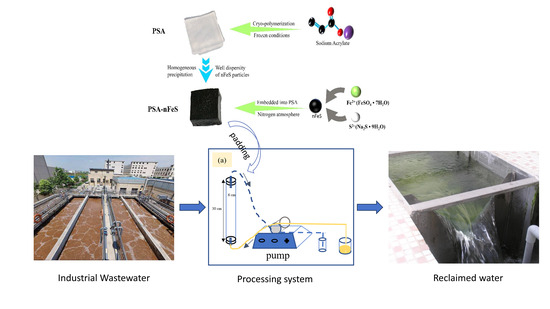
nFeS Embedded into Cryogels for High-Efficiency Removal of Cr(VI): From Mechanism to for Treatment of Industrial Wastewater
Abstract
1. Introduction
2. Results and Discussion
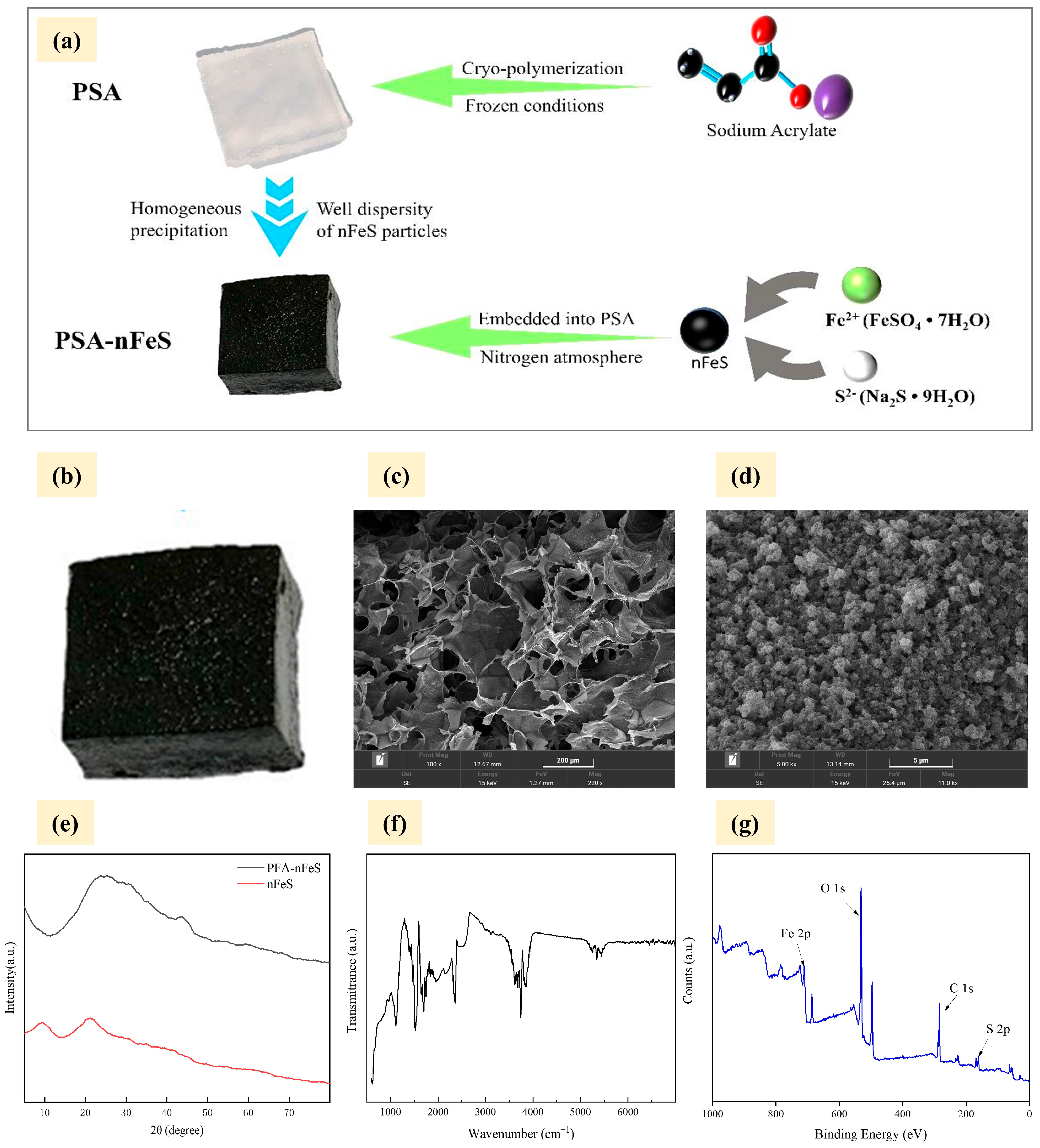
2.1. PSA-nFeS Composites Characterization
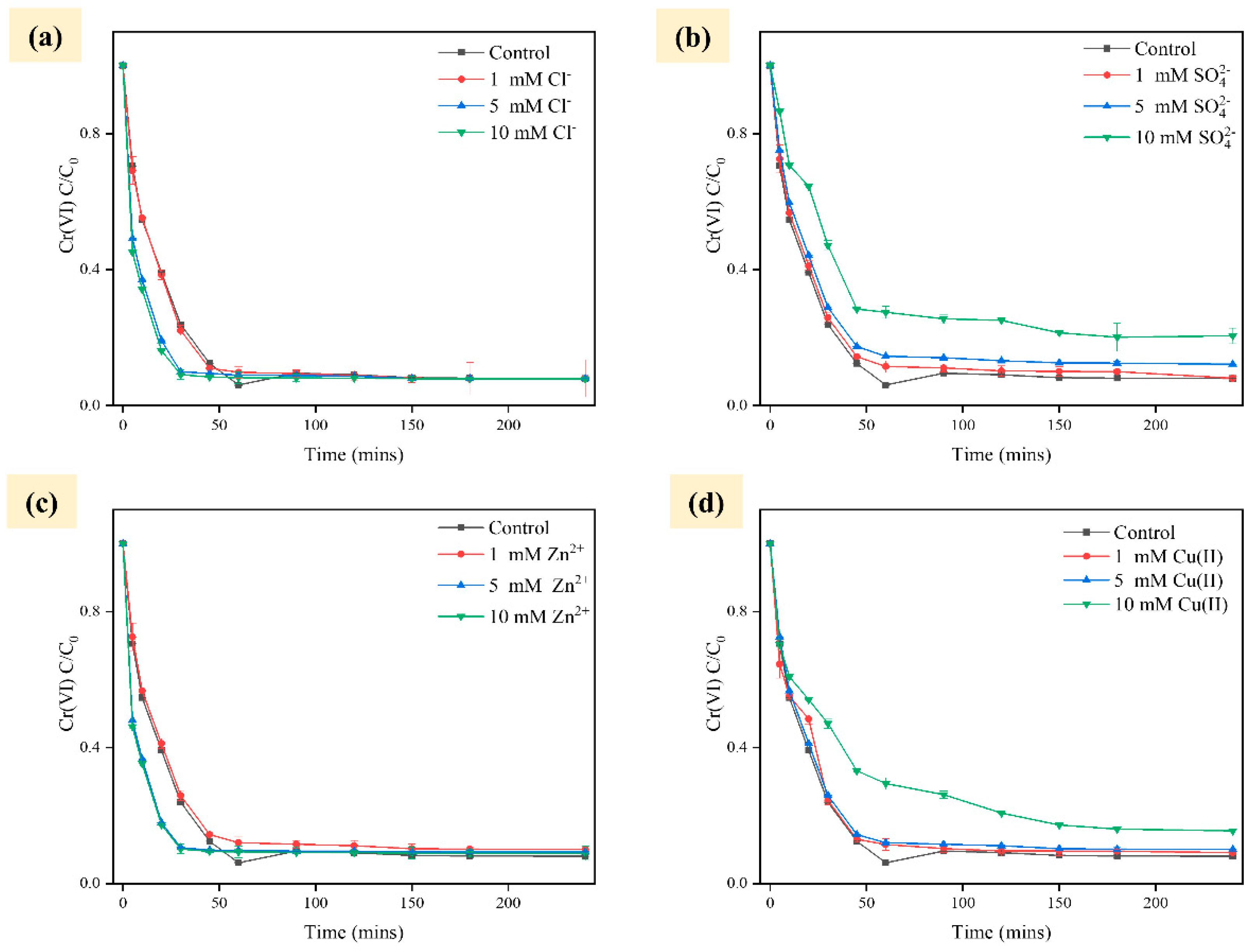
2.2. Effect of Environmental Factors on Cr Removal
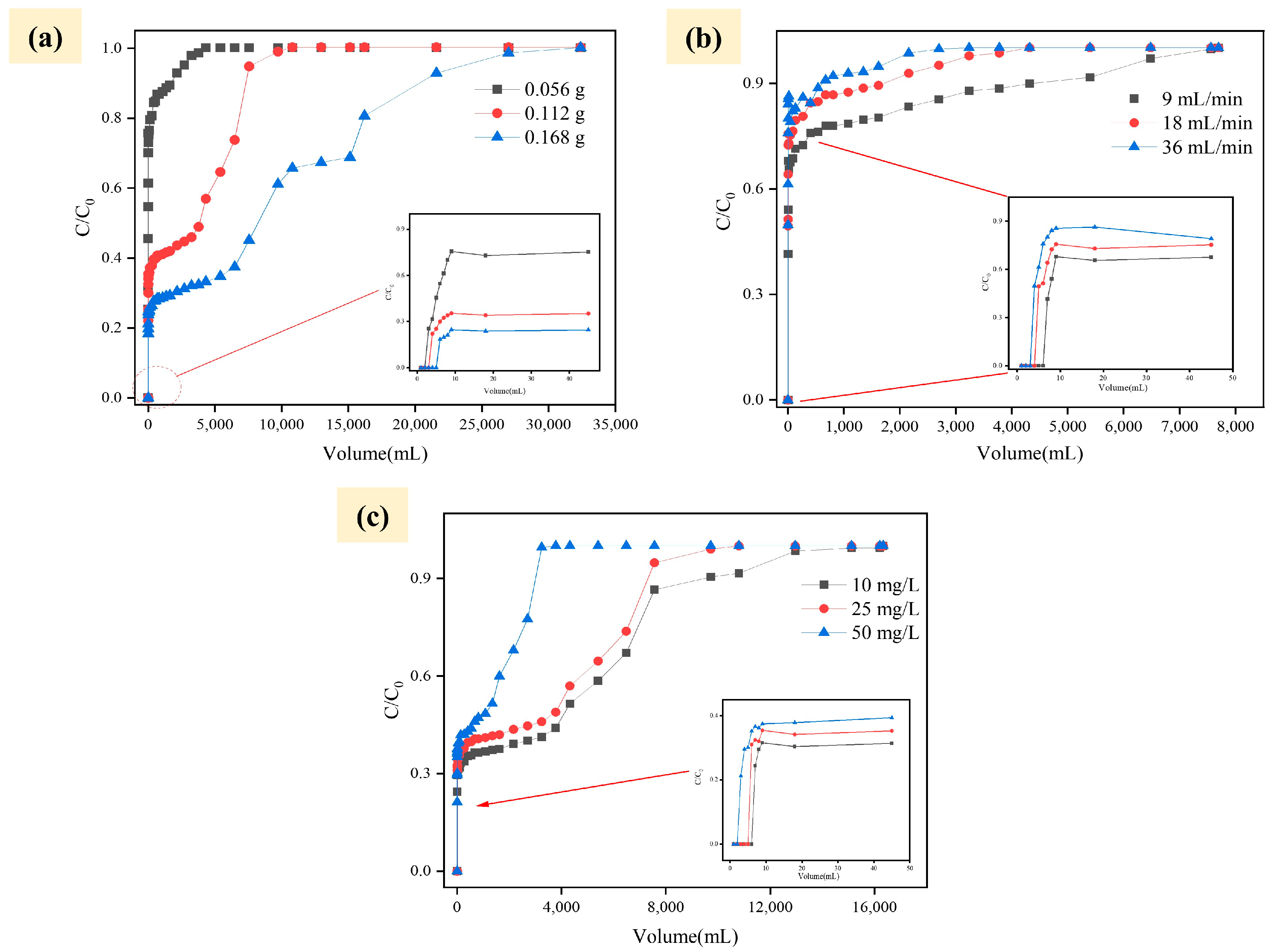
2.3. Columnar Experiment
2.4. Model Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of PSA-nFeS and Reactor
4.3. Environmental Factors
4.4. Column Experiments
4.5. Dynamic Experimental Model
4.6. Analysis Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Zhang, P.; Zeng, L.; Yu, L.; Li, D. Study on preparation of glass-ceramics from municipal solid waste incineration (MSWI) fly ash and chromium slag. J. Build. Eng. 2023, 68, 106080. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, C.; Srinivas, K.; Sekaran, G. Pyrolysis coupled pulse oxygen incineration for disposal of hazardous chromium impregnated fine particulate solid waste generated from leather industry. J. Environ. Chem. Eng. 2014, 2, 516–524. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean. Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Lv, D.; Zhou, J.S.; Cao, Z.; Xu, J.; Liu, Y.L.; Li, Y.Z.; Yang, K.L.; Zimo Lou, Z.M.; Lou, L.P.; Xu, X.H. Mechanism and influence factors of chromium(VI) removal by Sulfide-modified nanoscale zerovalent iron. Chemosphere 2019, 224, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Peng, X.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z. Liquid nitrogen activation of zero-valent iron and its enhanced Cr(VI) removal performance. Environ. Sci. Technol. 2019, 53, 8333–8341. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.K.; Han, Z.T.; Zhang, W.; Le Song, L.; Li, H. Synthesis of zeolite-supported microscale zero-valent iron for the removal of Cr6+ and Cd2+ from aqueous solution. J. Environ. Manag. 2016, 169, 84–90. [Google Scholar] [CrossRef]
