Engineered Dynamic Hydrogel Niches for the Regulation of Redox Homeostasis in Osteoporosis and Degenerative Endocrine Diseases
Abstract
:1. Introduction
2. The Design of Dynamic Hydrogels
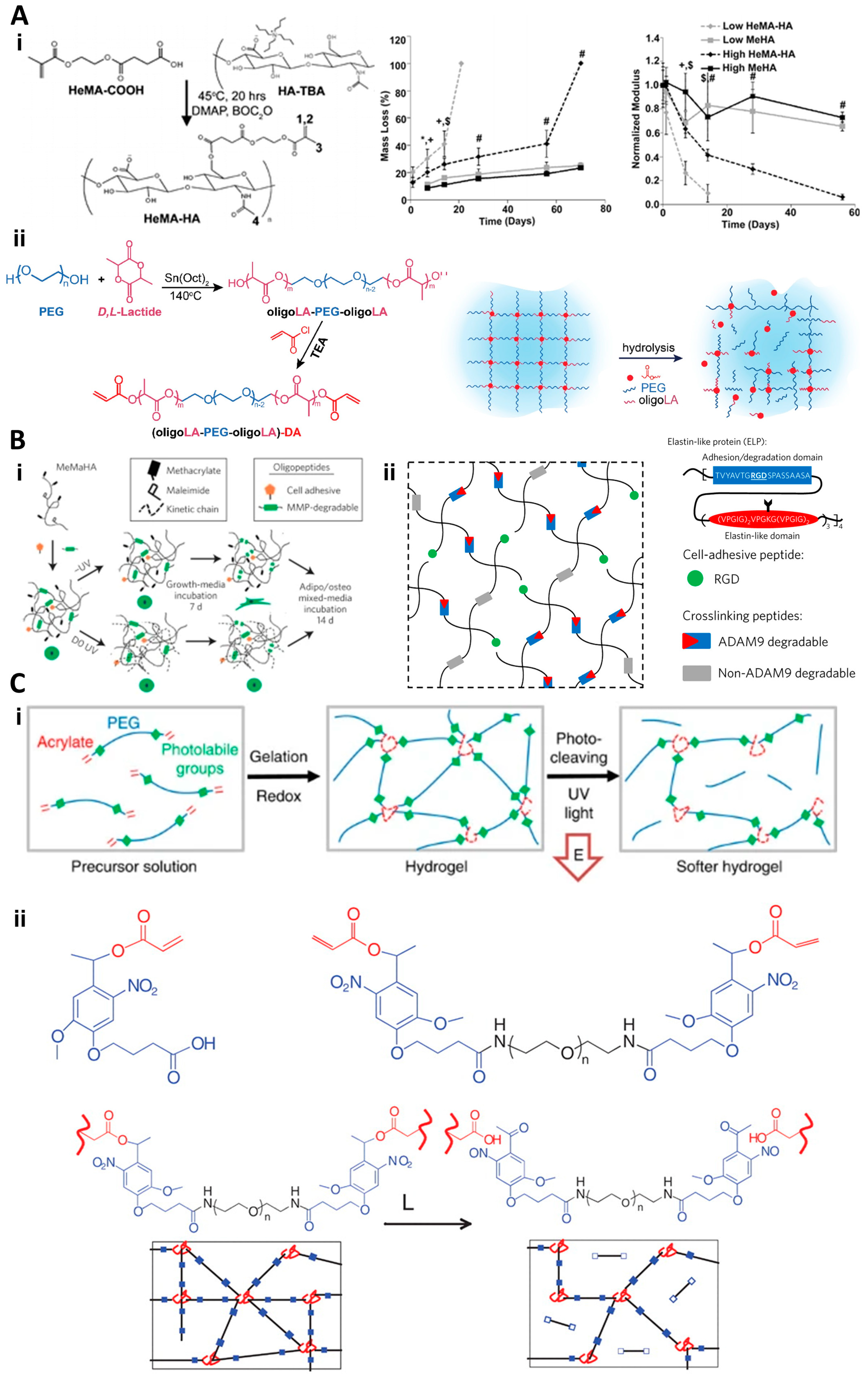
2.1. Degradation-Reliant Dynamic Hydrogels
2.2. Degradation-Independent Dynamic Hydrogels
2.3. The Advance of Engineered Dynamic Hydrogels
2.3.1. Revolutionizing Disease Management with Dynamic Hydrogels
2.3.2. Biophysical Regulation of Redox Homeostasis
2.3.3. Tailoring Hydrogels to Disease Specificities
2.3.4. The Role in Stem Cell Therapy and Regenerative Medicine
3. The Metabolism Regulation of Dynamic Hydrogels
3.1. Osteoporosis
3.2. Type II Diabetes
3.3. Other Degenerative Endocrine Diseases
4. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Regnier, M.; Van Hul, M.; Knauf, C.; Cani, P.D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. 2021, 248, R67–R82. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Ham, T.R.; Leipzig, N.D. Biomaterial strategies for limiting the impact of secondary events following spinal cord injury. Biomed. Mater. 2018, 13, 024105. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.T.A.; Kim, Y.M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, A.E.; Maldonado-Lasunción, I.; Oudega, M. Biomaterials for revascularization and immunomodulation after spinal cord injury. Biomed. Mater. 2018, 13, 044105. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Schirmer, L.; Chwalek, K.; Tsurkan, M.V.; Freudenberg, U.; Werner, C. Glycosaminoglycan-based hydrogels with programmable host reactions. Biomaterials 2020, 228, 119557. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, H.; Priimagi, A.; Ikkala, O. Programmable responsive hydrogels inspired by classical conditioning algorithm. Nat. Commun. 2019, 10, 3267. [Google Scholar] [CrossRef]
- Wang, Y. Programmable hydrogels. Biomaterials 2018, 178, 663–680. [Google Scholar] [CrossRef]
- Bryant, S.J.; Vernerey, F.J. Programmable Hydrogels for Cell Encapsulation and Neo-Tissue Growth to Enable Personalized Tissue Engineering. Adv. Healthc. Mater. 2018, 7, 1700605. [Google Scholar] [CrossRef] [PubMed]
- Zaviskova, K.; Tukmachev, D.; Dubisova, J.; Vackova, I.; Hejcl, A.; Bystronova, J.; Pravda, M.; Scigalkova, I.; Sulakova, R.; Velebny, V.; et al. Injectable hydroxyphenyl derivative of hyaluronic acid hydrogel modified with RGD as scaffold for spinal cord injury repair. J. Biomed. Mater. Res. Part A 2018, 106, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Huang, L.L.; Jiang, X.C.; Chen, J.C.; OuYang, H.W.; Gao, J.Q. Transplantation of BDNF Gene Recombinant Mesenchymal Stem Cells and Adhesive Peptide-modified Hydrogel Scaffold for Spinal Cord Repair. Curr. Gene Ther. 2018, 18, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S.; et al. A MnO2 Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano 2019, 13, 14283–14293. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.Z.; Zhang, G.W.; Xu, J.G.; Chen, S.; Wang, H.; Cao, L.L.; Liang, B.; Lian, X.F. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol. Sin. 2017, 38, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Han, I.B.; Thakor, D.K.; Ropper, A.E.; Yu, D.; Wang, L.; Kabatas, S.; Zeng, X.; Kim, S.W.; Zafonte, R.D.; Teng, Y.D. Physical impacts of PLGA scaffolding on hMSCs: Recovery neurobiology insight for implant design to treat spinal cord injury. Exp. Neurol. 2019, 320, 112980. [Google Scholar] [CrossRef] [PubMed]
