Development, Optimization, and Evaluation of New Gel Formulations with Cyclodextrin Complexes and Volatile Oils with Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis
2.2. Gels Characteristics
2.3. Rheological Data
2.4. Scanning Electron Microscopy
2.5. Antimicrobial Activity
2.5.1. Qualitative Assessment of the Antimicrobial Activity
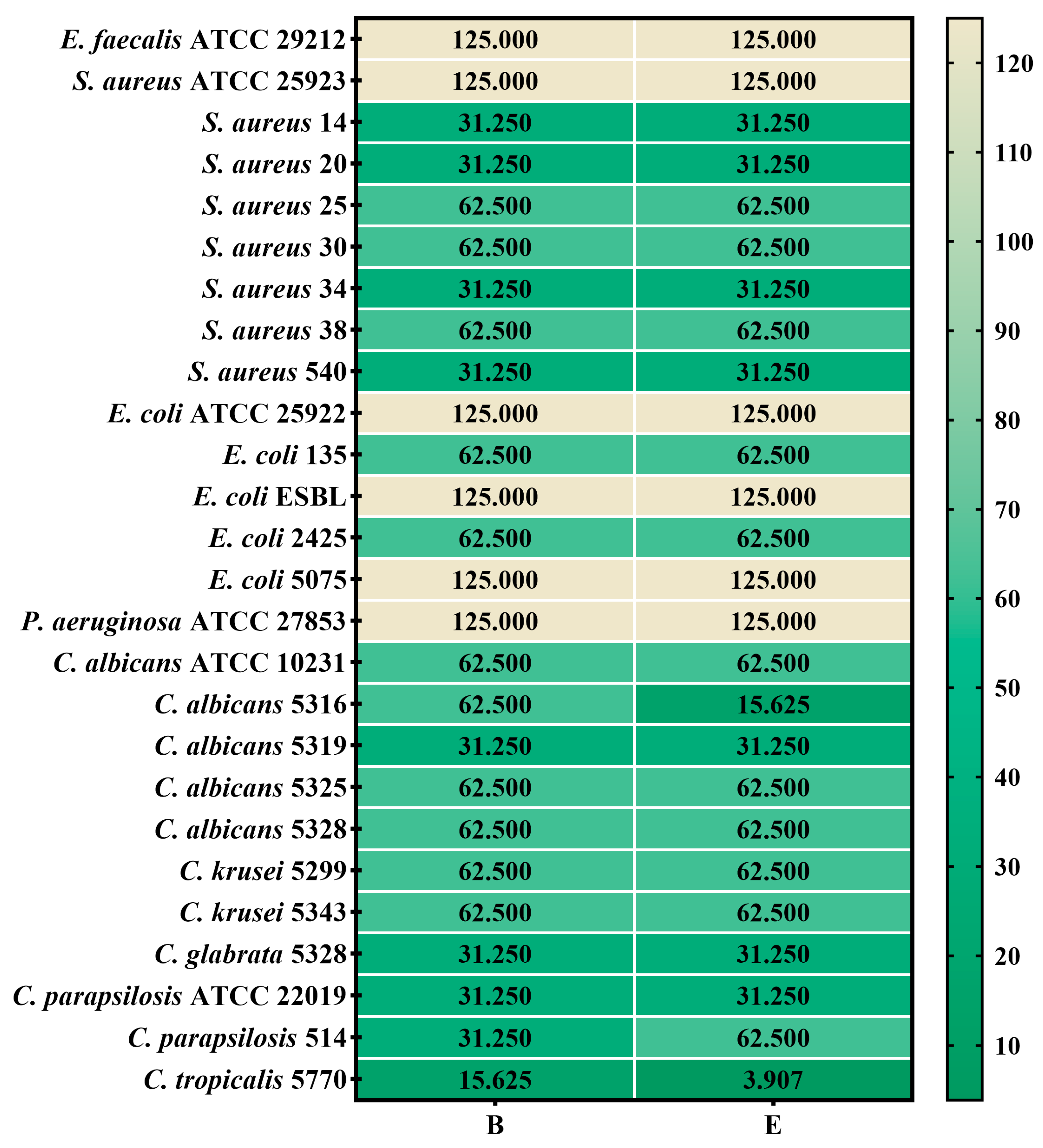
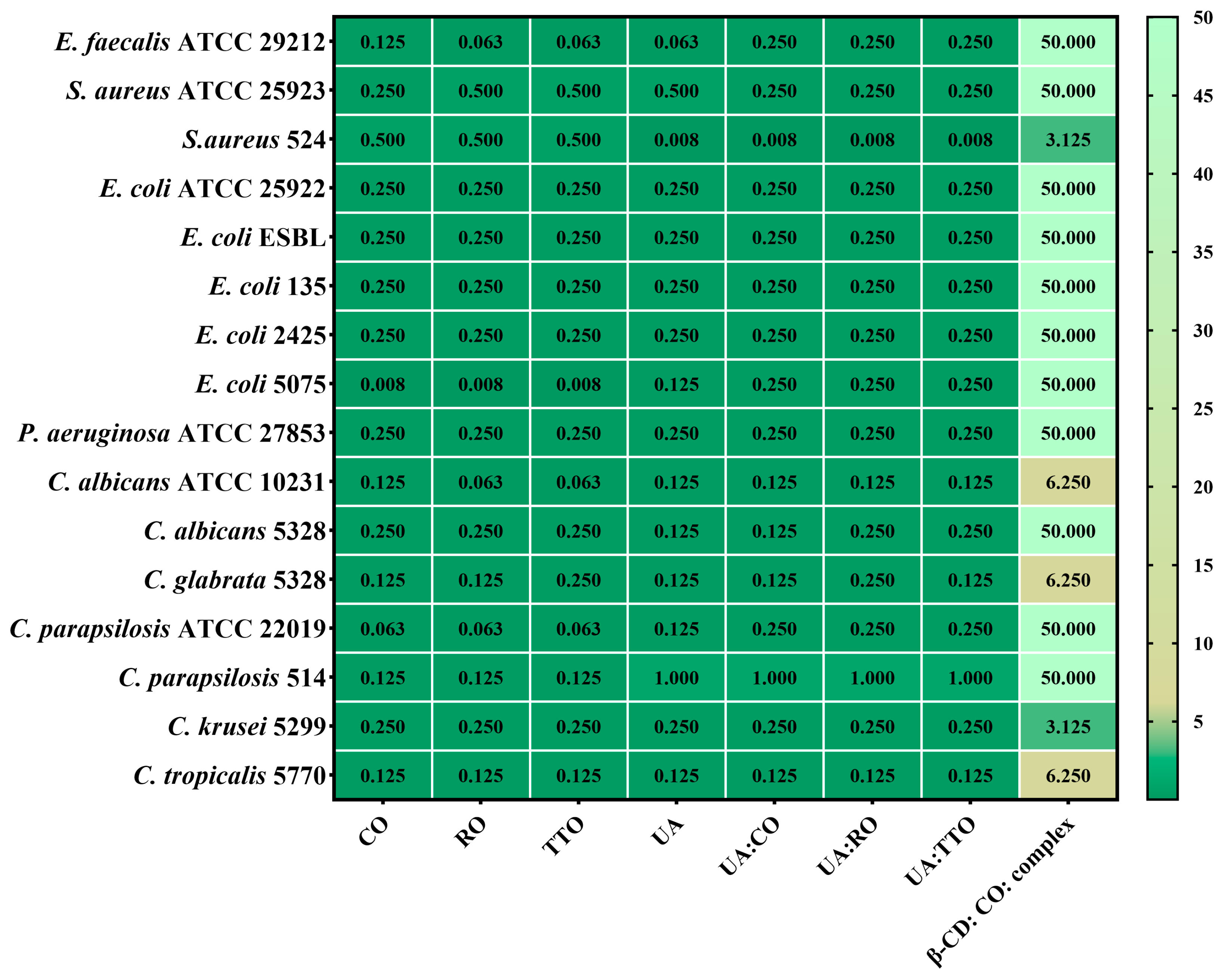
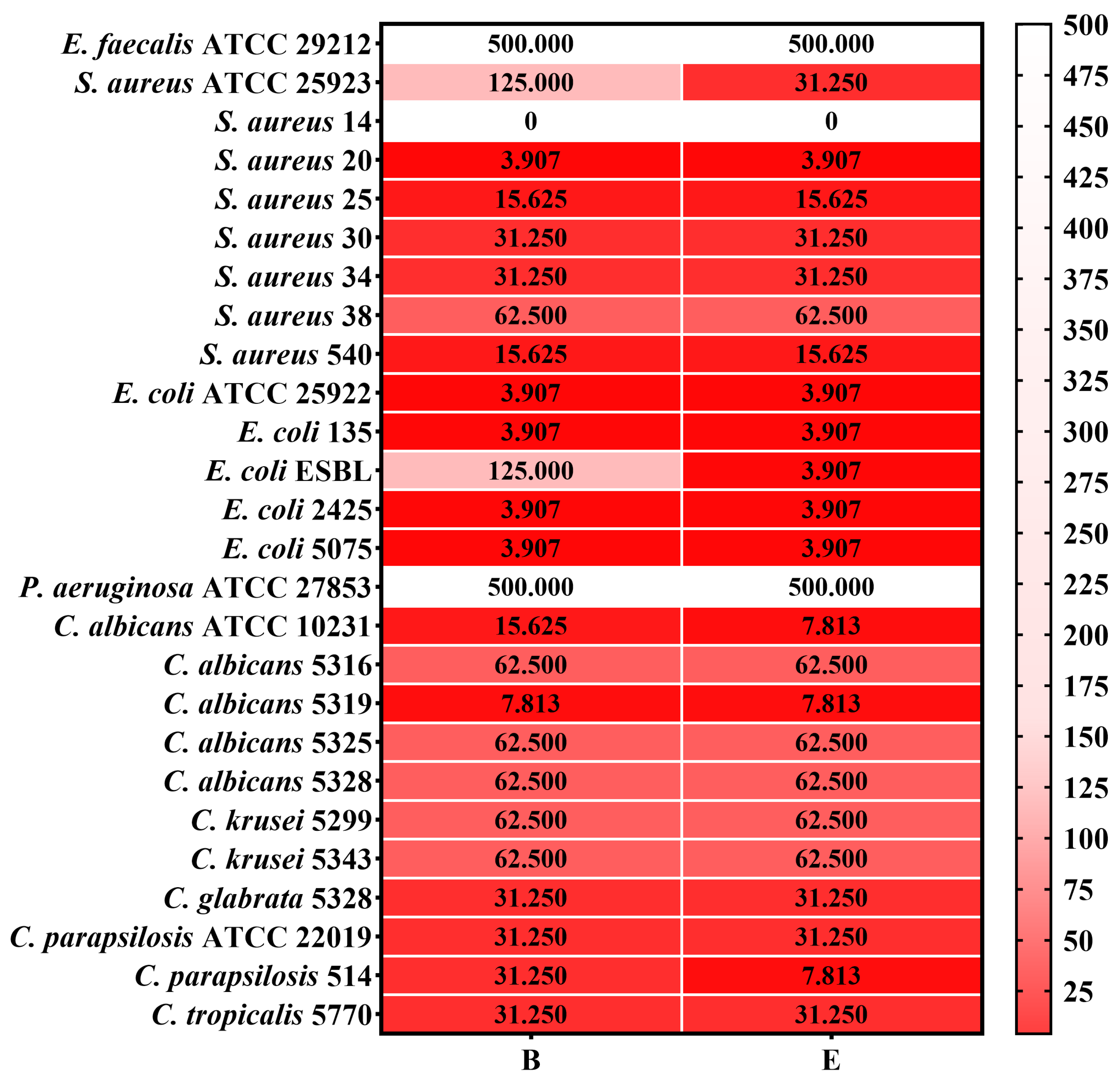
2.5.2. Quantitative Assessment of the Antimicrobial Activity
2.5.3. Semiquantitative Assessment of the Microbial Adherence to the Inert Substratum
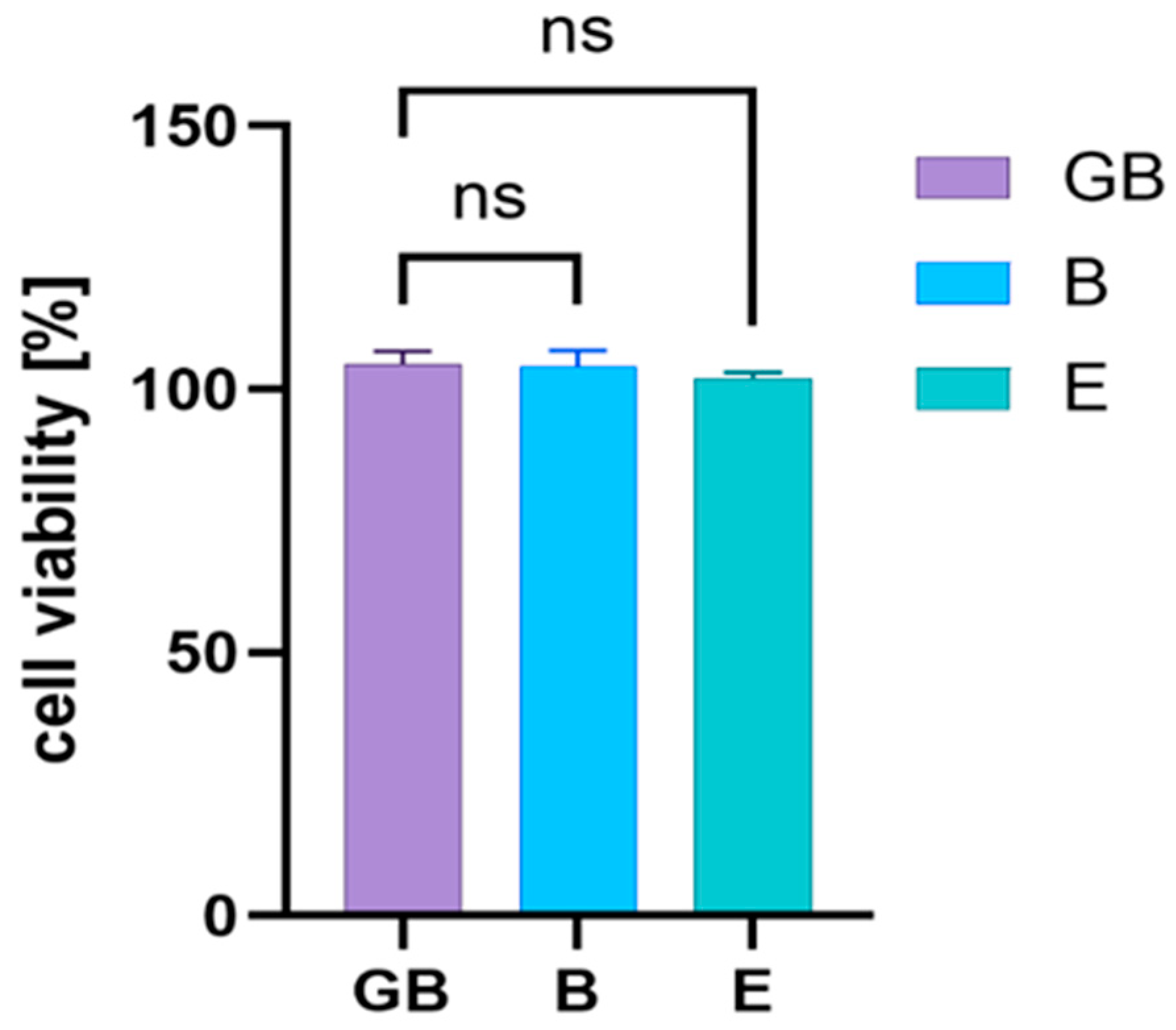
2.6. Cytotoxic Activity
3. Conclusions
4. Materials and Methods
4.1. GC-MS Analysis
4.2. Formulation of Hydrogels
- The determination of the appearance was made by examination with the magnifying glass (4.5×) of a sample stretched by a thin layer on a microscopic blade;
- The determination of the main characteristics was made according to Romanian Pharmacopoeia Xth edition [74];
- pH was determined after the gel samples were suitably processed, respectively after the dispersion of samples in water (1:5), followed by the measuring of the pH of an aqueous phase, as follows: 25 mL of distilled water were added to 5 g sample and the mixture was stirred into an Erlenmeyer glass with cork heating on the water bath at 60 °C for 10 min. After cooling on the watery phase, the pH was determined with Radelkis pH meter (Radelkis Ktsz.; Budapest; Hungary);
- The determination of the viscosity was performed with a rotational Brookfield LFV viscometer (AMETEK Brookfield, Middleboro, MA, USA);
- The stability was performed by maintaining the samples at 2 °C and 40 °C: 5 g of sample were introduced into a weighing ampoule with a lid, and then, the ampoule was maintained for 8 h at mentioned temperatures. Then, the appearance of the sample was examined.
4.3. Rheological Analysis and Data Modeling
4.4. SEM Analysis
4.5. Antimicrobial Activity
4.5.1. Qualitative Assay of the Antimicrobial Activity
4.5.2. Quantitative Assay of the Antimicrobial Activity
4.5.3. Semiquantitative Assessment of the Microbial Adherence to the Inert Substratum
4.6. Cytotoxic Activity
4.7. β-cyclodextrin-Eugenia caryophyllata Volatile Oil Complex Preparation
4.8. β-cyclodextrin-Eugenia caryophyllata Volatile Oil Complex Characteristics
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slater, J. The Effects of Co-Solvent Levels and Neutralisation on the Rheology of Carbopol Gels. In Third European Rheology Conference and Golden Jubilee Meeting of the British Society of Rheology; Oliver, D.R., Ed.; Springer: Dordrecht, The Netherlands, 1990; pp. 453–455. [Google Scholar]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological Characterization of Topical Carbomer Gels Neutralized to Different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Song, J.-Y.; Lee, E.-J.; Park, S.-K. Rheological properties and microstructures of Carbopol gel network system. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Laxton, P.B.; Berg, J.C. Gel trapping of dense colloids. J. Colloid Interface Sci. 2005, 285, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Oppong, F.K.; Rubatat, L.; Frisken, B.J.; Bailey, A.E.; de Bruyn, J.R. Microrheology and structure of a yield-stress polymer gel. Phys. Rev. E 2006, 73, 041405. [Google Scholar] [CrossRef] [PubMed]
- Piau, J.M. Carbopol gels: Elastoviscoplastic and slippery glasses made of individual swollen sponges. J. Non-Newton. Fluid Mech. 2007, 144, 1–29. [Google Scholar] [CrossRef]
- Garg, A.; Gupta, B.; Prakash, R.; Singh, S. Preparation and Characterization of Hydroxypropyl-b-Cyclodextrin Inclusion Complex of Eugenol: Differential Pulse Voltammetry and 1H-NMR. Chem. Pharm. Bull. 2010, 58, 1313–1319. [Google Scholar] [CrossRef]
- Garg, A.; Ahmad, J.; Hassan, M.Z. Inclusion complex of thymol and hydroxypropyl-β-cyclodextrin (HP-β-CD) in polymeric hydrogel for topical application: Physicochemical characterization, molecular docking, and stability evaluation. J. Drug Deliv. Sci. Technol. 2021, 64, 102609. [Google Scholar] [CrossRef]
- Bilensoy, E.; Rouf, M.A.; Vural, I.; Hincal, A.A. Thermosensitive vaginal gel formulation for the controlled release of clotrimazole via complexation to beta-cyclodextrin. J. Control Release 2006, 116, e107–e109. [Google Scholar] [CrossRef]
- Joita, F.A.; Oprea, E.; Musuc, A.M.; Mititelu, M.; Marinescu, F.; Lupuliasa, D.; Hincu, L.; Rosca, A.C.; Boroghina, S.C.; Popescu, I.A. Development of antimicrobial hydrogels using alginate-chitosan matrix enhanced with essential oils. Farmacia 2024, 72, 906–916. [Google Scholar]
- Jain, M.; Nowak, B.P.; Ravoo, B.J. Supramolecular Hydrogels Based on Cyclodextrins: Progress and Perspectives. Chemnanomat 2022, 8, e202200077. [Google Scholar] [CrossRef]
- Bendi, A.; Jafar Ahamed, A.; Jaison Jose, T.; Raghav, N.; Mujafarkani, N.; Atri, S. Synthesis, Characterization, and biological evaluation of a novel PTF terpolymer and its copper Polychelate: Antibacterial and antifungal efficacy. J. Mol. Liq. 2024, 408, 125403. [Google Scholar] [CrossRef]
- Carvalho-Silva, J.M.; Gaspar, C.S.; Dos Reis, A.C.; Teixeira, A.B.V. Denture stomatitis: Treatment with antimicrobial drugs or antifungal gels? A systematic review of clinical trials. J. Prosthet. Dent. 2024; in press. [Google Scholar]
- Aher, S.D.; Banerjee, S.K.; Gadhave, M.V.; Gaikwad, D.D. Emulgels—A New Dosage Form for Topical Drug Delivery. Int. J. Inst. Pharm. Life Sci. 2013, 3, 1–10. [Google Scholar]
- Preeti, B.; Gnanaranjan, G. Emulgel—A Novel Formulation Approach for Topical Delivery of Hydrophobic Drugs. Int. Res. J. Pharm. 2013, 4, 12–16. [Google Scholar]
- Baibhav, J.; Gurpreet, S.; Seema, S.; Vikas, S. Emulgel: A comprehensive review on the recent advances in topical drug delivery. Int. Res. J. Pharm. 2011, 2, 66–70. [Google Scholar]
- Kumar, D.; Singh, J.; Antil, M.; Kumar, V. Emulgel-Novel Topical Drug Delivery System—A comprehensive review. Int. J. Pharm. Sci. Res. 2016, 7, 4733–4742. [Google Scholar]
- Elmi, A.; Ventrella, D.; Barone, F.; Carnevali, G.; Filippini, G.; Pisi, A.; Benvenuti, S.; Scozzoli, M.; Bacci, M.L. In Vitro Effects of Tea Tree Oil (Melaleuca alternifolia Essential Oil) and its Principal Component Terpinen-4-ol on Swine Spermatozoa. Molecules 2019, 24, 1071. [Google Scholar] [CrossRef]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef]
- Hudaib, M.M.; Tawaha, K.A.; Hudaib, H.S.; Battah, A.H. Chemical Composition of Volatile Oil from the Aerial Parts of Rosmarinus officinalis L. Grown in Jordan. J. Essent. Oil Bear. Plants 2015, 18, 1282–1286. [Google Scholar] [CrossRef]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; De Souza Mendes, P. Rheological Characterization of Carbopol® Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Zheng, Y.; Ouyang, W.Q.; Wei, Y.P.; Syed, S.F.; Hao, C.S.; Wang, B.Z.; Shang, Y.H. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int. J. Nanomed. 2016, 11, 5971–5987. [Google Scholar] [CrossRef]
- Bassole, I.H.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Peter, S.; Aderibigbe, B.A. Polymer-Based Hydrogels Enriched with Essential Oils: A Promising Approach for the Treatment of Infected Wounds. Polymers 2022, 14, 3772. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Puvaca, N.; Milenkovic, J.; Galonja Coghill, T.; Bursic, V.; Petrovic, A.; Tanaskovic, S.; Pelic, M.; Ljubojevic Pelic, D.; Miljkovic, T. Antimicrobial Activity of Selected Essential Oils against Selected Pathogenic Bacteria: In Vitro Study. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef]
- Montenegro, L.; Pasquinucci, L.; Zappala, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol. 2006, 44, 616–625. [Google Scholar] [CrossRef]
- Castro, J.I.; Valencia-Llano, C.H.; Valencia Zapata, M.E.; Restrepo, Y.J.; Mina Hernandez, J.H.; Navia-Porras, D.P.; Valencia, Y.; Valencia, C.; Grande-Tovar, C.D. Chitosan/Polyvinyl Alcohol/Tea Tree Essential Oil Composite Films for Biomedical Applications. Polymers 2021, 13, 3753. [Google Scholar] [CrossRef]
- Anadon, A.; Martinez-Larranaga, M.R.; Martinez, M.A.; Ares, I.; Garcia-Risco, M.R.; Senorans, F.J.; Reglero, G. Acute oral safety study of rosemary extracts in rats. J. Food Prot. 2008, 71, 790–795. [Google Scholar] [CrossRef]
- Shalaby, S.E.M.; El-Din, M.M.; Mettwally, M.; Abo-Donia, S.A.; Attia, Z.A. Toxicological Affects of Essential Oils from Eucalyptus Eucalyptus globules and Clove Eugenia caryophyllus on Albino Rats. Pol. J. Environ. Stud. 2011, 20, 429–434. [Google Scholar]
- Saffron, C.M.; Park, J.-H.; Dale, B.E.; Voice, T.C. Kinetics of Contaminant Desorption from Soil: Comparison of Model Formulations Using the Akaike Information Criterion. Environ. Sci. Technol. 2006, 40, 7662–7667. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.I.; Villegas, M.; Cid, A.G.; Parentis, M.L.; Gonzo, E.E.; Bermudez, J.M. Validation of kinetic modeling of progesterone release from polymeric membranes. Asian J. Pharm. Sci. 2018, 13, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 4, 1–25. [Google Scholar]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Solid- and Vapor-Phase Antimicrobial Activities of Six Essential Oils: Susceptibility of Selected Foodborne Bacterial and Fungal Strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef]
- Tornuk, F.; Cankurt, H.; Ozturk, I.; Sagdic, O.; Bayram, O.; Yetim, H. Efficacy of various plant hydrosols as natural food sanitizers in reducing Escherichia coli O157:H7 and Salmonella typhimurium on fresh cut carrots and apples. Int. J. Food Microbiol. 2011, 148, 30–35. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Lysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules 2013, 18, 9334–9351. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Fabio, A.; Cermelli, C.; Fabio, G.; Nicoletti, P.; Quaglio, P. Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. Phytother. Res. 2007, 21, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.C.; Lima, J.A.d.; Silva, C.R.d.; Benvegnú, D.; Ferreira, J.; Burger, M.E.; Beck, R.C.R.; Rolim, C.M.B.; Rocha, M.I.U.M.; da Veiga, M.L.; et al. Hydrogels Containing Nanocapsules and Nanoemulsions of Tea Tree Oil Provide Antiedematogenic Effect and Improved Skin Wound Healing. J. Nanosci. Nanotechnol. 2015, 15, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and Characterization of PVA/Starch Hydrogel Membranes Incorporating Essential Oils Aimed to be Used in Wound Dressing Applications. J. Polym. Environ. 2020, 29, 156–174. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Junior, F.; Medeiros, D.; Nobrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-ol against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef]
- Shahina, Z.; Al Homsi, R.; Price, J.D.W.; Whiteway, M.; Sultana, T.; Dahms, T.E.S. Rosemary essential oil and its components 1,8-cineole and alpha-pinene induce ROS-dependent lethality and ROS-independent virulence inhibition in Candida albicans. PLoS ONE 2022, 17, e0277097. [Google Scholar] [CrossRef]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef]
- Farhanghi, A.; Aliakbarlu, J.; Tajik, H.; Mortazavi, N.; Manafi, L.; Jalilzadeh-Amin, G. Antibacterial interactions of pulegone and 1,8-cineole with monolaurin ornisin against Staphylococcus aureus. Food Sci. Nutr. 2022, 10, 2659–2666. [Google Scholar] [CrossRef]
- Kifer, D.; Muzinic, V.; Klaric, M.S. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Akhmouch, A.A.; Hriouech, S.; Mzabi, A.; Tanghort, M.; Chefchaou, H.; Remmal, A.; Chami, N. Synergistic Action of AMX Associated with 1,8-Cineole and Its Effect on the ESBL Enzymatic Resistance Mechanism. Antibiotics 2022, 11, 1002. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobis, O.; Lassoued, M.A. 1,8-Cineol (Eucalyptol) Disrupts Membrane Integrity and Induces Oxidative Stress in Methicillin-Resistant Staphylococcus aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Bashiri Dezfouli, A.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Khan, L.A.; Manzoor, N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J. Med. Microbiol. 2010, 59, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Elo, H.; Matikainen, J.; Pelttari, E. Potent activity of the lichen antibiotic (+)-usnic acid against clinical isolates of vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. Naturwissenschaften 2007, 94, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Maciag-Dorszynska, M.; Wegrzyn, G.; Guzow-Krzeminska, B. Antibacterial activity of lichen secondary metabolite usnic acid is primarily caused by inhibition of RNA and DNA synthesis. Fems Microbiol. Lett. 2014, 353, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.H.; Lucarini, R.; Mendes-Giannini, M.J. Effect of usnic acid on Candida orthopsilosis and C. parapsilosis. Antimicrob. Agents Chemother. 2012, 56, 595–597. [Google Scholar] [CrossRef]
- Nithyanand, P.; Beema Shafreen, R.M.; Muthamil, S.; Karutha Pandian, S. Usnic acid inhibits biofilm formation and virulent morphological traits of Candida albicans. Microbiol. Res. 2015, 179, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kiti, K.; Suwantong, O. Bilayer wound dressing based on sodium alginate incorporated with curcumin-beta-cyclodextrin inclusion complex/chitosan hydrogel. Int. J. Biol. Macromol. 2020, 164, 4113–4124. [Google Scholar] [CrossRef]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Pante, G.C.; Castro, J.C.; Lini, R.S.; Romoli, J.C.Z.; Pires, T.Y.; Garcia, F.P.; Nakamura, C.V.; Mulati, A.C.N.; Matioli, G.; Machinski Junior, M. Inclusion Complexes of Litsea cubeba (Lour.) Pers Essential Oil into beta-Cyclodextrin: Preparation, Physicochemical Characterization, Cytotoxicity and Antifungal Activity. Molecules 2024, 29, 1626. [Google Scholar] [CrossRef]
- Atyim, P.; Olah, N.K.; Osser, G.; Toma, C.C.; Morgovan, C.; Atyim, E. Practical Investigation of Gels Containing Aristolochia (Aristolochia Clematitis) Extract. Stud. Univ. Babes-Bolyai Chem. 2017, 62, 153–164. [Google Scholar] [CrossRef]
- Romanian Farmacopeia, Xth ed.; Editura Medicala: Bucharest, Romania, 1998; p. 1316.
- Ghica, M.V.; Albu, M.G.; Dinu-Pirvu, C.; Moisescu, S. In vitro Kinetic Release and Flow Behaviour of Some Collagen-Minocycline Topical Hydrogels. Rev. Chim. 2012, 63, 929–935. [Google Scholar]
- Ghica, M.V.; Hirjau, M.; Lupuleasa, D.; Dinu-Pirvu, C.E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef] [PubMed]
- Păunica-Panea, G.; Ficai, A.; Marin, M.M.; Marin, Ș.; Albu, M.G.; Constantin, V.D.; Dinu-Pîrvu, C.; Vuluga, Z.; Corobea, M.C.; Ghica, M.V. New Collagen-Dextran-Zinc Oxide Composites for Wound Dressing. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Puri, A.; Nguyen, H.X.; Banga, A.K. Microneedle-mediated intradermal delivery of epigallocatechin-3-gallate. Int. J. Cosmet. Sci. 2016, 38, 512–523. [Google Scholar] [CrossRef]
- Ilie, C.I.; Spoiala, A.; Geana, E.I.; Chircov, C.; Ficai, A.; Ditu, L.M.; Oprea, E. Bee Bread: A Promising Source of Bioactive Compounds with Antioxidant Properties-First Report on Some Antimicrobial Features. Antioxidants 2024, 13, 353. [Google Scholar] [CrossRef]
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; pp. 1–352.
- Wu, K.; Zhang, T.; Chai, X.; Duan, X.; He, D.; Yu, H.; Liu, X.; Tao, Z. Encapsulation Efficiency and Functional Stability of Cinnamon Essential Oil in Modified Beta-Cyclodextrins: In Vitro and In Silico Evidence. Foods 2022, 12, 45. [Google Scholar] [CrossRef]
- Devrnja, N.; Andelkovic, B.; Ljujic, J.; Cosic, T.; Stupar, S.; Milutinovic, M.; Savic, J. Encapsulation of Fennel and Basil Essential Oils in beta-Cyclodextrin for Novel Biopesticide Formulation. Biomolecules 2024, 14, 353. [Google Scholar] [CrossRef]
- Alizadeh, N.; Nazari, F. Thymol essential oil/β-cyclodextrin inclusion complex into chitosan nanoparticles: Improvement of thymol properties in vitro studies. J. Mol. Liq. 2022, 346, 118250. [Google Scholar] [CrossRef]





| Compounds | KI * | tR (min) ** | Relative Area (%) |
|---|---|---|---|
| α-Pinene | 930 | 6.01 | 1.02 |
| Sabinene (β-Thujene) | 969 | 6.72 | 0.72 |
| β-Pinene | 982 | 6.95 | 0.45 |
| α-Phellandrene | 997 | 7.23 | 0.27 |
| α-Terpinene | 1009 | 7.44 | 10.85 |
| p-Cymene | 1017 | 7.58 | 2.63 |
| Eucalyptol | 1026 | 7.73 | 3.76 |
| γ-Terpinene | 1051 | 8.17 | 18.77 |
| α-Terpinolene | 1078 | 8.64 | 3.43 |
| trans-Sabinene hydrate | 1090 | 8.84 | 0.15 |
| cis-2-p-Menthen-1-ol | 1113 | 9.21 | 0.43 |
| Borneol | 1162 | 9.99 | 0.01 |
| Terpinen-4-ol | 1175 | 10.20 | f36.32 |
| α-Terpineol | 1183 | 10.32 | 2.63 |
| cis-Piperitol | 1197 | 10.54 | 0.17 |
| Geraniol | 1238 | 11.15 | 0.01 |
| δ-EIemene | 1324 | 12.39 | 0.06 |
| α-Cubebene | 1336 | 12.55 | 0.09 |
| Eugenol | 1344 | 12.66 | 0.08 |
| α-Copaene | 1364 | 12.94 | 0.28 |
| Methyleugenol | 1384 | 13.23 | 0.09 |
| α-Gurjunene | 1398 | 13.42 | 0.75 |
| β-Caryophyllene | 1409 | 13.56 | 0.41 |
| γ-Elemene | 1430 | 13.82 | 1.95 |
| Alloaromadendrene | 1454 | 14.11 | 0.75 |
| γ-Muurolene | 1463 | 14.22 | 0.91 |
| β-Guaiene | 1480 | 14.43 | 0.53 |
| α-Muurolene | 1490 | 14.56 | 3.88 |
| γ-Cadinene | 1512 | 14.82 | 3.48 |
| δ-Cadinene | 1523 | 14.95 | 0.38 |
| Spathulenol | 1574 | 15.56 | 0.27 |
| Globulol | 1582 | 15.65 | 0.71 |
| Viridiflorol | 1590 | 15.75 | 0.63 |
| Rosifoliol | 1597 | 15.84 | 0.15 |
| Ledol | 1601 | 15.88 | 0.10 |
| Cubenol | 1614 | 16.12 | 0.55 |
| epi-Cubenol | 1621 | 16.25 | 0.15 |
| τ-Cadinol | 1623 | 16.29 | 0.45 |
| Total | 98.27 |
| Compounds | KI * | tR (min) ** | Relative Area (%) |
|---|---|---|---|
| α-Pinene | 932 | 6.03 | 11.83 |
| Camphene | 946 | 6.29 | 3.93 |
| Sabinene (β-Thujene) | 973 | 6.79 | 4.10 |
| β-Pinene | 982 | 6.96 | 0.71 |
| α-Phellandrene | 998 | 7.26 | 0.11 |
| p-Cymene | 1011 | 7.48 | 0.03 |
| Eucalyptol | 1030 | 7.80 | 49.70 |
| γ-Terpinene | 1049 | 8.14 | 0.52 |
| cis-Sabinene hydrate | 1060 | 8.33 | 0.04 |
| α-Terpinolene | 1078 | 8.64 | 0.35 |
| Linalool | 1087 | 8.79 | 0.63 |
| endo-Fenchol | 1107 | 9.12 | 0.03 |
| α-Campholenal | 1116 | 9.26 | 0.04 |
| Camphor | 1143 | 9.69 | 14.12 |
| Borneol | 1161 | 9.98 | 3.36 |
| Terpinen-4-ol | 1169 | 10.10 | 0.56 |
| α-Terpineol | 1183 | 10.32 | 2.00 |
| Myrtenol | 1189 | 10.41 | 0.04 |
| Verbenone | 1204 | 10.65 | 0.01 |
| cis-Carveol | 1208 | 10.71 | 0.01 |
| Thymol | 1271 | 11.62 | 0.02 |
| Bornyl acetate | 1275 | 11.68 | 0.75 |
| Carvacrol | 1287 | 11.86 | 0.02 |
| Eugenol | 1344 | 12.66 | 0.02 |
| α-Ylangene | 1359 | 12.88 | 0.03 |
| α-Copaene | 1364 | 12.94 | 0.02 |
| Methyleugenol | 1389 | 13.29 | 0.03 |
| β-Caryophyllene | 1412 | 13.60 | 5.80 |
| β-Ylangene | 1418 | 13.67 | 0.05 |
| α-Guaiene | 1431 | 13.83 | 0.03 |
| Aromadendrene | 1439 | 13.93 | 0.03 |
| α-Humulene | 1446 | 14.01 | 0.42 |
| γ-Muurolene | 1465 | 14.25 | 0.04 |
| α-Muurolene | 1491 | 14.57 | 0.15 |
| γ-Cadinene | 1511 | 14.81 | 0.03 |
| α-Calacorene | 1535 | 15.10 | 0.01 |
| Caryophyllene oxide | 1582 | 15.65 | 0.24 |
| Methyl jasmonate | 1621 | 16.26 | 0.04 |
| Total | 99.84 |
| Characteristic | Formula A | Formula B | Formula C |
|---|---|---|---|
| Initial macroscopic characteristics | appearance: homogenous; color: yellowish; smell: specific | appearance: homogenous; color: yellowish; smell: specific | appearance: homogenous; color: yellowish; smell: specific |
| Macroscopic characteristics after 60 days | appearance: homogenous; color: yellowish smell: specific | appearance: homogenous; color: yellowish; smell: specific | appearance: homogenous; color: yellowish; smell: specific |
| Initial pH | 6.50 | 6.00 | 6.70 |
| pH after 60 days | 6.40 | 6.60 | 6.60 |
| Characteristic | Formula D | Formula E | Formula F |
|---|---|---|---|
| Initial macroscopic characteristics | appearance: homogenous; color: yellowish; smell: specific | appearance: homogenous; color: yellowish; smell: specific | appearance: homogenous; color: yellowish; smell: specific |
| Macroscopic characteristics after 60 days | appearance: homogenous; color: yellowish smell: specific | appearance: homogenous; color: yellowish; smell: specific | appearance: homogenous; color: yellowish; smell: specific |
| Initial pH | 6.70 | 6.60 | 6.40 |
| pH after 60 days | 6.70 | 6.50 | 6.30 |
| Gel/Rheological Model | Casson | Bingham | Ostwald-de Waele | Herschel-Bulkley | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Adj R2 | AICc | R2 | Adj R2 | AICc | R2 | Adj R2 | AICc | R2 | Adj R2 | AICc | |
| Formula A | 0.9733 | 0.9715 | 33.29 | 0.8766 | 0.8684 | 59.29 | 0.9790 | 0.9776 | 29.16 | 0.9981 | 0.9978 | −7.961 |
| Formula B | 0.9648 | 0.9624 | 50.01 | 0.8556 | 0.8460 | 74.01 | 0.9847 | 0.9837 | 35.85 | 0.9986 | 0.9984 | −1.536 |
| Formula C | 0.9473 | 0.9437 | 77.29 | 0.8230 | 0.8112 | 97.87 | 0.9862 | 0.9853 | 54.51 | 0.9947 | 0.9939 | 41.870 |
| Formula D | 0.9605 | 0.9578 | 51.98 | 0.8462 | 0.8360 | 75.07 | 0.9832 | 0.9821 | 37.45 | 0.9969 | 0.9964 | 12.390 |
| Formula E | 0.9676 | 0.9654 | 47.99 | 0.8632 | 0.8541 | 72.47 | 0.9833 | 0.9822 | 36.73 | 0.9983 | 0.9981 | 1.090 |
| Formula F | 0.9672 | 0.9650 | 43.43 | 0.8603 | 0.8510 | 68.05 | 0.9840 | 0.9830 | 31.19 | 0.9990 | 0.9989 | −12.980 |
| Gel/ Flow Parameters | Yield Stress (Pa) (τ0—Pa) | Consistency Index (K—Pa·sn) | Flow Index (n) | Viscosity at 0.30 rpm (η0.3—Pa·s) | |
|---|---|---|---|---|---|
| Initial | After 60 Days | ||||
| Formula A | 29.518 | 20.021 | 0.34 | 489.20 | 472.10 |
| Formula B | 48.333 | 34.938 | 0.29 | 822.90 | 710.20 |
| Formula C | 80.150 | 76.884 | 0.25 | 1553.00 | 1432.80 |
| Formula D | 51.397 | 36.334 | 0.28 | 871.90 | 760.30 |
| Formula E | 43.268 | 31.869 | 0.31 | 738.10 | 682.00 |
| Formula F | 41.732 | 28.915 | 0.30 | 698.80 | 536.40 |
| Strains | Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GB | B | E | CO | RO | TTO | UA | Complex | UA:CO | UA:RO | UA:TTO | DMSO | |
| Gram-positive bacteria | ||||||||||||
| E. faecalis ATCC 29212 | - | + | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - |
| S. aureus ATCC 25923 | - | + | + | +++ | + | + | ++ | +++ | ++ | +++ | +++ | - |
| Gram-negative bacteria | ||||||||||||
| E. coli ATCC 25922 | + | + | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - |
| E. coli 135 | - | ++ | ++ | ++ | - | - | ++ | ++ | ++ | ++ | ++ | - |
| E. coli ESBL | - | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - |
| E. coli 2425 | + | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - |
| E. coli 5075 | - | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - |
| P. aeruginosa ATCC 27853 | - | + | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - |
| Yeasts | ||||||||||||
| C. albicans ATCC 10231 | - | ++ | ++ | + | + | ++ | ++ | ++ | ++ | ++ | ++ | - |
| C. albicans 5316 | - | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - |
| C. albicans 5319 | - | + | + | +++ | +++ | +++ | + | + | +++ | +++ | +++ | + |
| C. albicans 5325 | - | ++ | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - |
| C. albicans 5328 | - | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | +++ | ++ | - |
| C. krusei 5299 | - | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - |
| C. krusei 5343 | - | + | ++ | + | + | ++ | ++ | ++ | ++ | ++ | ++ | - |
| C. glabrata 5328 | - | + | + | + | + | + | ++ | - | ++ | ++ | ++ | - |
| C. parapsilosis ATCC 22019 | - | ++ | ++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ | - |
| C. parapsilosis 514 | - | + | + | ++ | ++ | ++ | ++ | ++ | +++ | +++ | +++ | + |
| C. tropicalis 5770 | - | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | - |
| Components | Formula A | Formula B | Formula C |
|---|---|---|---|
| Carbopol 940 | 0.8 g | 1 g | 1.2 g |
| Glycerin | 5 g | 5 g | 5 g |
| Triethanolamine | q.s. | q.s. | q.s. |
| Cyclodextrin complex with clove essential oil | 1 g | 1 g | 1 g |
| Usnic acid | 0.5 g | 0.5 g | 0.5 g |
| Tea tree essential oil | 1.5 g | 1.5 g | 1.5 g |
| Rosemary essential oil | 1.5 g | 1.5 g | 1.5 g |
| Purified water | until 100 g | until 100 g | until 100 g |
| Components | Formula D | Formula E | Formula F |
|---|---|---|---|
| Carbopol 940 | 1 g | 1 g | 1 g |
| Glycerin | 5 g | 5 g | 5 g |
| Triethanolamine | q.s. | q.s. | q.s. |
| Cyclodextrin complex with clove essential oil | 1 g | 1 g | 1 g |
| Usnic acid | 0.5 g | 0.5 g | 0.5 g |
| Tea tree essential oil | 1 g | 2 g | 2.5 g |
| Rosemary essential oil | 1 g | 2 g | 2.5 g |
| Purified water | until 100 g | until 100 g | until 100 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancu, A.I.; Oprea, E.; Dițu, L.M.; Ficai, A.; Ilie, C.-I.; Badea, I.A.; Buleandra, M.; Brîncoveanu, O.; Ghica, M.V.; Avram, I.; et al. Development, Optimization, and Evaluation of New Gel Formulations with Cyclodextrin Complexes and Volatile Oils with Antimicrobial Activity. Gels 2024, 10, 645. https://doi.org/10.3390/gels10100645
Stancu AI, Oprea E, Dițu LM, Ficai A, Ilie C-I, Badea IA, Buleandra M, Brîncoveanu O, Ghica MV, Avram I, et al. Development, Optimization, and Evaluation of New Gel Formulations with Cyclodextrin Complexes and Volatile Oils with Antimicrobial Activity. Gels. 2024; 10(10):645. https://doi.org/10.3390/gels10100645
Chicago/Turabian StyleStancu, Alina Ionela, Eliza Oprea, Lia Mara Dițu, Anton Ficai, Cornelia-Ioana Ilie, Irinel Adriana Badea, Mihaela Buleandra, Oana Brîncoveanu, Mihaela Violeta Ghica, Ionela Avram, and et al. 2024. "Development, Optimization, and Evaluation of New Gel Formulations with Cyclodextrin Complexes and Volatile Oils with Antimicrobial Activity" Gels 10, no. 10: 645. https://doi.org/10.3390/gels10100645
APA StyleStancu, A. I., Oprea, E., Dițu, L. M., Ficai, A., Ilie, C.-I., Badea, I. A., Buleandra, M., Brîncoveanu, O., Ghica, M. V., Avram, I., Pîrvu, C. E. D., & Mititelu, M. (2024). Development, Optimization, and Evaluation of New Gel Formulations with Cyclodextrin Complexes and Volatile Oils with Antimicrobial Activity. Gels, 10(10), 645. https://doi.org/10.3390/gels10100645