- Ifthikar, J.; Shahib, I.I.; Jiang, W.; Senthilnithy, R.; Elkhlifi, Z.; Wang, J.; Zhuqi Chen, Z.Q. Review on technologies for the development of effective and practical chromate removal from wastewaters. J. Environ. Chem. Eng. 2023, 11, 110735. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Zhao, R.R.; Zhou, Z.M.; Zhao, X.D.; Jing, G.H. Enhanced Cr(VI) removal from simulated electroplating rinse wastewater by amino-functionalized vermiculite-supported nanoscale zero-valent iron. Chemosphere 2019, 218, 458–467. [Google Scholar] [CrossRef]
- Chen, Y.N.; Liang, W.Y.; Li, Y.P.; Wu, Y.X.; Chen, Y.R.; Xiao, W.; Zhao, L.; Zhang, J.C.; Li, H. Modification, application and reaction mechanisms of nano-sized iron sulfide particles for pollutant removal from soil and water: A review. Chem. Eng. J. 2019, 362, 144–159. [Google Scholar] [CrossRef]
- Zhuang, M.; Wang, H.; Qi, L.; Cui, L.Q.; Quan, G.X.; Yan, J.L. Production of activated biochar via a self-blowing strategy-supported sulfidated nanoscale zerovalent iron with enhanced reactivity and stability for Cr(VI) reduction. J. Clean. Prod. 2021, 315, 128108. [Google Scholar] [CrossRef]
- Cong, Y.Q.; Shen, L.D.; Wang, B.M.; Cao, J.L.; Pan, Z.X.; Wang, Z.Y.; Wang, K.; Li, Q.B.; Li, X.C. Efficient removal of Cr(VI) at alkaline pHs by sulfite/iodide/UV: Mechanism and modeling. Water Res. 2022, 222, 118919. [Google Scholar] [CrossRef]
- Gao, J.; Yang, L.Z.; Liu, Y.Y.; Shao, F.L.; Liao, Q.J.H.; Shang, J.G. Scavenging of Cr(VI) from aqueous solutions by sulfide modified nanoscale zero-valent iron supported by biochar. J. Taiwan Inst. Chem. Eng. 2018, 91, 449–456. [Google Scholar] [CrossRef]
- Jia, Z.Z.; Shu, Y.H.; Huang, R.L.; Liu, J.G.; Liu, L.L. Enhanced reactivity of nZVI embedded into supermacroporous cryogels for highly efficient Cr(VI) and total Cr removal from aqueous solution. Chemosphere 2018, 199, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Jiang, S.J.; Zhong, J.; Chen, X.K.; Shu, Y.H. Reactivity enhancement of ferrous sulfide by Poly-Sodium Acrylate cryogels on aqueous Cr(VI) removal: Performance and mechanism. J. Environ. Chem. Eng. 2022, 10, 108783. [Google Scholar] [CrossRef]
- Ifthikar, J.; Chen, Z.; Chen, Z.; Jawad, A. A self-gating proton-coupled electron transfer reduction of hexavalent chromium by core-shell SBA-Dithiocarbamate chitosan composite. J. Hazard. Mater. 2020, 384, 121257. [Google Scholar] [CrossRef]
- Ifthikar, J.; Ibran Shahib, I.; Jawad, A.; Gendy, E.A.; Wang, S.; Wu, B.; Chen, Z.; Chen, Z. The excursion covered for the elimination of chromate by exploring the coordination mechanisms between chromium species and various functional groups, Coord. Chem. Rev. 2021, 437, 213868. [Google Scholar] [CrossRef]
- Liu, W.; Jin, L.; Xu, J.; Liu, J.; Li, Y.; Zhou, P.; Wang, C.; Dahlgren, R.A.; Wang, X. Insight into pH dependent Cr(VI) removal with magnetic Fe3S4. Chem. Eng. J. 2019, 359, 564–571. [Google Scholar] [CrossRef]
- Luo, H.; Fu, F.F.L.; Tang, B. Ferrous sulfide supported on modified diatomite for the removal of Cr(VI): Performance and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131538. [Google Scholar] [CrossRef]
- Ajouyed, O.; Hurel, C.; Ammari, M.; Allal, L.B.; Marmier, N. Sorption of Cr(VI) onto natural iron and aluminum (oxy)hydroxides: Effects of pH, ionic strength and initial concentration. J. Hazard. Mater. 2010, 174, 616–622. [Google Scholar] [CrossRef]
- Loo, S.L.; Krantz, W.B.; Fane, A.G.; Gao, Y.B.; Lim, T.T.; Hu, X. Bactericidal Mechanisms revealed for rapid water disinfection by super absorbent cryogels decorated with silver nanoparticles. Environ. Sci. Technol. 2015, 49, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, L.P.; Gao, L.Z. Nano-Sized Iron Sulfifide: Structure, Synthesis, Properties, and Biomedical Applications. Front. Chem. 2020, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.L.; Fane, A.G.; Lim, T.T.; Krantz, W.B.; Liang, Y.N.; Liu, X.; Hu, X. Super absorbent cryogels decorated with silver nanoparticles as a novel water technology for point-of-use disinfection. Environ. Sci. Technol. 2013, 47, 9363–9371. [Google Scholar] [CrossRef]
- Wang, W.H.; Hu, B.B.; Wang, C.; Liang, Z.J.; Cui, F.Y.; Zhiwei Zhao, Z.W.; Yang, C. Cr(VI) removal by micron-scale iron-carbon composite induced by ball milling: The role of activated carbon. Chem. Eng. J. 2020, 389, 122633. [Google Scholar] [CrossRef]
- Sapsford, D.; Barnes, A.; Dey, M.; Williams, K.; Jarvis, A.; Younger, P.; Liang, L. Iron and manganese removal in a vertical flow reactor for passive treatment of mine water. In Proceedings of the 7th International Conference on Acid Rock Drainage (ICARD), St. Louis, MO, USA, 26–30 March 2006. [Google Scholar] [CrossRef]
- Wang, W.H.; Gao, P.; Yang, C.; Zhao, Z.W.; Zhen, S.C.; Zhou, Y.X.; Zhang, T.T. Separable and reactivated magnetic mZVAl/nFe3O4 composite induced by ball milling for efficient adsorption-reduction-sequestration of aqueous Cr(VI). Sep. Purif. Technol. 2022, 288, 120689. [Google Scholar] [CrossRef]
- Lv, X.; Qin, X.; Wang, K.; Peng, Y.; Wang, P.; Jiang, G. Nanoscale zero valent iron supported on MgAl-LDH-decorated reduced graphene oxide: Enhanced performance in Cr(VI) removal, mechanism and regeneration. J. Hazard. Mater. 2019, 373, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Varadharajan, C.; Belle, H.R.; Bill, M.; Brodie, E.L.; Conrad, M.E.; Han, R.Y.; Irwin, C.; Larsen, J.T.; Lim, H.C.; Molins, S.; et al. Reoxidation of Chromium(III) products formed under different biogeochemical regimes. Environ. Sci. Technol. 2017, 51, 4918–4927. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, K.; Min, D.W.; Choi, W.Y. Freezing-Enhanced Dissolution of Iron Oxides: Effects of Inorganic Acid Anions. Environ. Sci. Technol. 2015, 49, 12816–12822. [Google Scholar] [CrossRef]
- Lv, J.F.; Tong, X.; Zheng, Y.X.; Xie, X.; Huang, L.Y. Reduction of Cr(VI) with a relative high concentration using different kinds of zero-valent iron powders: Focusing on effect of carbon content and structure on reducibility. J. Cent. South Univ. 2018, 25, 2119–2130. [Google Scholar] [CrossRef]
- Maleh, H.K.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Li Fu, L.; Sillanpää, M. Recent advances in removal techniques of Cr(VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Z.; Wang, B.; Shen, J.; Zhang, J.; Li, D. Cr(VI) removal using different reducing agents combined with fly ash leachate: A comparative study of their efficiency and potential mechanisms. Chemosphere 2018, 213, 172–181. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.P.; Yin, H.L.; Jin, S.; Liu, F.; Chen, H.H. Mechanism Study of humic acid functional groups for Cr(VI) retention: Two-dimensional FTIR and C-13 CP/MAS NMR correlation spectroscopic analysis. Environ. Pollut. 2017, 225, 86–92. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, J.; Liang, S.; Chang, S.W.; Nguyen, D.D.; Liu, Y. Application of a breakthrough biosorbent for removing heavy metals from synthetic and real wastewaters in a lab-scale continuous fixed-bed column. Bioresour. Technol. 2017, 229, 78–87. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Batch and continuous flow studies of Cr(VI) adsorption from synthetic and real wastewater by magnetic pine cone composite. Chem. Eng. Res. Des. 2020, 153, 806–818. [Google Scholar] [CrossRef]