- Kubinová, S.; Horák, D.; Hejčl, A.; Plichta, Z.; Kotek, J.; Syková, E. Highly superporous cholesterol-modified poly(2-hydroxyethyl methacrylate) scaffolds for spinal cord injury repair. J. Biomed. Mater. Res. Part A 2011, 99, 618–629. [Google Scholar] [CrossRef]
- Hejčl, A.; Růžička, J.; Proks, V.; Macková, H.; Kubinová, Š.; Tukmachev, D.; Cihlář, J.; Horák, D.; Jendelová, P. Dynamics of tissue ingrowth in SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores after bridging a spinal cord transection. J. Mater. Sci. Mater. Med. 2018, 29, 89. [Google Scholar] [CrossRef]
- Chen, C.M.; Tang, J.C.; Gu, Y.; Liu, L.L.; Liu, X.Z.; Deng, L.F.; Martins, C.; Sarmento, B.; Cui, W.G.; Chen, L. Bioinspired Hydrogel Electrospun Fibers for Spinal Cord Regeneration. Adv. Funct. Mater. 2019, 29, 1806899. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Haggerty, A.E.; Yan, J.; Lan, M.; Seu, M.; Yang, M.; Marlow, M.M.; Maldonado-Lasuncion, I.; Cho, B.; et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials 2020, 245, 119978. [Google Scholar] [CrossRef]
- Yang, L.T.; Conley, B.M.; Cerqueira, S.R.; Pongkulapa, T.; Wang, S.Q.; Lee, J.K.; Lee, K.B. Effective Modulation of CNS Inhibitory Microenvironment using Bioinspired Hybrid-Nanoscaffold-Based Therapeutic Interventions. Adv. Mater. 2020, 32, 2002578. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, W.; Wu, X.; Zhang, N.; Zhang, Y.; Ouyang, S.; Song, X.; Fang, X.; Seeram, R.; Xue, W.; et al. Functional Self-Assembling Peptide Nanofiber Hydrogels Designed for Nerve Degeneration. ACS Appl. Mater. Interfaces 2016, 8, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; LeSavage, B.L.; Dewi, R.E.; Dinh, C.B.; Stowers, R.S.; Khariton, M.; Lampe, K.J.; Nguyen, D.; Chaudhuri, O.; Enejder, A.; et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017, 16, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; LeSavage, B.L.; Dewi, R.E.; Lampe, K.J.; Heilshorn, S.C. Matrix Remodeling Enhances the Differentiation Capacity of Neural Progenitor Cells in 3D Hydrogels. Adv. Sci. 2019, 6, 1801716. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, C.E.; Messersmith, P.B. Enzymatically Degradable Mussel-Inspired Adhesive Hydrogel. Biomacromolecules 2011, 12, 4326–4334. [Google Scholar] [CrossRef]
- Chong, S.F.; Sexton, A.; De Rose, R.; Kent, S.J.; Zelikin, A.N.; Caruso, F. A paradigm for peptide vaccine delivery using viral epitopes encapsulated in degradable polymer hydrogel capsules. Biomaterials 2009, 30, 5178–5186. [Google Scholar] [CrossRef]
- Dobner, S.; Bezuidenhout, D.; Govender, P.; Zilla, P.; Davies, N. A Synthetic Non-degradable Polyethylene Glycol Hydrogel Retards Adverse Post-infarct Left Ventricular Remodeling. J. Card. Fail. 2009, 15, 629–636. [Google Scholar] [CrossRef]
- He, X.Z.; Jabbari, E. Material properties and cytocompatibility of injectable MMP degradable poly(lactide ethylene oxide fumarate) hydrogel as a carrier for marrow stromal cells. Biomacromolecules 2007, 8, 780–792. [Google Scholar] [CrossRef]
- Holland, T.A.; Bodde, E.W.H.; Baggett, L.S.; Tabata, Y.; Mikos, A.G.; Jansen, J.A. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J. Biomed. Mater. Res. A 2005, 75A, 156–167. [Google Scholar] [CrossRef]
