Sol–Gel Synthesis of TiO2 with Pectin and Their Efficiency in Solar Cells Sensitized by Quantum Dots
Abstract
:1. Introduction
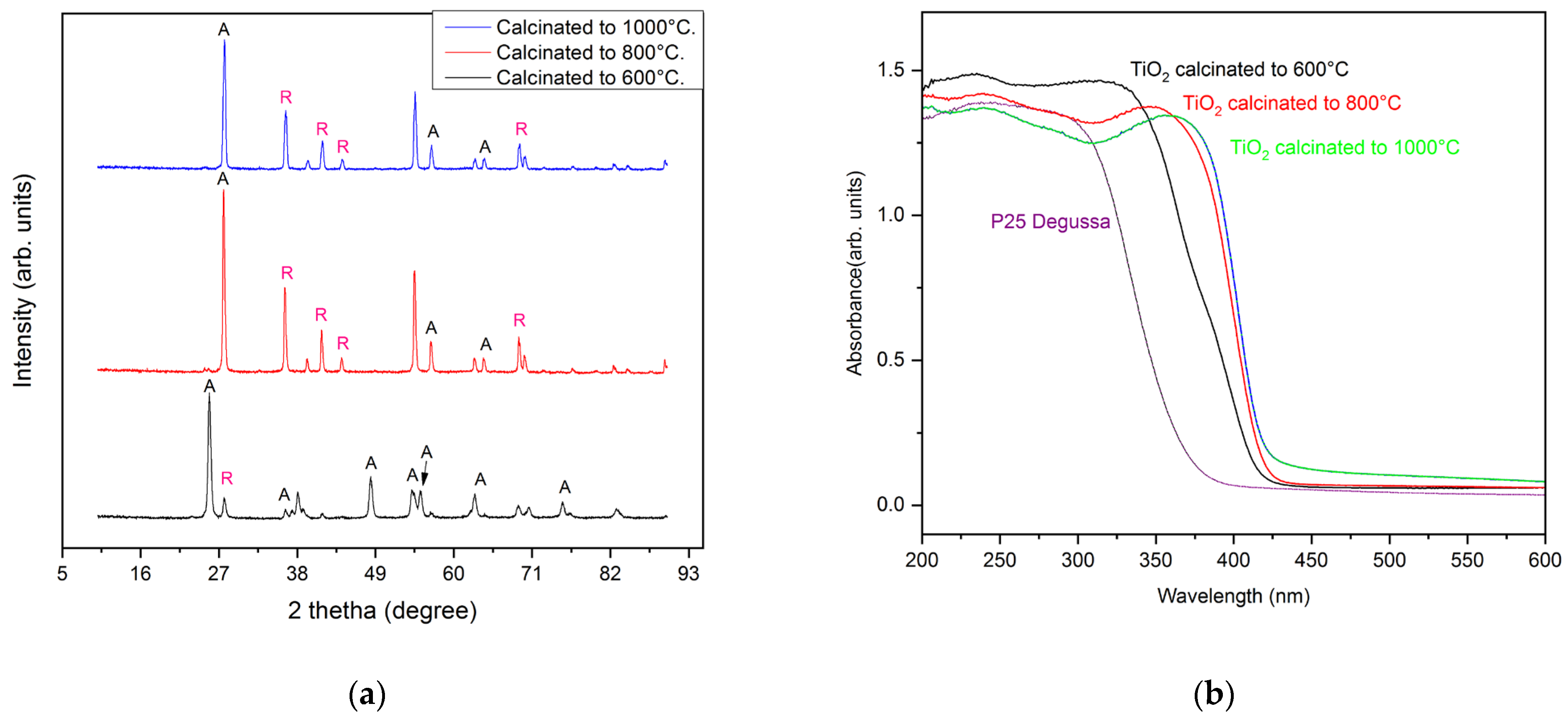
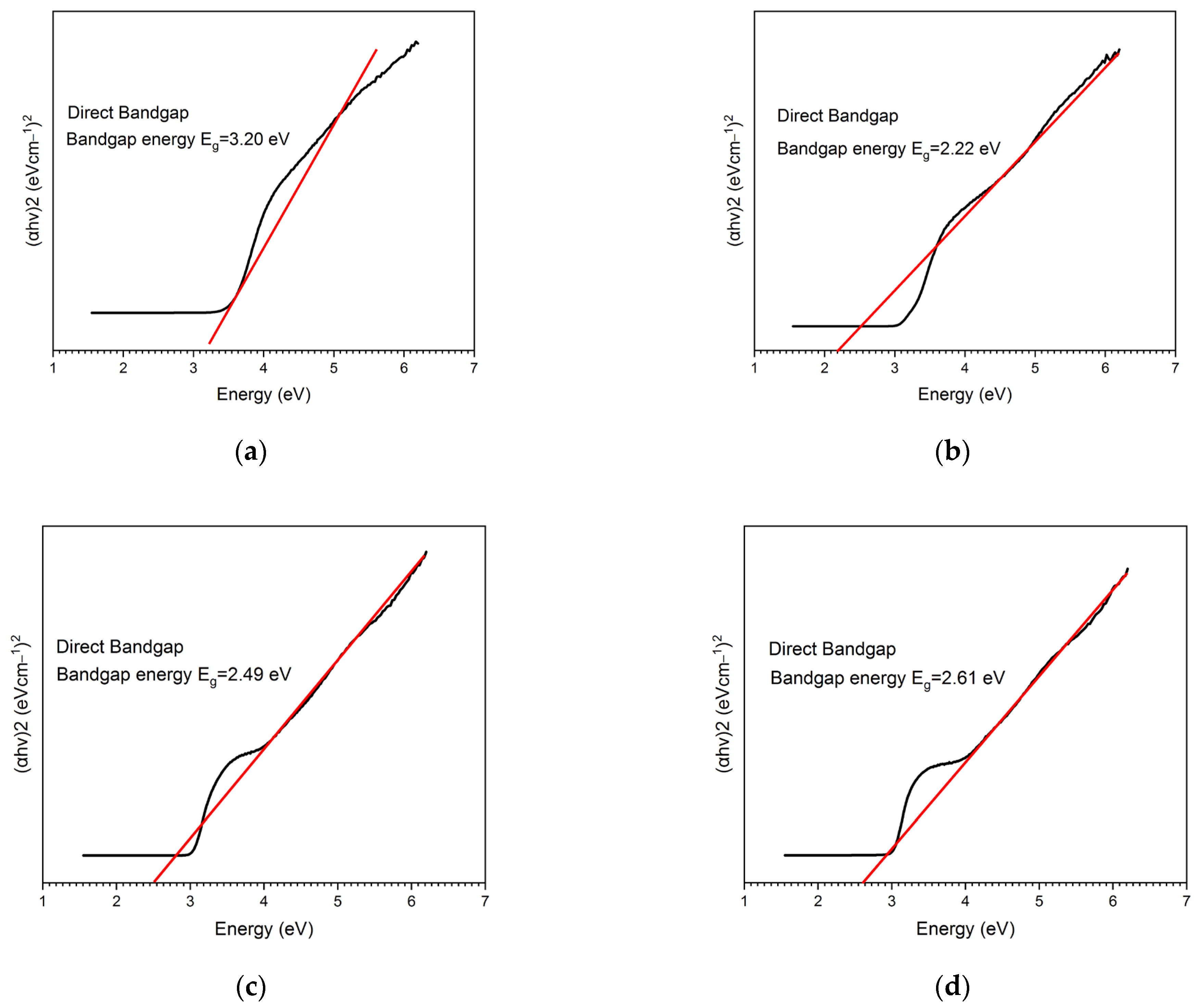
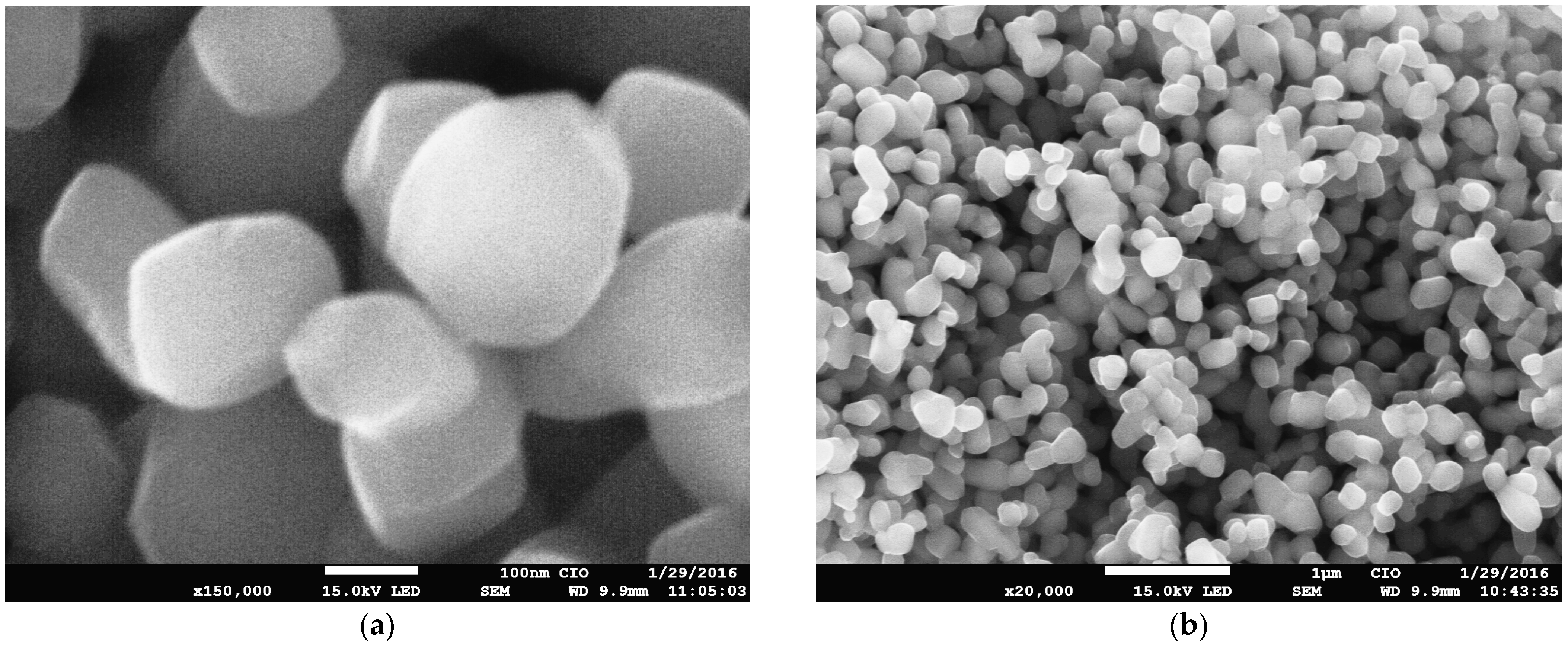
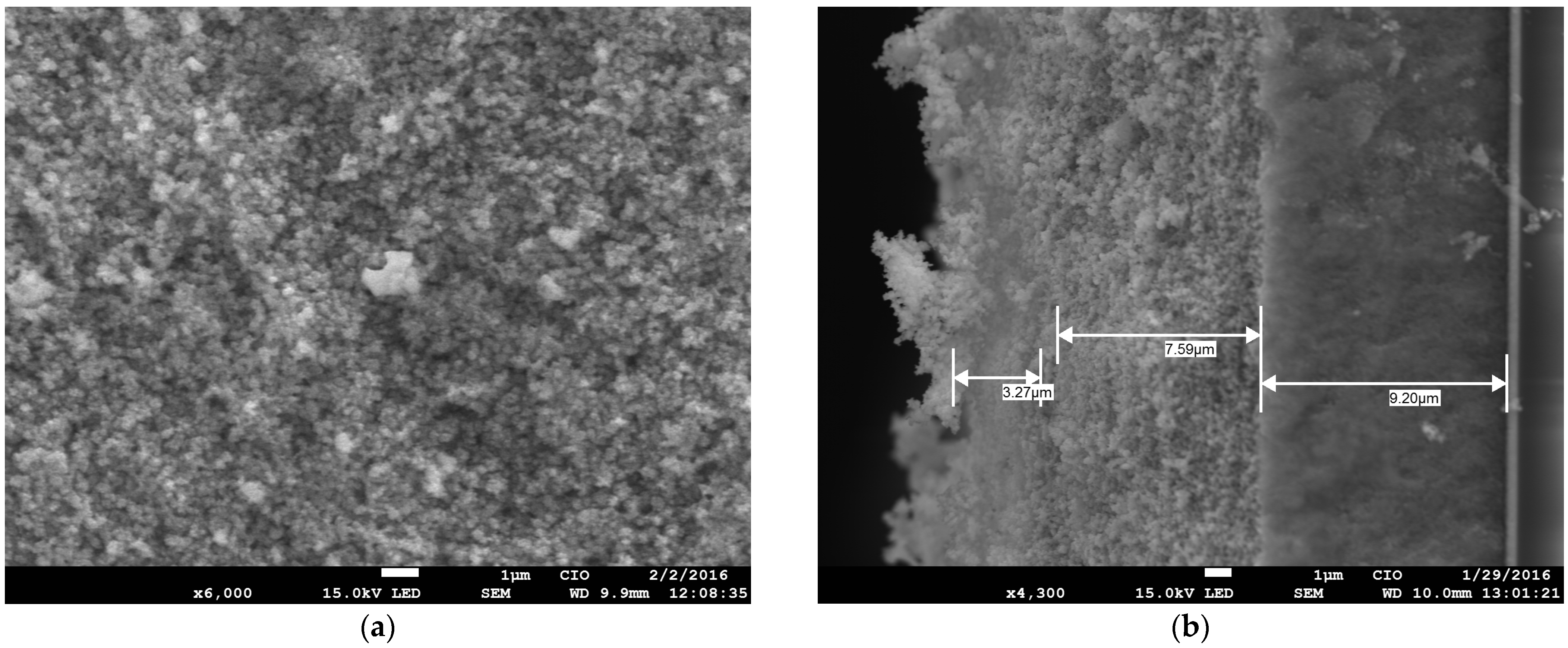
2. Results and Discussion
2.1. Characterization of the Sol–Gel Synthesis of TiO2 with Pectin
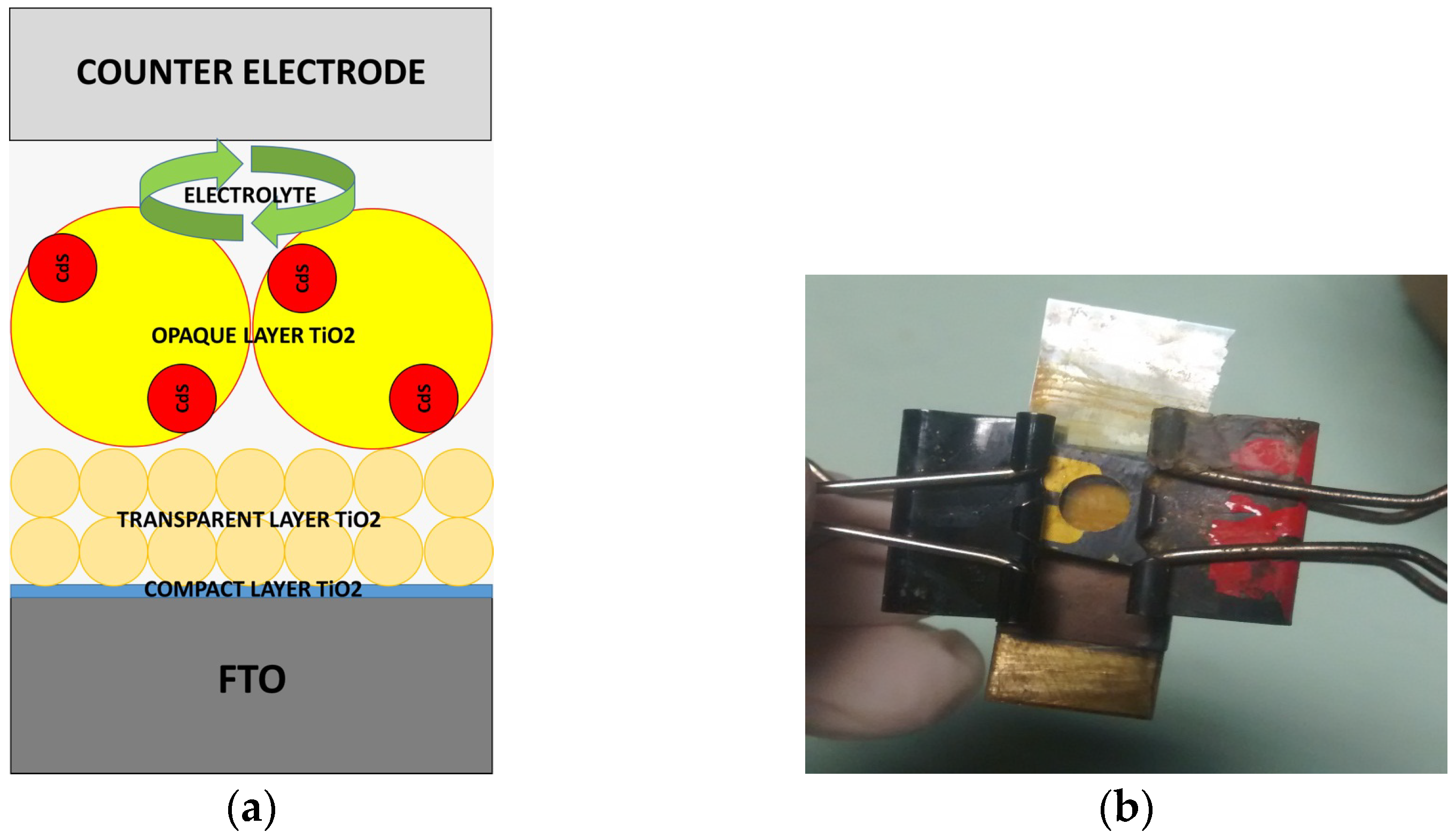
Characterization of the Performance of a DSSC Cell with the Pectin-Based Paste
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sol–Gel Synthesis of TiO2 with Pectin
4.3. Formulation of the Pectin-Based Paste
4.4. Fabrication of Photoelectrode Using Pectin Synthesized TiO2 NPs
4.4.1. Sensitization with Quantum Dots
4.4.2. Fabrication of Counter Electrode
4.5. Cell Configuration Assembly
4.6. Characterization of the Sol–Gel Synthesis of TiO2 with Pectin
4.7. Characterization of a DSSC Cell with the Synthesis of TiO2
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García Gutiérrez, D.I.; Navarro Garza, M.A.; Peláez Cienfuegos, R.F.; Guerrero Chávez, L. Aplicaciones de La Nanotecnología En Fuentes Alternas de Energía. Ingenierias 2010, 13, 53–62. [Google Scholar]
- Matsumoto, Y.; Valdés, M.; Urbano, J.A.; Kobayashi, T.; López, G.; Peña, R. Global Solar Irradiation in North Mexico City and Some Comparisons with the South. Energy Procedia 2014, 57, 1179–1188. [Google Scholar] [CrossRef]
- Perea-Moreno, A.J.; Hernandez-Escobedo, Q.; Garrido, J.; Verdugo-Diaz, J.D. Stand-Alone Photovoltaic System Assessment in Warmer Urban Areas in Mexico. Energies 2018, 11, 284. [Google Scholar] [CrossRef]
- Apeh, O.O.; Meyer, E.L.; Overen, O.K. Contributions of Solar Photovoltaic Systems to Environmental and Socioeconomic Aspects of National Development—A Review. Energies 2022, 15, 5963. [Google Scholar] [CrossRef]
- Jun, H.K.; Careem, M.A.; Arof, A.K. Quantum Dot-Sensitized Solar Cells-Perspective and Recent Developments: A Review of Cd Chalcogenide Quantum Dots as Sensitizers. Renew. Sustain. Energy Rev. 2013, 22, 148–167. [Google Scholar] [CrossRef]
- Sasani Ghamsari, M.; Bahramian, A.R. High Transparent Sol-Gel Derived Nanostructured TiO2 Thin Film. Mater. Lett. 2008, 62, 361–364. [Google Scholar] [CrossRef]
- Qian, X.; Qin, D.; Bai, Y.; Li, T.; Tang, X.; Wang, E.; Dong, S. Photosensitization of TiO2 Nanoparticulate Thin Film Electrodes by CdS Nanoparticles. J. Solid State Electrochem. 2001, 5, 562–567. [Google Scholar] [CrossRef]
- Irwin, M.D.; Buchholz, D.B.; Hains, A.W.; Chang, R.P.H.; Marks, T.J. P-Type Semiconducting Nickel Oxide as an Efficiency-Enhancing Anode Interfacial Layer in Polymer Bulk-Heterojunction Solar Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 2783–2787. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737. [Google Scholar] [CrossRef]
- Nelson, J. Solar Cells by Self-Assembly? Sci. New Ser. 2001, 293, 1059–1060. [Google Scholar] [CrossRef]
- West, J.L.; Halas, N.J. Engineered Nanomaterials for Biophotonics Applications: Improving Sensing, Imaging, and Therapeutics. Annu. Rev. Biomed. Eng. 2003, 5, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.; Arkhipov, V. Thin Film Solar Cells Fabrication, Characterization and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780470091289. [Google Scholar]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, G. Hierarchically Structured Photoelectrodes for Dye-Sensitized Solar Cells. J. Mater. Chem. 2011, 21, 6769–6774. [Google Scholar] [CrossRef]
- Lu, J.G.; Chang, P.; Fan, Z. Quasi-One-Dimensional Metal Oxide Materials-Synthesis, Properties and Applications. Mater. Sci. Eng. R Rep. 2006, 52, 49–91. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, F. Organic Semiconductors for the New Millennium. Philos. Trans. R. Soc. London Ser. A Math. Phys. Eng. Sci. 2000, 358, 173–192. [Google Scholar] [CrossRef]
- Fall, A.; Ngom, I.; Bakayoko, M.; Sylla, N.F.; Elsayed Ahmed Mohamed, H.; Jadvi, K.; Kaviyarasu, K.; Ngom, B.D. