.M. Coelomycete systematic with special reference to Colletotrichum. Mycoscience 2008, 49, 345–350. [Google Scholar] [CrossRef]
- Alizadeh, A.; Javan-Nikkhah, M.; Zare, R.; Fotouhifar, K.B.; Damm, U.; Stukenbrock, E.H. New records of Colletotrichum species for the mycobiota of Iran. Mycol. Iran. 2015, 2, 95–109. [Google Scholar]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.S.; Hyde, K.D.; Chen, Y.J.; Papp, V.; Palla, B.; Papp, D.; Bhunjun, C.S.; Hurdeal, V.G.; Senwanna, C.; Manawasinghe, I.S. One stop shop IV: Taxonomic update with molecular phylogeny for important phytopathogenic genera: 76–100. Fungal Divers. 2020, 103, 87–218. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Bhunjun, C.S.; Hyde, K.D.; Gentekaki, E.; Itthayakorn, P. Colletotrichum: Lifestyles, biology, morpho-species, species complexes and accepted species. Mycosphere 2021, 12, 519–669. [Google Scholar] [CrossRef]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; He, L.; Zhu, J.; Wu, B.; Liu, F.; Mu, W. Activity of the novel fungicide mefentrifluconazole against Colletotrichum scovillei. Plant Dis. 2021, 105, 1522–1530. [Google Scholar] [CrossRef]
- Kim, C.H.; Hassan, O.; Chang, T. Diversity, pathogenicity, and fungicide sensitivity of Colletotrichum species associated with apple anthracnose in South Korea. Plant Dis. 2020, 104, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Mora-Aguilera, J.A.; Ríos-López, E.G.; Yáñez-Zúñiga, M.; Rebollar-Alviter, A.; Tovar-Pedraza, J.M. Sensitivity to MBC fungicides and prochloraz of Colletotrichum gloeosporioides species complex isolates from mango orchards in Mexico. J. Plant Dis. Prot. 2021, 128, 481–491. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Gao, Y.; Li, B.; Mu, W.; Liu, F. Characterization and fungicide sensitivity of Colletotrichum spp. from different hosts in Shandong, China. Plant Dis. 2019, 103, 34–43. [Google Scholar] [CrossRef]
- Chen, S.N.; Luo, C.X.; Hu, M.J.; Schnabel, G. Sensitivity of Colletotrichum species, including C. fioriniae and C. nymphaeae, from peach to demethylation inhibitor fungicides. Plant Dis. 2016, 100, 2434–2441. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.R.; Zhang, M.J.; Chen, F.M. Occurrence of Pestalotiopsis lushanensis causing leaf blight on Buddhist pine in China. Eur. J. Plant Pathol. 2022, 162, 655–665. [Google Scholar] [CrossRef]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity tests. Appl. Environ. Microbiol. 1996, 62, 1014–1020. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Silva, D.N.; Talhinhas, P.; Várzea, V.; Cai, L.; Paulo, O.S.; Batista, D. Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: An example from coffee (Coffea spp.) hosts. Mycologia 2012, 104, 396–409. [Google Scholar] [CrossRef]
- Templeton, M.D.; Rikkerink, E.H.A.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenaseencoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Zhang, D.; Gao, F.L.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Quaedvlieg, W.; Binder, M.; Groenewald, J.Z.; Summerell, B.A.; Carnegie, A.J.; Burgess, T.I.; Crous, P.W. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia 2014, 33, 1–40. [Google Scholar] [CrossRef]
- Johnston, P.R.; Jones, D. Relationships among Colletotrichum isolates from fruit rots assessed using rDNA sequences. Mycologia 1997, 89, 420–430. [Google Scholar] [CrossRef]
- Liu, F.; Ma, Z.Y.; Hou, L.W.; Diao, Y.Z.; Wu, W.P.; Damm, U.; Song, S.; Cai, L. Updating species diversity of Colletotrichum, with a phylogenomic overview. Stud. Mycol. 2022, 101, 1–56. [Google Scholar] [CrossRef]
- Huang, F.; Chen, G.Q.; Hou, X.; Fu, Y.S.; Cai, L.; Hyde, K.D.; Li, H.Y. Colletotrichum species associated with cultivated citrus in China. Fungal Divers. 2013, 61, 61–74. [Google Scholar] [CrossRef]
- Wang, Y.C.; Hao, X.Y.; Wang, L.; Xiao, B.; Wang, X.C.; Yang, Y.J. Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci. Rep. 2016, 6, 35287. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Crous, P.W.; Bai, Q.; Zhang, P.F.; Xiang, J.; Guo, Y.S.; Zhao, F.F.; Yang, M.M.; Hong, N.; Xu, W.X.; et al. Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia 2019, 42, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Prihastuti, H.; Cai, L.; Chen, H.; McKenzie, E.H.C.; Hyde, K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009, 39, 89–109. [Google Scholar]
- Guarnaccia, V.; Groenewald, J.Z.; Polizzi, G.; Crous, P.W. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 2017, 39, 32. [Google Scholar] [CrossRef]
- Crouch, J.A.; Tredway, L.P.; Clarke, B.B.; Hillman, B.I. Phylogenetic and population genetic divergence correspond with habitat for the pathogen Colletotrichum cereale and allied taxa across diverse grass communities. Mol. Ecol. 2009, 18, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Amsden, B.; Dixon, E.; Vaillancourt, L.; Gauthier, N.A.W. Characterization of Colletotrichum species causing bitter rot of apples in Kentucky orchards. Plant Dis. 2016, 100, 2194–2203. [Google Scholar] [CrossRef]
- Chongo, G.; Bernier, C.C. Effects of host, inoculum concentration, wetness duration, growth stage, and temperature on anthracnose of lentil. Plant Dis. 2000, 84, 544–548. [Google Scholar] [CrossRef]
- Li, H.; Li, S.Z.; Wang, Y.C.; Liu, J.A.; Xu, J.P.; Zhou, G.Y. Identification of the pathogens causing anthracnose of Camellia oleifera in Nursery and their resistance to fungicides. Sci. Silvae Sin. 2019, 55, 85–94. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, M.; Gao, Z.Y.; Zhang, Z.K.; Yang, F.Z.; Xie, Y.X.; Hu, M.J.; Yang, Y. Screening and cross-resistance analysis of alternative fungicides against carbendazim-resistant Colletotrichum gloeosporioides Penz. from mango (Mangifera indica L.). Acta Hortic. 2013, 992, 415–422. [Google Scholar] [CrossRef]
- European Commission Implementing Regulation (Eu) 2019/29. 2019. Available online: https://eur-lex.europa.eu/eli/reg_impl/2019/291/oj (accessed on 7 July 2023).
- Xu, X.M.; Chen, J.Y.; Li, B.R.; Tang, L.J. Carbendazim residues in vegetables in China between 2014 and 2016 and a chronic carbendazim exposure risk assessment. Food Control 2018, 91, 20–25. [Google Scholar] [CrossRef]




| Gene | PCR Primers (Forward/Reverse) | PCR: Thermal Cycles: (Annealing Temp. in Bold) |
|---|---|---|
| ITS | ITS1/ITS4 | 94 °C: 3 min, (94 °C: 30 s, 55 °C: 30 s, 72 °C: 45 s) × 33 cycles, 72 °C: 10 min |
| CAL | CL-1C/CL-2C | 95 °C: 3 min, (95 °C: 30 s, 55 °C: 30 s, 72 °C: 30 s) × 35 cycles, 72 °C: 10 min |
| ACT | ACT-512F/ACT-783R | 94 °C: 3 min, (94 °C: 30 s, 58 °C: 30 s, 72 °C: 45 s) × 35 cycles, 72 °C: 10 min |
| TUB2 | T1/Bt-2b | 95 °C: 3 min, (95 °C: 30 s, 55 °C: 30 s, 72 °C: 30 s) × 35 cycles, 72 °C: 10 min |
| CHS-1 | CHS-79F/CHS-354R | 94 °C: 3 min, (94 °C: 30 s, 58 °C: 30 s, 72 °C: 45 s) × 35 cycles, 72 °C: 10 min |
| ApMat | AM-F/AM-R | 94 °C: 3 min, (94 °C: 1 min, 55 °C: 30 s, 72 °C: 1 min) × 35 cycles, 72 °C: 10 min |
| GAPDH | GD-F1/GD-R1 | 94 °C: 3 min, (94 °C: 30 s, 58 °C: 30 s, 72 °C: 45 s) × 35 cycles, 72 °C: 10 min |
| Media | Colony Growth (mm/d) | ||
|---|---|---|---|
| SHS 1-3 | SHS 1-6 | SHS 1-7 | |
| CM | 16.41 ± 0.24 b * | 15.78 ± 0.14 b | 17.87 ± 0.18 b |
| PDA | 17.33 ± 0.23 a | 17.93 ± 0.12 a | 15.24 ± 0.16 a |
| MM | 5.48 ± 0.08 d | 5.01 ± 0.05 d | 6.59 ± 0.12 d |
| CZA | 13.36 ± 0.17 c | 12.51 ± 0.13 c | 11.25 ± 0.21 c |
| Temperature | Colony Growth (mm/d) | ||
|---|---|---|---|
| SHS 1-3 | SHS 1-6 | SHS 1-7 | |
| 15 °C | 8.48 ± 0.26 d* | 9.92 ± 0.06 e | 9.53 ± 0.07 e |
| 20 °C | 15.43 ± 0.40 a | 14.17 ± 0.27 c | 15.4 ± 0.29 c |
| 25 °C | 13.70 ± 0.15 b | 18.09 ± 0.19 a | 17.14 ± 0.19 a |
| 30 °C | 12.42 ± 0.30 c | 16.05 ± 0.31 b | 16.14 ± 0.07 b |
| 35 °C | 7.78 ± 0.27 c | 12.15 ± 0.30 d | 10.89 ± 0.23 d |
| Isolate | Species | Lesion Length (mm) |
|---|---|---|
| SHS 1-3 | C. siamense | 3.97 ± 0.90 a * |
| SHS 1-6 | C. siamense | 4.06 ± 0.96 a |
| SHS 1-7 | C. siamense | 3.99 ± 1.04 a |
| Fungicide | EC50 Values (μg/mL) | ||
|---|---|---|---|
| SHS 1-3 | SHS 1-6 | SHS 1-7 | |
| Prochloraz | 0.0033 ± 0.0003 f * | 0.0047 ± 0.00029 e | 0.0039 ± 0.00030 d |
| Tebuconazole | 0.13 ± 0.0010 cd | 0.086 ± 0.0016 d | 0.10 ± 0.0013 c |
| Myclobutanil | 0.52 ± 0.038 b | 0.22 ± 0.053 b | 0.57 ± 0.044 b |
| Pyraclostrobine | 0.096 ± 0.022 de | 0.072 ± 0.017 de | 0.067 ± 0.0019 cd |
| Carbendazim | 0.071± 0.0011 e | 0.051 ± 0.0023 de | 0.044 ± 0.0014 cd |
| Flusilazole | 0.068 ± 0.038 e | 0.052 ± 0.041de | 0.12 ± 0.0036 c |
| Iprodione | 15.15 ± 1.25 a | 12.27 ± 1.44 a | 14.05 ± 0.96 a |
| Difenoconazole | 0.15 ± 0.018 c | 0.13 ± 0.031 cd | 0.10 ± 0.020 c |
| Imazalil | 0.17 ± 0.022 c | 0.14 ± 0.035 bc | 0.14 ± 0.017 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liao, Y.-C.-Z.; Wan, Y.; Li, D.-W.; Zhu, L.-H. Colletotrichum siamense, a Novel Causal Agent of Viburnum odoratissimum Leaf Blotch and Its Sensitivity to Fungicides. J. Fungi 2023, 9, 882. https://doi.org/10.3390/jof9090882
Li H, Liao Y-C-Z, Wan Y, Li D-W, Zhu L-H. Colletotrichum siamense, a Novel Causal Agent of Viburnum odoratissimum Leaf Blotch and Its Sensitivity to Fungicides. Journal of Fungi. 2023; 9(9):882. https://doi.org/10.3390/jof9090882
Chicago/Turabian StyleLi, Hui, Yang-Chun-Zi Liao, Yu Wan, De-Wei Li, and Li-Hua Zhu. 2023. "Colletotrichum siamense, a Novel Causal Agent of Viburnum odoratissimum Leaf Blotch and Its Sensitivity to Fungicides" Journal of Fungi 9, no. 9: 882. https://doi.org/10.3390/jof9090882