- Gheju, M.; Iovi, A. Kinetics of hexavalent chromium reduction by scrap iron. J. Hazard. Mater. 2006, 135, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Melitas, N.; Chuffe-Moscoso, O.; Farrell, J. Kinetics of soluble chromium removal from contaminated water by zerovalent iron media: Corrosion inhibition and passive oxide effects. Environ. Sci. Technol. 2001, 35, 3948–3953. [Google Scholar] [CrossRef]
- Lyu, S.L.; Liu, T.; Wang, X.; Zuo, K.X.; Xie, Y.H. Removal and mechanism study of Cr(VI) in water by sludge biochar-supported nano-ferrous sulfide. China Environ. Sci. 2023, 43, 3935–3945. [Google Scholar]
- Lin, C.L.; Zhong, L.Y.; Zhong, X.L.; Wei, B.Y.; Yin, J.Y. Adsorption-reduction reaction between bagasse-prepared biochar and Cr(VI). J. Agro-Environ. Sci. 2023, 42, 2335–2345. [Google Scholar]
- Liu, S.Y.; Han, J.C.; Ma, D.Q.; Liu, H.X.; Fang, M.; Tan, X.L. MXene@MOF for synergetic removal of Cr(VI) by adsorption and reduction. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132438. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, Y.N.; Guo, J.Z.; Chen, L.; Li, B. Enhanced removal of Cr(VI) by polyethyleneimine-modified bamboo hydrochar. Environ. Sci. Pollut. Res. 2023, 30, 94185–94194. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.F.; Zhang, Y.X.; Liu, J.Q.; Zhang, Y.Q.; Yang, S.Y. Ethanol-assisted mechanical activation of zero-valent aluminum for fast and highly efficient removal of Cr(VI). Appl. Surf. Sci. 2020, 533, 147543. [Google Scholar] [CrossRef]
- Chen, H.X.; Dou, J.F.; Xu, H.B. Removal of Cr(VI) ions by sewage sludge compost biomass from aqueous solutions: Reduction to Cr(III) and biosorption. Appl. Surf. Sci. 2017, 425, 728–735. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Fu, H.F.; Yi, X.H.; Wang, P.; Zhao, C.; Wang, C.C.; Zheng, W.W. Simultaneous Cr(VI) reduction and Cr(III) removal of bifunctional MOF/Titanate nanotube composites. Environ. Pollut. 2019, 249, 502–511. [Google Scholar] [CrossRef]


