The Effect of Acetic Acid as a Solvent on the Structure and Properties of Poly(3-hydroxybutyrate)—Based Dried Gels
Abstract
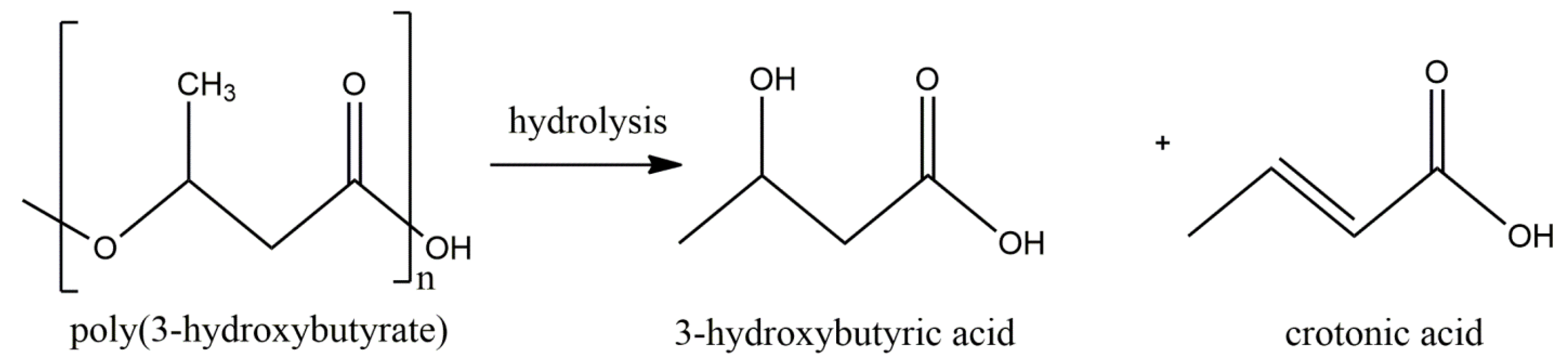
:1. Introduction
2. Results and Discussion
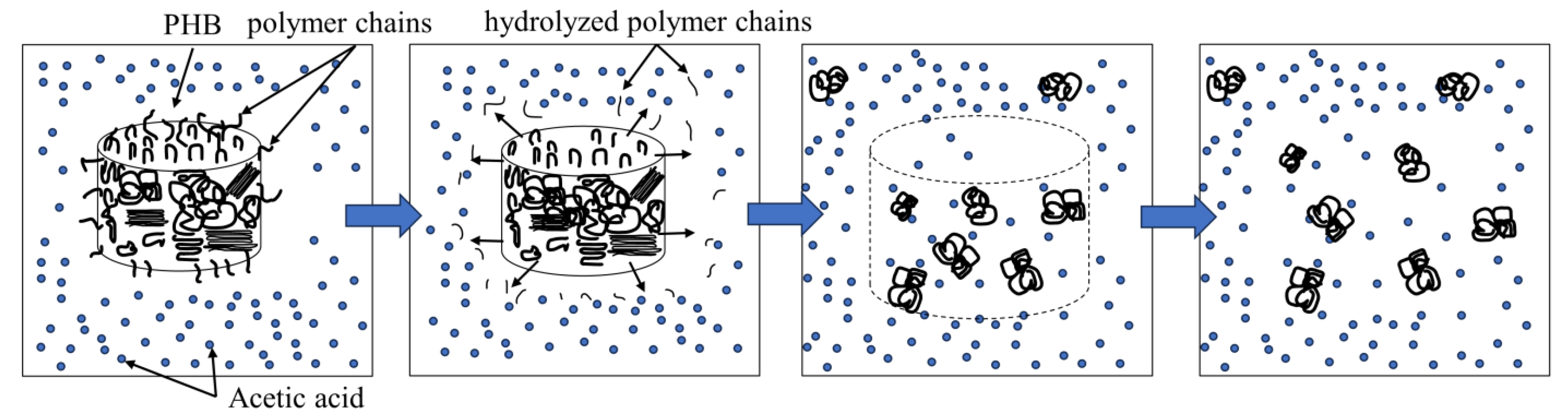
2.1. Gel Preparation
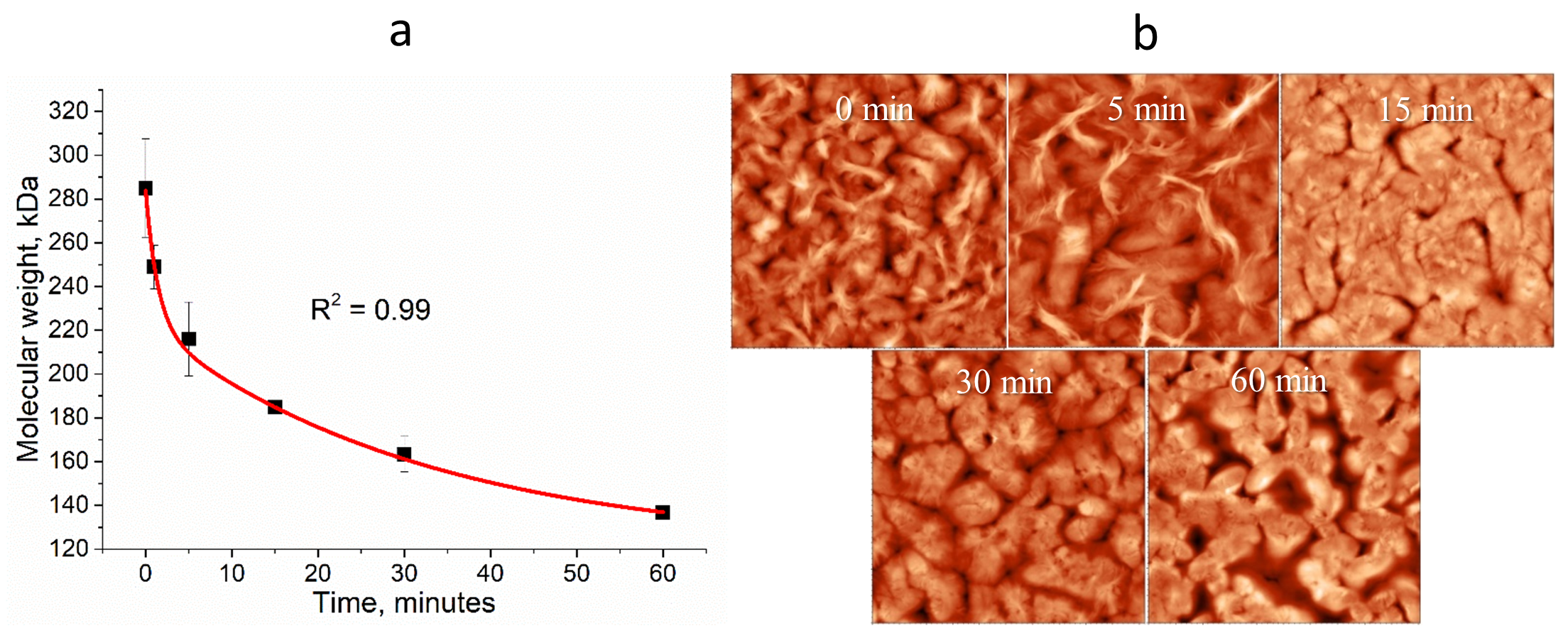
2.2. Change in Molecular Weight of PHB aa
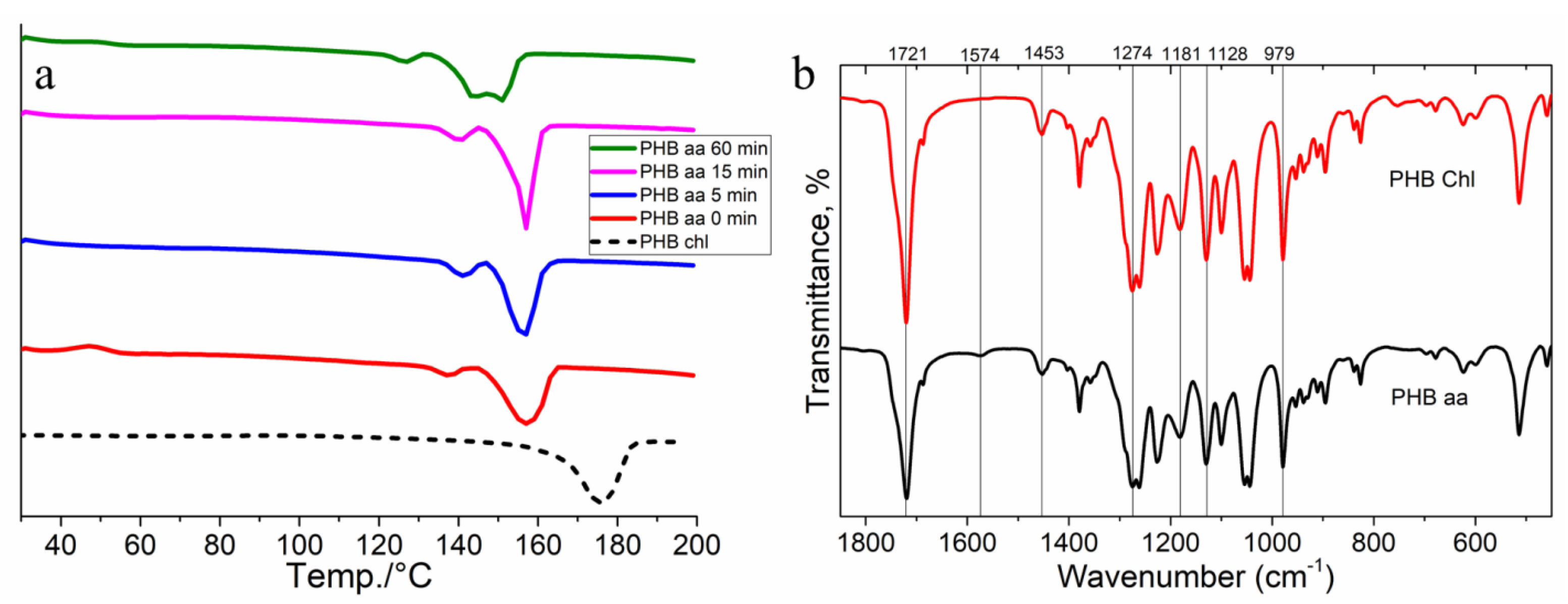
2.3. Differential Scanning Calorimetry (DSC)
2.4. FTIR Spectroscopy
2.5. Degradation of PHB Gels
2.6. Scanning Electron Microscopy (SEM) of Dried Gels
2.7. Structure Parameters of PHB-Based Gels
2.8. Rheological Behavior and Stiffness of Gels
2.9. Biocompatibility Tests of Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PHB Dried Gels
4.2.1. Preparation of Gels from PHB aa
4.2.2. Preparation of Gels from PHB chl
4.3. Molecular Weight Measurement
4.4. Atomic Force Microscopy (AFM)
4.5. Differential Scanning Calorimetry (DSC)
4.6. FTIR Spectroscopy
4.7. Enzymatic Degradation
4.8. Scanning Electron Microscopy (SEM)
4.9. Determination of Swelling Ratio, Porosity and Density of Gels
4.10. Rheological Tests
4.11. Biocompatibility In Vitro Assay
4.12. Statistical Processing of the Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazur, F.; Pham, A.-H.; Chandrawati, R. Polymer Materials as Catalysts for Medical, Environmental, and Energy Applications. Appl. Mater. Today 2023, 35, 101937. [Google Scholar] [CrossRef]
- Bekchanov, D.; Mukhamediev, M.; Yarmanov, S.; Lieberzeit, P.; Mujahid, A. Functionalizing Natural Polymers to Develop Green Adsorbents for Wastewater Treatment Applications. Carbohydr. Polym. 2024, 323, 121397. [Google Scholar] [CrossRef]
- Haleem, A.; Pan, J.-M.; Shah, A.; Hussain, H.; He, W. A Systematic Review on New Advancement and Assessment of Emerging Polymeric Cryogels for Environmental Sustainability and Energy Production. Sep. Purif. Technol. 2023, 316, 123678. [Google Scholar] [CrossRef]
- Li, T.-T.; Cheng, S.-B.; Feng, L.-F.; Gu, X.-P.; Duan, J.-T.; Jiang, M.-Z.; Zhang, C.-L. A Review on the Free Radical Grafting of Vinyl Monomers onto Polyethylene and Polypropylene by Reactive Extrusion. Chem. Eng. Sci. 2023, 278, 118916. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; Furton, K.G.; Tzanavaras, P.D.; Zacharis, C.K. In Situ Synthesis of Monolithic Sol–Gel Polyethylene Glycol-Based Sorbent Encapsulated in Porous Polypropylene Microextraction Capsules and Its Application for Selective Extraction of Antifungal and Anthelmintic Drugs from Human Urine. Microchem. J. 2022, 180, 107594. [Google Scholar] [CrossRef]