- Holland, T.A.; Bodde, E.W.H.; Cuijpers, V.; Baggett, L.S.; Tabata, Y.; Mikos, A.G.; Jansen, J.A. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthr. Cartil. 2007, 15, 187–197. [Google Scholar] [CrossRef]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release 2005, 101, 111–125. [Google Scholar] [CrossRef]
- Rizzi, S.C.; Ehrbar, M.; Halstenberg, S.; Raeber, G.P.; Schmoekel, H.G.; Hagenmuller, H.; Muller, R.; Weber, F.E.; Hubbell, J.A. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part II: Biofunctional characteristics. Biomacromolecules 2006, 7, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, S.C.; Hubbell, J.A. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part 1: Development and physicochernical characteristics. Biomacromolecules 2005, 6, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, B.V.; Brock, J.L.; Silver, J.S.; Leight, J.L.; Randolph, M.A.; Anseth, K.S. Development of a Cellularly Degradable PEG Hydrogel to Promote Articular Cartilage Extracellular Matrix Deposition. Adv. Healthc. Mater. 2015, 4, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.A.; Williams, C.G.; Li, Q.A.; Sharma, B.; Elisseeff, J.H. Synthesis and characterization of a novel degradable phosphate-containing hydrogel. Biomaterials 2003, 24, 3969–3980. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Leach, J.B. Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Tous, E.; Ifkovits, J.L.; Koomalsingh, K.J.; Shuto, T.; Soeda, T.; Kondo, N.; Gorman, J.H.; Gorman, R.C.; Burdick, J.A. Influence of Injectable Hyaluronic Acid Hydrogel Degradation Behavior on Infarction-Induced Ventricular Remodeling. Biomacromolecules 2011, 12, 4127–4135. [Google Scholar] [CrossRef]
- Peng, Y.M.; Liu, Q.J.; He, T.L.; Ye, K.; Yao, X.; Ding, J.D. Degradation rate affords a dynamic cue to regulate stem cells beyond varied matrix stiffness. Biomaterials 2018, 178, 467–480. [Google Scholar] [CrossRef]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013, 12, 458–465. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef]
- Rodin, S.; Domogatskaya, A.; Strom, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.F.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Accardo, J.V.; Kalow, J.A. Reversibly tuning hydrogel stiffness through photocontrolled dynamic covalent crosslinks. Chem. Sci. 2018, 9, 5987–5993. [Google Scholar] [CrossRef] [PubMed]
- Amir, F.; Liles, K.P.; Delawder, A.O.; Colley, N.D.; Palmquist, M.S.; Linder, H.R.; Sell, S.A.; Barnes, J.C. Reversible Hydrogel Photopatterning: Spatial and Temporal Control over Gel Mechanical Properties Using Visible Light Photoredox Catalysis. ACS Appl. Mater. Inter. 2019, 11, 24627–24638. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.H.; Ma, Q.; Yu, H.X.; Zhang, Y.F.; Yan, Z.C.; Liu, F.Y.; Liu, C.Y.; Jiang, H.F.; Chen, Y.M. Macroscopic Organohydrogel Hybrid from Rapid Adhesion between Dynamic Covalent Hydrogel and Organogel. ACS Macro. Lett. 2015, 4, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Du, W.B.; Deng, A.M.; Guo, J.; Chen, J.; Li, H.M.; Gao, Y. An injectable self-healing hydrogel-cellulose nanocrystals conjugate with excellent mechanical strength and good biocompatibility. Carbohydr. Polym. 2019, 223, 115084. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.Y.; Nau, W.M.; Hoogenboom, R. Reversible covalent locking of a supramolecular hydrogel via UV-controlled anthracene dimerization. Polym. Chem. 2021, 12, 307–315. [Google Scholar] [CrossRef]
- Li, B.; Lin, C.L.; Lu, C.J.; Zhang, J.J.; He, T.; Qiu, H.Y.; Yin, S.C. A rapid and reversible thermochromic supramolecular polymer hydrogel and its application in protected quick response codes. Mater. Chem. Front. 2020, 4, 869–874. [Google Scholar] [CrossRef]
- Popescu, M.T.; Liontos, G.; Avgeropoulos, A.; Voulgari, E.; Avgoustakis, K.; Tsitsilianis, C. Injectable Hydrogel: Amplifying the pH Sensitivity of a Triblock Copolypeptide by Conjugating the N-Termini via Dynamic Covalent Bonding. ACS Appl. Mater. Inter. 2016, 8, 17539–17548. [Google Scholar] [CrossRef]
- Ren, B.W.; Chen, X.Y.; Ma, Y.; Du, S.K.; Qian, S.B.; Xu, Y.J.; Yan, Z.L.; Li, J.L.; Jia, Y.; Tan, H.P.; et al. Dynamical release nanospheres containing cell growth factor from biopolymer hydrogel via reversible covalent conjugation. J. Biomater. Sci. -Polym. Ed. 2018, 29, 1344–1359. [Google Scholar] [CrossRef]
- Roberts, M.C.; Hanson, M.C.; Massey, A.P.; Karren, E.A.; Kiser, P.F. Dynamically restructuring hydrogel networks formed with reversible covalent crosslinks. Adv. Mater. 2007, 19, 2503–2507. [Google Scholar] [CrossRef]
- Teng, L.J.; Chen, Y.H.; Jia, Y.G.; Ren, L. Supramolecular and dynamic covalent hydrogel scaffolds: From gelation chemistry to enhanced cell retention and cartilage regeneration. J. Mater. Chem. B 2019, 7, 6705–6736. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.R.; Jin, L.L.; Oliveira, J.M.; Reis, R.L.; Zhong, Q.; Mao, Z.W.; Gao, C.Y. Adaptable hydrogel with reversible linkages for regenerative medicine: Dynamic mechanical microenvironment for cells. Bioact. Mater. 2021, 6, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Yesilyurt, V.; Ayoob, A.M.; Appel, E.A.; Borenstein, J.T.; Langer, R.; Anderson, D.G. Mixed Reversible Covalent Crosslink Kinetics Enable Precise, Hierarchical Mechanical Tuning of Hydrogel Networks. Adv. Mater. 2017, 29, 1605947. [Google Scholar] [CrossRef]
- Feng, Z.B.; Zuo, H.L.; Gao, W.S.; Ning, N.Y.; Tian, M.; Zhang, L.Q. A Robust, Self-Healable, and Shape Memory Supramolecular Hydrogel by Multiple Hydrogen Bonding Interactions. Macromol. Rapid Commun. 2018, 39, e1800138. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.Y.; Liang, Q.F.; Li, H.F.; Peng, L.Q.; Wu, M.M.; Zhao, X.L.; Cui, X.; Ruan, C.S.; Liu, W.G. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef]
- Liu, J.; Scherman, O.A. Cucurbit n uril Supramolecular Hydrogel Networks as Tough and Healable Adhesives. Adv. Funct. Mater. 2018, 28, 1800848. [Google Scholar] [CrossRef]
- Roth-Konforti, M.E.; Comune, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Shabat, D.; Adler-Abramovich, L. UV Light-Responsive Peptide-Based Supramolecular Hydrogel for Controlled Drug Delivery. Macromol. Rapid Commun. 2018, 39, e1800588. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Fujisaku, T.; Tanaka, W.; Kubota, R.; Minami, S.; Urayama, K.; Hamachi, I. An adaptive supramolecular hydrogel comprising self-sorting double nanofibre networks. Nat. Nanotechnol. 2018, 13, 165–172. [Google Scholar] [CrossRef]