| Materials | Time (s) | References |
|---|---|---|
| PSA-Ag | 15 | [24] |
| PSA-AgNP | 10 | [22] |
| PSA-nFeS | 9 | This study |
| Co/ (mg/L) | Filling Doses/ (g nFeS) | Q/ (L/h) | Thomas Model | Yoon–Nelson Model | |||
|---|---|---|---|---|---|---|---|
| KT/(L/h·mg) | q0/(mg/g) | R2 | q0/(mg/g) | R2 | |||
| 25 | 0.056 | 0.54 | 0.4884 | 256.5410 | 0.9137 | 233.7130 | 0.9381 |
| 25 | 0.056 | 1.08 | 0.5357 | 214.7280 | 0.9915 | 214.4920 | 0.9941 |
| 25 | 0.056 | 1.62 | 1.3206 | 206.0150 | 0.9318 | 195.7140 | 0.9567 |
| 25 | 0.056 | 1.08 | 0.5357 | 214.7280 | 0.9915 | 214.4920 | 0.9941 |
| 25 | 0.112 | 1.08 | 0.1921 | 230.2450 | 0.9508 | 218.7330 | 0.9762 |
| 25 | 0.168 | 1.08 | 0.1285 | 241.0290 | 0.9606 | 228.9770 | 0.9862 |
| 10 | 0.112 | 1.08 | 0.1923 | 235.7700 | 0.9529 | 228.6160 | 0.9783 |
| 25 | 0.112 | 1.08 | 0.1921 | 230.2450 | 0.9508 | 218.8690 | 0.9762 |
| 50 | 0.112 | 1.08 | 0.2763 | 170.3140 | 0.9412 | 161.7990 | 0.9456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Jiang, S. nFeS Embedded into Cryogels for High-Efficiency Removal of Cr(VI): From Mechanism to for Treatment of Industrial Wastewater. Gels 2024, 10, 56. https://doi.org/10.3390/gels10010056
Xu P, Jiang S. nFeS Embedded into Cryogels for High-Efficiency Removal of Cr(VI): From Mechanism to for Treatment of Industrial Wastewater. Gels. 2024; 10(1):56. https://doi.org/10.3390/gels10010056
Chicago/Turabian StyleXu, Peng, and Shaojun Jiang. 2024. "nFeS Embedded into Cryogels for High-Efficiency Removal of Cr(VI): From Mechanism to for Treatment of Industrial Wastewater" Gels 10, no. 1: 56. https://doi.org/10.3390/gels10010056
APA StyleXu, P., & Jiang, S. (2024). nFeS Embedded into Cryogels for High-Efficiency Removal of Cr(VI): From Mechanism to for Treatment of Industrial Wastewater. Gels, 10(1), 56. https://doi.org/10.3390/gels10010056