Thixotropic Supramolecular Gel Based on l-Lysine Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis

2.2. Gelation Test
| Solvent | A | B | C |
|---|---|---|---|
| n-Hexane | GT(6) * | GT(8) * | I |
| n-Dodecane | GT(8) * | GTL(20) | P |
| Liquid paraffin | S | P | P |
| Squalane | P | P | P |
| Cyclohexane | GT(20) * | GT(6) * | I |
| Ethanol | GO(30) | P | GO(20) |
| Ethylene glycol | GO(30) | GO(30) * | – |
| IPM | GT(20) | GTL(10) | – |
| Toluene | GT(80) * | GT(15) * | GT(10) |
| Chloroform | GT(20) * | GTL(30) * | GT(10) |
| Castor oil | GT(40) | GT(80) | GT(80) |
| Kerosene | GT(15) * | GTL(10) * | GTL(40) |
| Light oil | GT(20) * | GT(6) * | GTL(40) |
2.3. Morphology, Gel Strength and Gel-to-Sol Transition Temperature (Tgel)


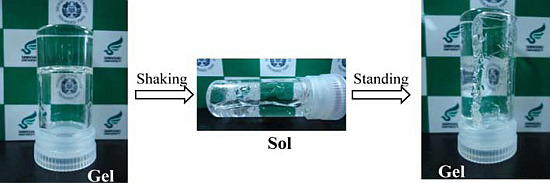
2.4. Simple Thixotropy Test of Supramolecular Gels

2.5. Rheological Studies


2.6. Appearance of Thixotropic Property

2.7. Mechanism of Thixotropic Behavior

3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Apparatus for Measurements
4.3. Gelation Test
4.4. Gel Strength
4.5. Gel-to-Sol Transition Temperature (Tgel)
4.6. Rheological Analysis
4.7. FE-SEM
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Terech, P.; Weiss, R.G. Low Molecular mass gelators of organic liquids and the properties of their gels. Chem. Rev. 1997, 97, 3133–3159. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K. Lost in translation? Chirality effects in the self-assembly of nanostructured gel-phase materials. Chem. Soc. Rev. 2009, 38, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hanabusa, K. l-Lysine-based low-molecular-weight gelators. Chem. Soc. Rev. 2009, 38, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-gelators and their applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Fages, F. (Ed.) Low Molecular Mass Gelators: Design, Self-Assembly, Function; Topics in Current Chemistry 256; Springer-Verlag: Berlin, Germany, 2005.
- Terech, P.; Weiss, R.G. (Eds.) Molecular Gels, Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006.
- Escuder, B. (Ed.) Functional Molecular Gels; Royal Society of Chemistry: Cambridge, UK, 2014.
- Naota, T.; Koori, H. Molecules that assemble by sound: an application to the instant gelation of stable organic fluids. J. Am. Chem. Soc. 2005, 127, 9324–9325. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Sallenave, X.; Fages, F.; Mieden-Gundert, G.; Müller, W.M.; Müller., U.; Vögtle, F.; Pozzo, J.L. Multiaddressable self-assembling organogelators based on 2H-Chromene and N-Acyl-1,ö-amino acid units. Langmuir 2002, 18, 7096–7101. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Ueki, K.; Yoshida, M.; Tamaoki, N.; Nakamura, T.; Sakai, H.; Abe, M. Assembly and photoinduced organization of mono- and oligopeptide molecules containing an azobenzene moiety. Adv. Funct. Mater. 2007, 17, 1507–1514. [Google Scholar] [CrossRef]
- Verdejo, B.; Rodríguez-Llansola, F.; Escuder, B.; Miravet, J.F.; Ballester, P. Sodium and pH responsive hydrogel formation by the supramolecular system calix[4]pyrrole derivative/tetramethylammonium cation. Chem. Commun. 2011, 47, 2017–2019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Gao, Y.; Kuang, Y.; Shi, J.F.; Du, X.W.; Zhou, J.; Wang, H.M.; Yang, Z.M.; Xu, B. Dephosphorylation of D-peptide derivatives to form biofunctional, supramolecular nanofibers/hydrogels and their potential applications for intracellular imaging and intratumoral chemotherapy. J. Am. Chem. Soc. 2013, 135, 9907–9914. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and anion-binding supramolecular gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, P.L.; Yan, J.L.; Fang, X.H.; Peng, J.X.; Liu, K.Q.; Fang, Y. An. Organometallic super-gelator with multiple-stimulus responsive properties. Adv. Mater. 2008, 20, 2508–2511. [Google Scholar] [CrossRef]
- Afrasiabi, R.; Kraatz, H.-B. Stimuli-responsive supramolecular gelation in ferrocene–peptide conjugates. Chem. Eur. J. 2013, 19, 17296–17300. [Google Scholar] [CrossRef] [PubMed]
- Because a common supramolecular gel is a physical gel, most of the gels is reformed by heating-cooling process.
- Shirakawa, M.; Norifumi Fujita, N.; Shinkai, S. A stable single piece of unimolecularly π-stacked porphyrin aggregate in a thixotropic low molecular weight gel: A one-dimensional molecular template for polydiacetylene wiring up to several tens of micrometers in length. J. Am. Chem. Soc. 2005, 127, 4164–4165. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Raghavan, S.R.; Terech, P.; Weiss, R.G. Distinct kinetic pathways generate organogel networks with contrasting fractality and thixotropic properties. J. Am. Chem. Soc. 2006, 128, 15341–15352. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, L.; Zhang, M.; Yi, T. Low-molecular-mass gels responding to ultrasound and mechanical stress: towards self-healing materials. Chem. Soc. Rev. 2014, 43, 5346–5371. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Gao, D.; Yan, J.; Ma, Y.; Liu, K.; Fang, Y. Novel dimeric cholesteryl derivatives and their smart thixotropic gels. Langmuir 2011, 27, 12156–12163. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Baral, A.; Banerjee, A. An amino-acid-based self-healing hydrogel: Modulation of the self-healing properties by incorporating carbon-based nanomaterials. Chem. Eur. J. 2013, 19, 14950–14957. [Google Scholar] [CrossRef] [PubMed]
- Ohsedo, Y.; Oono, M.; Saruhashi, K.; Watanabe, H. N-alkylamido-d-glucamine-based gelators for the generation of thixotropic gels. RSC Adv. 2014, 4, 48554–48558. [Google Scholar] [CrossRef]
- Dawn, A.; Shiraki, T.; Ichikawa, H.; Takada, A.; Takahashi, Y.; Tsuchiya, Y.; Lien, L.T.N.; Shinkai, S. Stereochemistry-dependent, mechanoresponsive supramolecular host assemblies for fullerenes: A guest-induced enhancement of thixotropy. J. Am. Chem. Soc. 2012, 134, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Ohsedo, Y.; Oono, M.; Tanaka, A.; Watanabe, H. Mixing induced thixotropy of a two-component system of alkylurea organogelators having different alkyl chains. New J. Chem. 2013, 37, 2250–2253. [Google Scholar] [CrossRef]
- Yan, C.; Altunbas, A.; Yucel, T.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable solid hydrogel: Mechanism of shear-tinning and immediate recovery of injectable β–hairpin peptide hydrogels. Soft Matter 2010, 6, 5143–5156. [Google Scholar] [CrossRef] [PubMed]
- Hoshizawa, H.; Suzuki, M.; Hanabusa, K. Cyclo(l-aspartyl-l-phenylalanyl)-containing poly(dimethylsiloxane)-based thixotropic organogels. Chem. Lett. 2011, 40, 1143–1145. [Google Scholar] [CrossRef]
- Hoshizawa, H.; Minemura, Y.; Yoshikawa, K.; Suzuki, M.; Hanabusa, K. Thixotropic Hydrogelators Based on a Cyclo(dipeptide) Derivative. Langmuir 2013, 29, 14666–14673. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yumoto, M.; Kimura, M.; Shirai, H.; Hanabusa, K. New low-molecular-weight gelators based on an l-Lysine: Amphiphilic gelators and water-soluble organogelators. Helv. Chim. Acta 2004, 87, 1–10. [Google Scholar] [CrossRef]
- See supporting information.
- For all gels, the FTIR spectroscopies showed the typical IR peaks are about 3300 cm−1 and 1640 cm−1, arising from hydrogen bonded amide groups, and 2920 cm−1 and 2850 cm−1, arising from the alkyl groups with van der Waals interaction.
- Gelator A-H form a dimer structure in the organogels through the hydrogen bonding between carboxylic acid groups that is confirmed by a FT-IR spectroscopy [30]. In contrast, a portion of gelator B-H forms a dimer structure.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, M.; Hayakawa, Y.; Hanabusa, K. Thixotropic Supramolecular Gel Based on l-Lysine Derivatives. Gels 2015, 1, 81-93. https://doi.org/10.3390/gels1010081
Suzuki M, Hayakawa Y, Hanabusa K. Thixotropic Supramolecular Gel Based on l-Lysine Derivatives. Gels. 2015; 1(1):81-93. https://doi.org/10.3390/gels1010081
Chicago/Turabian StyleSuzuki, Masahiro, Yuta Hayakawa, and Kenji Hanabusa. 2015. "Thixotropic Supramolecular Gel Based on l-Lysine Derivatives" Gels 1, no. 1: 81-93. https://doi.org/10.3390/gels1010081
APA StyleSuzuki, M., Hayakawa, Y., & Hanabusa, K. (2015). Thixotropic Supramolecular Gel Based on l-Lysine Derivatives. Gels, 1(1), 81-93. https://doi.org/10.3390/gels1010081