- Tan, J.L.; Zhang, M.; Hai, Z.J.; Wu, C.F.; Lin, J.; Kuang, W.; Tang, H.; Huang, Y.L.; Chen, X.D.; Liang, G.L. Sustained Release of Two Bioactive Factors from Supramolecular Hydrogel Promotes Periodontal Bone Regeneration. ACS Nano 2019, 13, 5616–5622. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.N.; Zeng, H.; Ji, X.F.; Xie, T.; Yan, X.Z.; Wu, Z.L.; Huang, F.H. Reversible Ion-Conducting Switch in a Novel Single-Ion Supramolecular Hydrogel Enabled by Photoresponsive Host-Guest Molecular Recognition. Adv. Mater. 2019, 31, e1807328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Ren, Y.P.; Zhu, Y.; Hao, L.J.; Chen, Y.H.; An, G.; Wu, H.K.; Shi, X.T.; Mao, C.B. A Rapidly Self-Healing Host-Guest Supramolecular Hydrogel with High Mechanical Strength and Excellent Biocompatibility. Angew. Chem. Int. Ed. 2018, 57, 9008–9012. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Wang, H.B.; Gao, F.; Xu, Z.Y.; Dai, F.Y.; Liu, W.G. An Injectable Supramolecular Polymer Nanocomposite Hydrogel for Prevention of Breast Cancer Recurrence with Theranostic and Mammoplastic Functions. Adv. Funct. Mater. 2018, 28, 1801000. [Google Scholar] [CrossRef]
- Zhang, X.W.; Wang, J.; Jin, H.; Wang, S.T.; Song, W.L. Bioinspired Supramolecular Lubricating Hydrogel Induced by Shear Force. J. Am. Chem. Soc. 2018, 140, 3186–3189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Zhang, H.; Zou, Q.L.; Xing, R.R.; Jiao, T.F.; Yan, X.H. An injectable dipeptide-fullerene supramolecular hydrogel for photodynamic antibacterial therapy. J. Mater. Chem. B 2018, 6, 7335–7342. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Lie, Y. A polysaccharide/tetraphenylethylene-mediated blue-light emissive and injectable supramolecular hydrogel. Chin. Chem. Lett. 2018, 29, 84–86. [Google Scholar] [CrossRef]
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1, 15012. [Google Scholar] [CrossRef]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef]
- Lou, J.Z.; Liu, F.; Lindsay, C.D.; Chaudhuri, O.; Heilshorn, S.C.; Xia, Y. Dynamic Hyaluronan Hydrogels with Temporally Modulated High Injectability and Stability Using a Biocompatible Catalyst. Adv. Mater. 2018, 30, e1705215. [Google Scholar] [CrossRef]
- Lou, J.Z.; Stowers, R.; Nam, S.M.; Xia, Y.; Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 2018, 154, 213–222. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef]
- Shi, L.Y.; Carstensen, H.; Holzl, K.; Lunzer, M.; Li, H.; Hilborn, J.; Ovsianikov, A.; Ossipov, D.A. Dynamic Coordination Chemistry Enables Free Directional Printing of Biopolymer Hydrogel. Chem. Mater. 2017, 29, 5816–5823. [Google Scholar] [CrossRef]
- Lee, J.; Chang, K.; Kim, S.; Gite, V.; Chung, H.; Sohn, D. Phase Controllable Hyaluronic Acid Hydrogel with Iron(III) Ion Catechol Induced Dual Cross-Linking by Utilizing the Gap of Gelation Kinetics. Macromolecules 2016, 49, 7450–7459. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Rodell, C.B.; MacArthur, J.W.; Dorsey, S.M.; Wade, R.J.; Wang, L.L.; Woo, Y.J.; Burdick, J.A. Shear-Thinning Supramolecular Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties In Vivo. Adv. Funct. Mater. 2015, 25, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.Y.; Zhang, G.P.; Zeng, X.L.; Li, J.H.; Li, G.; Huang, W.P.; Sun, R.; Wong, C.P. High-Strength, Tough, Fatigue Resistant, and Self-Healing Hydrogel Based on Dual Physically Cross-Linked Network. ACS Appl. Mater. Inter. 2016, 8, 24030–24037. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.P.; Xiao, C.; Sun, J.C.; Xiong, D.S.; Hu, X.H. Biological self-assembly of injectable hydrogel as cell scaffold via specific nucleobase pairing. Chem. Commun. 2012, 48, 10289–10291. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, dgaa048. [Google Scholar] [CrossRef]
- Watts, N.B.; Adler, R.A.; Bilezikian, J.P.; Drake, M.T.; Eastell, R.; Orwoll, E.S.; Finkelstein, J.S.; Endocrine, S. Osteoporosis in men: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1802–1822. [Google Scholar] [CrossRef]
- Delitala, A.P.; Scuteri, A.; Doria, C. Thyroid Hormone Diseases and Osteoporosis. J. Clin. Med. 2020, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Schiellerup, S.P.; Skov-Jeppesen, K.; Windelov, J.A.; Svane, M.S.; Holst, J.J.; Hartmann, B.; Rosenkilde, M.M. Gut Hormones and Their Effect on Bone Metabolism. Potential Drug Therapies in Future Osteoporosis Treatment. Front. Endocrinol. 2019, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Grady, D.; Herrington, D.; Bittner, V.; Blumenthal, R.; Davidson, M.; Hlatky, M.; Hsia, J.; Hulley, S.; Herd, A.; Khan, S.; et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002, 288, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Eastell, R.; Adams, A.L. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. Reply. N. Engl. J. Med. 2020, 383, 2189–2190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, Q.; Xu, J.; Xu, X.; Tian, F.; Yeung, K.W.K.; Bian, L. Self-Assembled Injectable Nanocomposite Hydrogels Stabilized by Bisphosphonate-Magnesium (Mg2+) Coordination Regulates the Differentiation of Encapsulated Stem Cells via Dual Crosslinking. Adv. Funct. Mater. 2017, 27, 1701642. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, Z.; Yang, B.; Feng, Q.; Xu, X.; Yuan, W.; Li, X.; Chen, X.; Duan, L.; Wang, D. Adaptable Hydrogels Mediate Cofactor-Assisted Activation of Biomarker-Responsive Drug Delivery via Positive Feedback for Enhanced Tissue Regeneration. Adv. Sci. 2018, 5, 1800875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lin, S.; Feng, Q.; Dong, C.; Yang, Y.; Li, G.; Bian, L. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017, 64, 389–400. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yuan, W.H.; Wei, K.C.; Yang, B.G.; Chen, X.Y.; Li, Z.; Zhang, Z.Y.; Bian, L.M. Highly Dynamic Nanocomposite Hydrogels Self-Assembled by Metal Ion-Ligand Coordination. Small 2019, 15, e1900242. [Google Scholar] [CrossRef]
- Nafee, N.; Zewail, M.; Boraie, N. Alendronate-loaded, biodegradable smart hydrogel: A promising injectable depot formulation for osteoporosis. J. Drug Target. 2018, 26, 563–575. [Google Scholar] [CrossRef]
- Li, D.W.; Zhou, J.; Zhang, M.M.; Ma, Y.Z.; Yang, Y.Y.; Han, X.; Wang, X. Long-term delivery of alendronate through an injectable tetra-PEG hydrogel to promote osteoporosis therapy. Biomater. Sci. 2020, 8, 3138–3146. [Google Scholar] [CrossRef]
- Kuang, L.J.; Huang, J.H.; Liu, Y.T.; Li, X.L.; Yuan, Y.; Liu, C.S. Injectable Hydrogel with NIR Light-Responsive, Dual-Mode PTH Release for Osteoregeneration in Osteoporosis. Adv. Funct. Mater. 2021, 31, 2105383. [Google Scholar] [CrossRef]
- Amani, N.; Javar, H.A.; Dorkoosh, F.A.; Rouini, M.R.; Amini, M.; Sharifzadeh, M.; Boumi, S. Preparation and Pulsatile Release Evaluation of Teriparatide-Loaded Multilayer Implant Composed of Polyanhydride-Hydrogel Layers Using Spin Coating for the Treatment of Osteoporosis. J. Pharm. Innov. 2021, 16, 337–358. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, C.; Shi, B.B.; Chen, C.Y.; Yang, C.; Mao, G.F.; Shi, F.F. Extracorporeal Shock Wave Combined with Teriparatide-Loaded Hydrogel Injection Promotes Segmental Bone Defects Healing in Osteoporosis. Tissue Eng. Regen. Med. 2021, 18, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Yu, H.; Li, Z.; Ye, W.; Liu, Z.Q.; Gao, J.N.; Wang, Y.; Li, X.; Zhang, L.; Alenina, N.; et al. Oxidative RNA Damage in the Pathogenesis and Treatment of Type 2 Diabetes. Front. Physiol. 2022, 13, 725919. [Google Scholar] [CrossRef] [PubMed]
- Ouidir, M.; Zeng, X.H.; Chatterjee, S.; Zhang, C.L.; Tekola-Ayele, F. Ancestry-Specific Genetic Risk Scores of Type 2 Diabetes and Longitudinal Fetal Growth in a Race-Ethnic Diverse Population. Diabetes 2021, 70. [Google Scholar] [CrossRef]
- Udell, J.A.; Cavender, M.A.; Bhatt, D.L.; Chatterjee, S.; Farkouh, M.E.; Scirica, B.M. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015, 3, 356–366. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.N.; Chan, S.Y.; Du, Z.Y.; Yan, Y.J.; Wang, T.J.; Li, P.; Huang, W. Hydrogel-based flexible materials for diabetes diagnosis, treatment, and management. NPJ Flex. Electron. 2021, 5, 26. [Google Scholar] [CrossRef]
- Wang, J.Q.; Ye, Y.Q.; Yu, J.C.; Kahkoska, A.R.; Zhang, X.D.; Wang, C.; Sun, W.J.; Corder, R.D.; Chen, Z.W.; Khan, S.A.; et al. Core-Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, X.X.; Liu, X.Y.; Sun, B.; Li, L.; Zhao, Y.J. Responsive Hydrogel Microcarrier-Integrated Microneedles for Versatile and Controllable Drug Delivery. Adv. Healthc. Mater. 2021, 10, 2002249. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, Z.J.; Chen, G.J.; Wang, Y.F.; Ci, T.Y.; Li, H.J.; Liu, X.S.; Zhou, D.J.; Kahkoska, A.R.; Zhou, Z.X.; et al. Injectable Biodegradable Polymeric Complex for Glucose-Responsive Insulin Delivery. ACS Nano 2021, 15, 4294–4304. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.S.; Chen, S.Y.; Yi, J.; Zhang, H.F.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Hoshi, R.; Chen, S.Y.; Yi, J.; Duan, C.W.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J. Control. Release 2016, 238, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.K.; Xu, P.C.; Yao, Z.X.; Cui, X.; Lei, X.X.; Li, L.L.; Dong, Y.Q.; Zhu, W.D.; Guo, R.; Cheng, B. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021, 124, 205–218. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Chiu, A.; Flanders, J.A.; Song, W.; Shou, D.H.; Lu, Y.C.; Grunnet, L.G.; Winkel, L.; Ingvorsen, C.; Christophersen, N.S.; et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, E263–E272. [Google Scholar] [CrossRef]
- Sirc, J.; Hrib, J.; Vetrik, M.; Hobzova, R.; Zak, A.; Stankova, B.; Slanar, O.; Hromadka, R.; Sandrikova, V.; Michalek, J. The Use of a Hydrogel Matrix for Controlled Delivery of Niacin to the Gastrointestinal Tract for Treatment of Hyperlipidemia. Physiol. Res. 2015, 64, S51–S60. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Lee, Y.J.; Baek, S.; Chung, Y.S.; Kim, D.H.; Lee, J.H.; Shin, Y.C.; Shin, Y.M.; Ryu, C.; Kim, H.S.; et al. Hormone autocrination by vascularized hydrogel delivery of ovary spheroids to rescue ovarian dysfunctions. Sci. Adv. 2021, 7, eabe8873. [Google Scholar] [CrossRef]
- Hsiao, A.Y.; Okitsu, T.; Teramae, H.; Takeuchi, S. 3D Tissue Formation of Unilocular Adipocytes in Hydrogel Microfibers. Adv. Healthc. Mater. 2016, 5, 548–556. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Wang, L.; Zhou, Z.F.; Sun, Q.; Liu, D.L.; Chen, Y.; Hu, H.; Cai, Y.; Lin, S.X.; Yu, Z.R.; et al. Simultaneous incorporation of PTH(1-34) and nano-hydroxyapatite into Chitosan/Alginate Hydrogels for efficient bone regeneration. Bioact. Mater. 2021, 6, 1839–1851. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, Y.; Jin, Y.M.; Kim, G.; Jung, S.C.; Park, Y.J.; Park, K.D.; Jo, I. Sustained release of parathyroid hormone via in situ cross-linking gelatin hydrogels improves the therapeutic potential of tonsil-derived mesenchymal stem cells for hypoparathyroidism. J. Tissue Eng. Regen. Med. 2018, 12, E1747–E1756. [Google Scholar] [CrossRef]






| Type | Sub-Type | Key Features | Mechanism of Action | Specific Examples and References | Biomedical Applications |
|---|---|---|---|---|---|
| Degradation-reliant dynamic hydrogels | Hydrolytic | Maintain integrity, allow controlled degradation | Hydrolysis of covalent interactions, tunable degradation rate | HEMA-modified polysaccharide hydrogels [37], PEG-oriented hydrogels [38] | Drug delivery, controlled exposure of embedded cells |
| Enzymatic | Feature enzyme-cleavable peptide sequences | Remodeling via cell-mediated enzymatic degradation | MMP-sensitive HA hydrogels [39], enzyme-responsive protein structures [23] | Replicating natural tissue dynamics, supporting cell adhesion and proliferation | |
| Light responsive | Degrade upon specific light exposure | Light-induced breakdown, spatial/temporal control | Nitrobenzyl-crosslinked hydrogels [39,40] | Wound healing, localized therapeutic interventions | |
| Degradation-independent dynamic hydrogels | Reversible covalent | Reversible bonds under certain conditions | Schiff base, boronate, Diels–Alder reactions | Hydrazone-bonded HA hydrogels [70] | Injectable platforms, cell therapy |
| Supramolecular physical | Formed by non-covalent interactions | Metal–ligand coordination, host–guest complexation, hydrogen bonding | Metal–ligand coordinated hydrogels [72], guest–host interaction hydrogels [75] | Stimuli-responsive hydrogels, phase adaptability in response to environmental changes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Xu, J.; Yang, N.; Wang, H.; Li, J.; Zhang, M.; Zhu, M. Engineered Dynamic Hydrogel Niches for the Regulation of Redox Homeostasis in Osteoporosis and Degenerative Endocrine Diseases. Gels 2024, 10, 31. https://doi.org/10.3390/gels10010031
Yuan W, Xu J, Yang N, Wang H, Li J, Zhang M, Zhu M. Engineered Dynamic Hydrogel Niches for the Regulation of Redox Homeostasis in Osteoporosis and Degenerative Endocrine Diseases. Gels. 2024; 10(1):31. https://doi.org/10.3390/gels10010031
Chicago/Turabian StyleYuan, Weihao, Jiankun Xu, Na Yang, Han Wang, Jinteng Li, Mengyao Zhang, and Meiling Zhu. 2024. "Engineered Dynamic Hydrogel Niches for the Regulation of Redox Homeostasis in Osteoporosis and Degenerative Endocrine Diseases" Gels 10, no. 1: 31. https://doi.org/10.3390/gels10010031
APA StyleYuan, W., Xu, J., Yang, N., Wang, H., Li, J., Zhang, M., & Zhu, M. (2024). Engineered Dynamic Hydrogel Niches for the Regulation of Redox Homeostasis in Osteoporosis and Degenerative Endocrine Diseases. Gels, 10(1), 31. https://doi.org/10.3390/gels10010031