- Jo, Y.; Garcia, C.V.; Ko, S.; Lee, W.; Shin, G.H.; Choi, J.C.; Park, S.-J.; Kim, J.T. Characterization and Antibacterial Properties of Nanosilver-Applied Polyethylene and Polypropylene Composite Films for Food Packaging Applications. Food Biosci. 2018, 23, 83–90. [Google Scholar] [CrossRef]
- Cui, M.; Chai, Z.; Lu, Y.; Zhu, J.; Chen, J. Developments of Polyurethane in Biomedical Applications: A Review. Resour. Chem. Mater. 2023, 2, 262–276. [Google Scholar] [CrossRef]
- Peter, N.J.; Griesshaber, E.; Reisecker, C.; Hild, S.; Oliveira, M.V.G.; Schmahl, W.W.; Schneider, A.S. Biocrystal Assembly Patterns, Biopolymer Distribution and Material Property Relationships in Mytilus Galloprovincialis, Bivalvia, and Haliotis Glabra, Gastropoda, Shells. Materialia 2023, 28, 101749. [Google Scholar] [CrossRef]
- Christina, K.; Subbiah, K.; Arulraj, P.; Krishnan, S.K.; Sathishkumar, P. A Sustainable and Eco-Friendly Approach for Environmental and Energy Management Using Biopolymers Chitosan, Lignin and Cellulose—A Review. Int. J. Biol. Macromol. 2024, 257, 128550. [Google Scholar] [CrossRef]
- Sridharan, S.; Kumar, M.; Saha, M.; Kirkham, M.B.; Singh, L.; Bolan, N.S. The Polymers and Their Additives in Particulate Plastics: What Makes Them Hazardous to the Fauna? Sci. Total Environ. 2022, 824, 153828. [Google Scholar] [CrossRef]
- Badar, I.H.; Wang, Z.; Liu, H.; Chen, Q.; Xia, X.; Liu, Q.; Kong, B. Future Prospects of High Internal Phase Pickering Emulsions Stabilized by Natural Modified Biopolymers as a Potential Fat Substitute in Meat Products. Trends Food Sci. Technol. 2023, 140, 104176. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Y. Biopolymer-Based Encapsulation of Anthocyanins as Reinforced Natural Colorants for Food Applications. J. Agric. Food Res. 2023, 11, 100488. [Google Scholar] [CrossRef]
- da Costa, R.C.; Daitx, T.S.; Mauler, R.S.; da Silva, N.M.; Miotto, M.; Crespo, J.S.; Carli, L.N. Poly(Hydroxybutyrate-Co-Hydroxyvalerate)-Based Nanocomposites for Antimicrobial Active Food Packaging Containing Oregano Essential Oil. Food Packag. Shelf Life 2020, 26, 100602. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Szydłowska-Czerniak, A.; Nowaczyk, J.; Olewnik-Kruszkowska, E. Development and Characterization of Active Packaging Films Based on Chitosan, Plasticizer, and Quercetin for Repassed Oil Storage. Food Chem. 2023, 399, 133934. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Guibal, E.; Alghamdi, H.M.; Elwakeel, K.Z. Use of Biopolymers in Wastewater Treatment: A Brief Review of Current Trends and Prospects. Chin. J. Chem. Eng. 2023, 64, 292–320. [Google Scholar] [CrossRef]
- Shabir, R.; Li, Y.; Megharaj, M.; Chen, C. Biopolymer as an Additive for Effective Biochar-Based Rhizobial Inoculant. Sci. Total Environ. 2024, 912, 169263. [Google Scholar] [CrossRef]
- Bhatt, R.; Jaffe, M. Biopolymers in Medical Implants. In Excipient Applications in Formulation Design and Drug Delivery; Springer International Publishing: Cham, Switzerland, 2015; pp. 311–348. [Google Scholar]
- Gerasimenko, A.Y.; Kurilova, U.E.; Savelyev, M.S.; Murashko, D.T.; Glukhova, O.E. Laser Fabrication of Composite Layers from Biopolymers with Branched 3D Networks of Single-Walled Carbon Nanotubes for Cardiovascular Implants. Compos. Struct. 2021, 260, 113517. [Google Scholar] [CrossRef]
- Anjana; Raturi, G.; Shree, S.; Sharma, A.; Panesar, P.S.; Goswami, S. Recent Approaches for Enhanced Production of Microbial Polyhydroxybutyrate: Preparation of Biocomposites and Applications. Int. J. Biol. Macromol. 2021, 182, 1650–1669. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Techno-Economic Assessment of a Sustainable and Cost-Effective Bioprocess for Large Scale Production of Polyhydroxybutyrate. Chemosphere 2021, 284, 131371. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Bonartsev, A.P.; Makhina, T.K.; Myshkina, V.L.; Voinova, V.V.; Bonartseva, G.A.; Shaitan, K.V. Hydrolytic Degradation of Poly(3-Hydroxybutyrate) and Its Copolymer with 3-Hydroxyvalerate of Different Molecular Weights in Vitro. Biophysics 2018, 63, 169–176. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Bonartsev, A.P.; Bagrov, D.V.; Yakovlev, S.G.; Myshkina, V.L.; Makhina, T.K.; Bessonov, I.V.; Kopitsyna, M.N.; Morozov, A.S.; Rusakov, A.A.; et al. Mechanics and Surface Ultrastructure Changes of Poly(3-Hydroxybutyrate) Films during Enzymatic Degradation in Pancreatic Lipase Solution. Mol. Cryst. Liq. Cryst. 2017, 648, 236–243. [Google Scholar] [CrossRef]
- Gogolewski, S.; Jovanovic, M.; Perren, S.M.; Dillon, J.G.; Hughes, M.K. Tissue Response and in Vivo Degradation of Selected Polyhydroxyacids: Polylactides (PLA), Poly(3-hydroxybutyrate) (PHB), and Poly(3-hydroxybutyrate- Co -3-hydroxyvalerate) (PHB/VA). J. Biomed. Mater. Res. 1993, 27, 1135–1148. [Google Scholar] [CrossRef]
- Iordanskii, A.; Bonartseva, G.; Makhina, T.; Sklyanchuk, E.; Zaikov, G. Bacterial Poly(3-Hydroxybutyrate) as a Biodegradable Polymer for Biomedicine. In Physical Chemistry Research for Engineering and Applied Sciences, Volume One; Apple Academic Press: Palm Bay, FL, USA, 2015; pp. 1–44. [Google Scholar]
- Bhende, P.P.; Chauhan, R.; Waigaonkar, S.; Bragança, J.M.; Ganguly, A. Composites of Bacillus Megaterium H16 Derived Poly-3-Hydroxybutyrate as a Biomaterial for Skin Tissue Engineering. Int. J. Biol. Macromol. 2023, 244, 125355. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, C.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Enhanced Crystallinity and Antibacterial of PHBV Scaffolds Incorporated with Zinc Oxide. J. Nanomater. 2020, 2020, 6014816. [Google Scholar] [CrossRef]
- Wei, L.; Liang, S.; McDonald, A.G. Thermophysical Properties and Biodegradation Behavior of Green Composites Made from Polyhydroxybutyrate and Potato Peel Waste Fermentation Residue. Ind. Crops Prod. 2015, 69, 91–103. [Google Scholar] [CrossRef]
- Kanabenja, W.; Passarapark, K.; Subchokpool, T.; Nawaaukkaratharnant, N.; Román, A.J.; Osswald, T.A.; Aumnate, C.; Potiyaraj, P. 3D Printing Filaments from Plasticized Polyhydroxybutyrate/Polylactic Acid Blends Reinforced with Hydroxyapatite. Addit. Manuf. 2022, 59, 103130. [Google Scholar] [CrossRef]
- Babaniyi, R.B.; Afolabi, F.J.; Obagunwa, M.P. Recycling of Used Polyethylene through Solvent Blending of Plasticized Polyhydroxybutyrate and Its Degradation Potential. Compos. Part C Open Access 2020, 2, 100021. [Google Scholar] [CrossRef]
- Ankush, K.; Pugazhenthi, G.; Mohit, K.; Vasanth, D. Experimental Study on Fabrication, Biocompatibility and Mechanical Characterization of Polyhydroxybutyrate-Ball Clay Bionanocomposites for Bone Tissue Engineering. Int. J. Biol. Macromol. 2022, 209, 1995–2008. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Ren, M.; Ding, C.; Chen, P.; Gu, Q.; Wu, Q. Evaluation of PHBHHx and PHBV/PLA Fibers Used as Medical Sutures. J. Mater. Sci. Mater. Med. 2014, 25, 561–571. [Google Scholar] [CrossRef]
- Luklinska, Z.B.; Schluckwerder, H. In Vivo Response to HA-polyhydroxybutyrate/Polyhydroxyvalerate Composite. J. Microsc. 2003, 211, 121–129. [Google Scholar] [CrossRef]
- Akhila, N.S.; Krishnan, V.G.; Parukoor Thomas, J.; Gowd, E.B. Unprecedented Thermally Induced Structural Changes in Aerogels of Poly(3-Hydroxybutyrate) during Heating. ACS Appl. Polym. Mater. 2024, 6, 6864–6874. [Google Scholar] [CrossRef]
- Zharkova, I.I.; Volkov, A.V.; Muraev, A.A.; Makhina, T.K.; Voinova, V.V.; Ryabova, V.M.; Gazhva, Y.V.; Kashirina, A.S.; Kashina, A.V.; Bonartseva, G.A.; et al. Poly(3-Hydroxybutyrate) 3D-Scaffold–Conduit for Guided Tissue Sprouting. Int. J. Mol. Sci. 2023, 24, 6965. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Wang, D.-M.; Lai, J.-Y. Nonsolvent-Induced Gelation and Its Effect on Membrane Morphology. Macromolecules 2002, 35, 6697–6706. [Google Scholar] [CrossRef]
- Kang, J.; Gi, H.; Choe, R.; Yun, S. Il Fabrication and Characterization of Poly(3-Hydroxybutyrate) Gels Using Non-Solvent-Induced Phase Separation. Polymer 2016, 104, 61–71. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.-Y.; Huh, M.; Yun, S. Il Porous Poly(3-Hydroxybutyrate) Scaffolds Prepared by Non-Solvent-Induced Phase Separation for Tissue Engineering. Macromol. Res. 2020, 28, 835–843. [Google Scholar] [CrossRef]
- Turchetto, A.; Cesàro, A. Gel-Sol Phase Transition of Poly(3-Hydroxybutyrate) in Dimethylformamide. Thermochim. Acta 1995, 269–270, 307–317. [Google Scholar] [CrossRef]
- Pich, A.; Schiemenz, N.; Boyko, V.; Adler, H.-J.P. Thermoreversible Gelation of Biodegradable Polyester (PHBV) in Toluene. Polymer 2006, 47, 553–560. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Bi, Z.; Zhou, B.; Wang, L.; Li, Q.; Turng, L.-S. Expanded Polytetrafluoroethylene/Poly(3-Hydroxybutyrate) (EPTFE/PHB) Triboelectric Nanogenerators and Their Potential Applications as Self-Powered and Sensing Vascular Grafts. Chem. Eng. J. 2023, 455, 140494. [Google Scholar] [CrossRef]
- Mohammadalipour, M.; Asadolahi, M.; Mohammadalipour, Z.; Behzad, T.; Karbasi, S. Plasma Surface Modification of Electrospun Polyhydroxybutyrate (PHB) Nanofibers to Investigate Their Performance in Bone Tissue Engineering. Int. J. Biol. Macromol. 2023, 230, 123167. [Google Scholar] [CrossRef]
- Budurova, D.; Ublekov, F.; Penchev, H. The Use of Formic Acid as a Common Solvent for Electrospinning of Hybrid PHB/Soy Protein Fibers. Mater. Lett. 2021, 301, 130313. [Google Scholar] [CrossRef]
- Ol’khov, A.A.; Iordanskii, A.L.; Fel’dshtein, M.M. Effect of Certain Solvent Parameters on the Structure of Polyhydroxybutyrate Films. Int. Polym. Sci. Technol. 2005, 32, 24–26. [Google Scholar] [CrossRef]
- Terada, M.; Marchessault, R.H. Determination of Solubility Parameters for Poly(3-Hydroxyalkanoates). Int. J. Biol. Macromol. 1999, 25, 207–215. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the Properties of Polyhydroxybutyrate Films Using Acetic Acid via Solvent Casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef]
- Chanda, S. Acetic Acid. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 25–26. [Google Scholar]
- Anbukarasu, P.; Sauvageau, D.; Elias, A.L. Enzymatic Degradation of Dimensionally Constrained Polyhydroxybutyrate Films. Phys. Chem. Chem. Phys. 2017, 19, 30021–30030. [Google Scholar] [CrossRef]
- Edwards, C.O.; Mandelkern, L. Crystallization–Gelation Process of Homopolymers and Copolymers from Solution. J. Polym. Sci. Polym. Lett. Ed. 1982, 20, 355–359. [Google Scholar] [CrossRef]
- Myshkina, V.L.; Nikolaeva, D.A.; Makhina, T.K.; Bonartsev, A.P.; Bonartseva, G.A. Effect of Growth Conditions on the Molecular Weight of Poly-3-Hydroxybutyrate Produced by Azotobacter Chroococcum 7B. Appl. Biochem. Microbiol. 2008, 44, 482–486. [Google Scholar] [CrossRef]
- Cohen-Arazi, N.; Domb, A.J.; Katzhendler, J. New Biocompatible Polyesters Derived from α-Amino Acids: Hydrolytic Degradation Behavior. Polymers 2010, 2, 418–439. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.-G. Theory of Polymer Chains in Poor Solvent: Single-Chain Structure, Solution Thermodynamics, and Θ Point. Macromolecules 2014, 47, 4094–4102. [Google Scholar] [CrossRef]
- Abramova, A.A.; Glagolev, M.K.; Vasilevskaya, V.V. Structured Globules with Twisted Arrangement of Helical Blocks: Computer Simulation. Polymer 2022, 253, 124974. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Okamura, K.; Su, C.J. Physical Properties of Poly (β-Hydroxy Butyrate). II. Conformational Aspects in Solution. Macromolecules 1970, 3, 735–740. [Google Scholar] [CrossRef]
- Hong, S.-G.; Hsu, H.-W.; Ye, M.-T. Thermal Properties and Applications of Low Molecular Weight Polyhydroxybutyrate. J. Therm. Anal. Calorim. 2013, 111, 1243–1250. [Google Scholar] [CrossRef]
- Zhuikova, Y.V.; Zhuikov, V.A.; Makhina, T.K.; Efremov, Y.M.; Aksenova, N.A.; Timashev, P.S.; Bonartseva, G.A.; Varlamov, V.P. Preparation and Characterization of Poly(3-Hydroxybutyrate)/Chitosan Composite Films Using Acetic Acid as a Solvent. Int. J. Biol. Macromol. 2023, 248, 125970. [Google Scholar] [CrossRef]
- Randriamahefa, S.; Renard, E.; Guérin, P.; Langlois, V. Fourier Transform Infrared Spectroscopy for Screening and Quantifying Production of PHAs by Pseudomonas Grown on Sodium Octanoate. Biomacromolecules 2003, 4, 1092–1097. [Google Scholar] [CrossRef]
- Vishnuvardhan Reddy, S.; Thirumala, M.; Mahmood, S.K. Production of PHB and P (3HB-Co-3HV) Biopolymers by Bacillus Megaterium Strain OU303A Isolated from Municipal Sewage Sludge. World J. Microbiol. Biotechnol. 2009, 25, 391–397. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.-H.; Yang, R.; Wu, Q.; Chen, G.-Q.; Zhang, Z.-M. In Situ FTIR Study on Melting and Crystallization of Polyhydroxyalkanoates. Polymer 2002, 43, 6893–6899. [Google Scholar] [CrossRef]
- Kann, Y.; Shurgalin, M.; Krishnaswamy, R.K. FTIR Spectroscopy for Analysis of Crystallinity of Poly(3-Hydroxybutyrate-Co-4 -Hydroxybutyrate) Polymers and Its Utilization in Evaluation of Aging, Orientation and Composition. Polym. Test. 2014, 40, 218–224. [Google Scholar] [CrossRef]
- Kalita, N.K.; Damare, N.A.; Hazarika, D.; Bhagabati, P.; Kalamdhad, A.; Katiyar, V. Biodegradation and Characterization Study of Compostable PLA Bioplastic Containing Algae Biomass as Potential Degradation Accelerator. Environ. Chall. 2021, 3, 100067. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Akoulina, E.A.; Chesnokova, D.V.; Wenhao, Y.; Makhina, T.K.; Demyanova, I.V.; Zhuikova, Y.V.; Voinova, V.V.; Belishev, N.V.; Surmenev, R.A.; et al. The Growth of 3t3 Fibroblasts on Phb, Pla and Phb/Pla Blend Films at Different Stages of Their Biodegradation in Vitro. Polymers 2021, 13, 108. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin, A.A.; Bonartsev, A.P.; et al. Comparative Structure-Property Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)s Films under Hydrolytic and Enzymatic Degradation: Finding a Transition Point in 3-Hydroxyvalerate Content. Polymers 2020, 12, 728. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; McLaughlin, J.; Bonallo, R. Effect of Pore Size, Morphology and Orientation on the Bulk Stiffness of a Porous Ti35Nb4Sn Alloy. J. Mater. Eng. Perform. 2018, 27, 2899–2909. [Google Scholar] [CrossRef]
- Bejarano, J.; Boccaccini, A.R.; Covarrubias, C.; Palza, H. Effect of Cu- and Zn-Doped Bioactive Glasses on the In Vitro Bioactivity, Mechanical and Degradation Behavior of Biodegradable PDLLA Scaffolds. Materials 2020, 13, 2908. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Kanungo, B.P.; Gibson, L.J. Density–Property Relationships in Mineralized Collagen–Glycosaminoglycan Scaffolds. Acta Biomater. 2009, 5, 1006–1018. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Dvoinina, L.M.; Sukovatyi, A.G.; Sukhanova, A.A.; Ertiletskaya, N.L. Improvement of Biocompatibility of High Molecular Weight Poly-3-Hydroxybutyrate by Blending with Its Functionalized Oligomers. Chim. Techno Acta 2024, 11, 202411101. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, X.; Chen, G.Q.; Chen, J.C. Effect of Lipase Treatment on the Biocompatibility of Microbial Polyhydroxyalkanoates. J. Mater. Sci. Mater. Med. 2002, 13, 849–854. [Google Scholar] [CrossRef]
- Joseph Ufere, S.K.; Sultana, N. Fabrication And Characterization Of Pcl/Ha/Ppy Composite Scaffold Using Freeze-Drying Technique. J. Teknol. 2016, 78, 89–94. [Google Scholar] [CrossRef]
- Valente, B.F.A.; Silvestre, A.J.D.; Neto, C.P.; Vilela, C.; Freire, C.S.R. Improving the Processability and Performance of Micronized Fiber-Reinforced Green Composites through the Use of Biobased Additives. Polymers 2022, 14, 3451. [Google Scholar] [CrossRef]
- Dudun, A.; Akoulina, E.; Zhuikov, V.; Makhina, T.; Voinova, V.; Belishev, N.; Khaydapova, D.; Shaitan, K.; Bonartseva, G.; Bonartsev, A. Competitive Biosynthesis of Bacterial Alginate Using Azotobacter Vinelandii 12 for Tissue Engineering Applications. Polymers 2021, 14, 131. [Google Scholar] [CrossRef]
- Akoulina, E.A.; Demianova, I.V.; Zharkova, I.I.; Voinova, V.V.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; Makhina, T.K.; et al. Growth of Mesenchymal Stem Cells on Poly(3-Hydroxybutyrate) Scaffolds Loaded with Simvastatin. Bull. Exp. Biol. Med. 2021, 171, 172–177. [Google Scholar] [CrossRef]

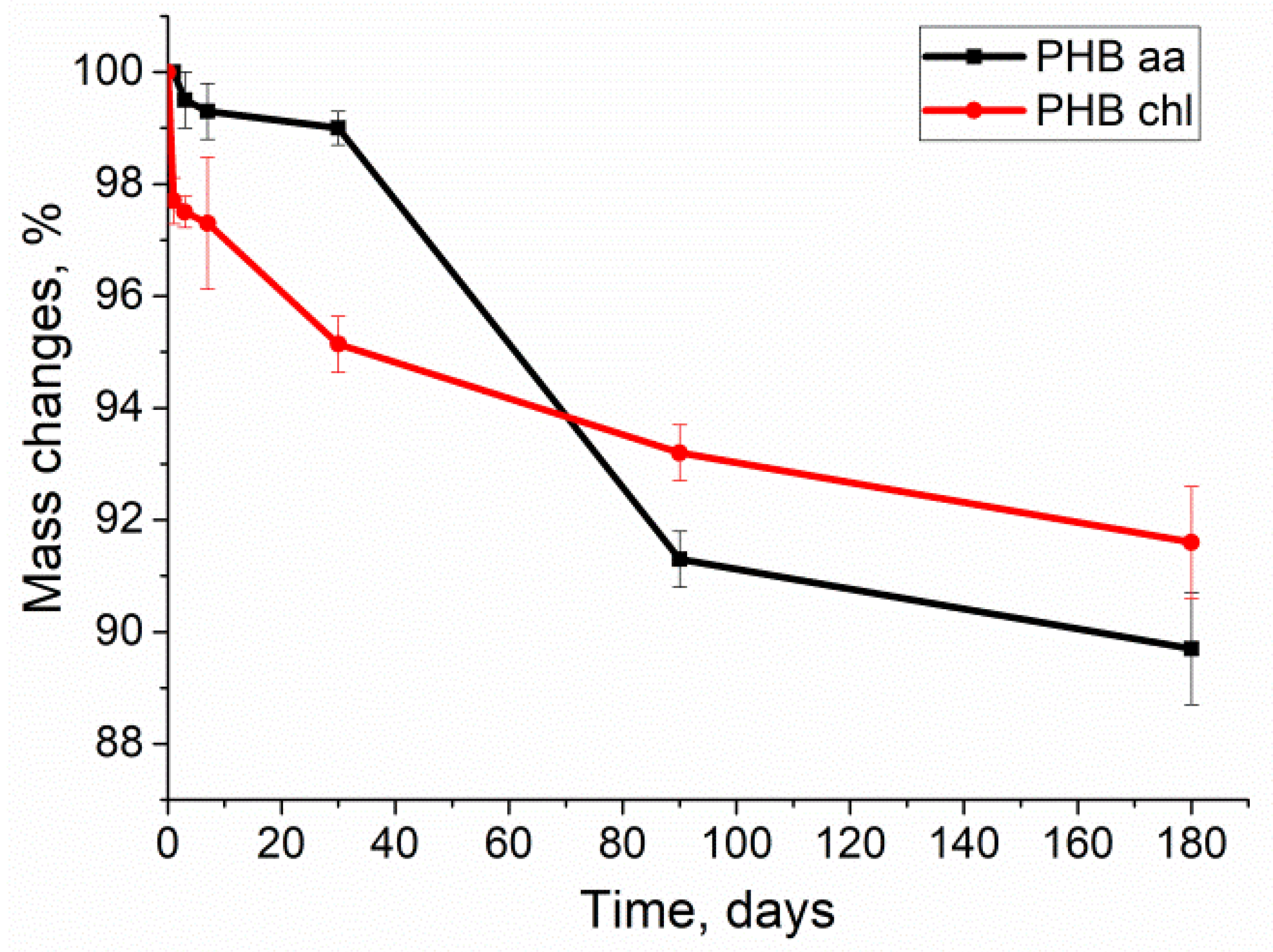

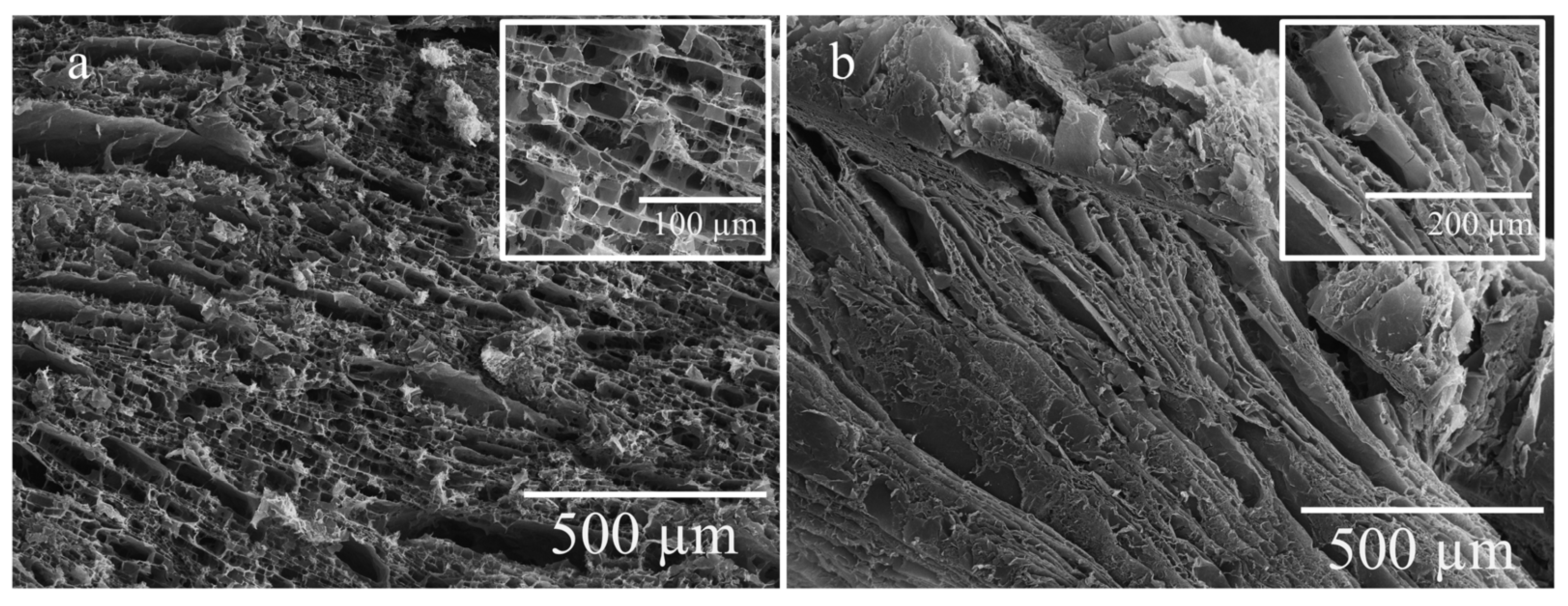
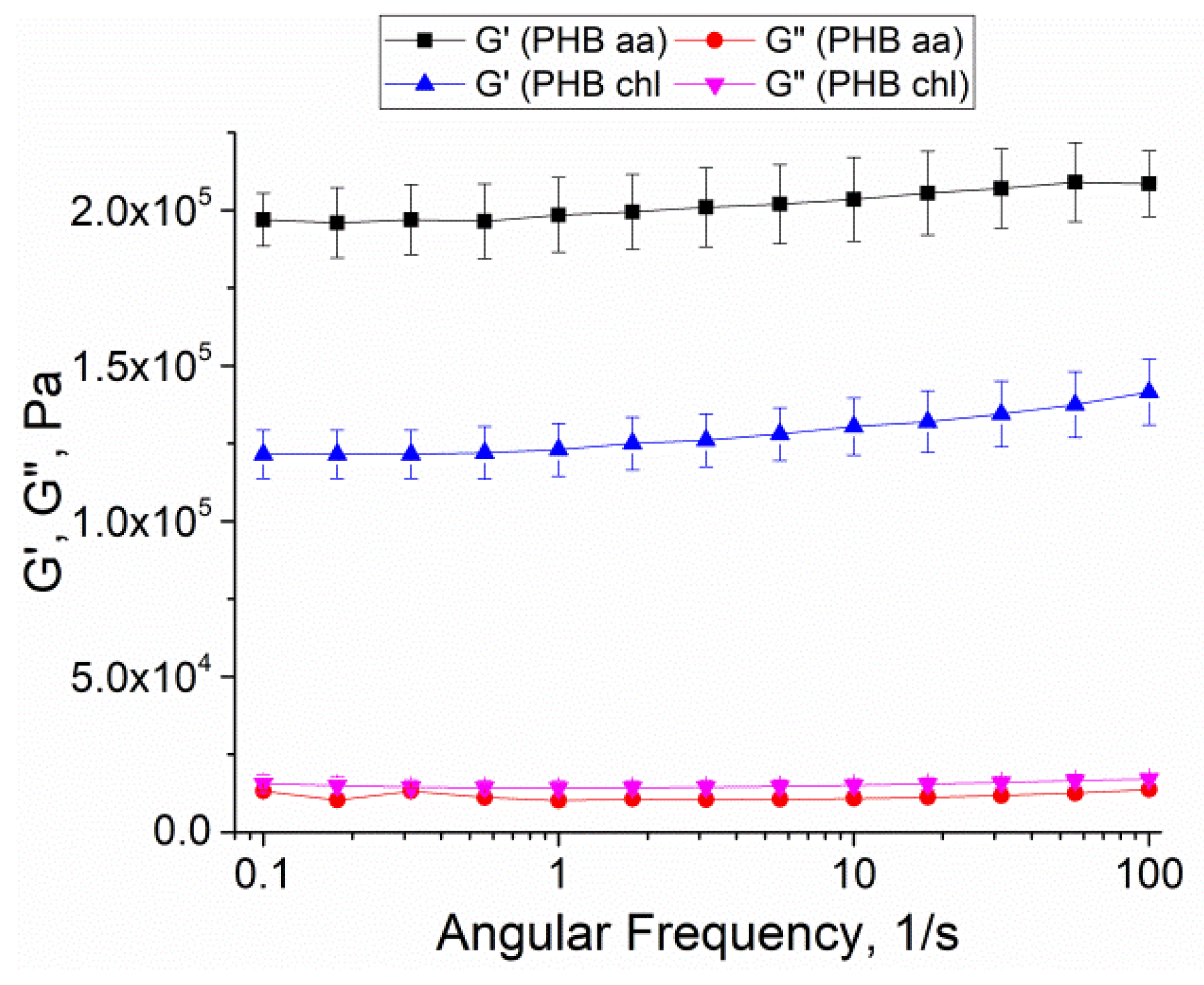

| Sample | Porosity, % | Density (d, g/cm3) | Swelling Ratio, % |
|---|---|---|---|
| PHB aa | 97.7 ± 0.9 | 0.040 ± 0.009 | 200 ± 45 |
| PHB chl | 92.5 ± 1.0 | 0.077 ± 0.010 | 125 ± 10 |
| Sample | Complex Shear Modulus (G*), kPa | Young’s Modulus, kPa |
|---|---|---|
| PHB aa | 198 ± 12 * | 101.5 ± 0.6 ** |
| PHB chl | 126 ± 8 | 41.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuikov, V.; Zhuikova, Y. The Effect of Acetic Acid as a Solvent on the Structure and Properties of Poly(3-hydroxybutyrate)—Based Dried Gels. Gels 2024, 10, 664. https://doi.org/10.3390/gels10100664
Zhuikov V, Zhuikova Y. The Effect of Acetic Acid as a Solvent on the Structure and Properties of Poly(3-hydroxybutyrate)—Based Dried Gels. Gels. 2024; 10(10):664. https://doi.org/10.3390/gels10100664
Chicago/Turabian StyleZhuikov, Vsevolod, and Yulia Zhuikova. 2024. "The Effect of Acetic Acid as a Solvent on the Structure and Properties of Poly(3-hydroxybutyrate)—Based Dried Gels" Gels 10, no. 10: 664. https://doi.org/10.3390/gels10100664
APA StyleZhuikov, V., & Zhuikova, Y. (2024). The Effect of Acetic Acid as a Solvent on the Structure and Properties of Poly(3-hydroxybutyrate)—Based Dried Gels. Gels, 10(10), 664. https://doi.org/10.3390/gels10100664







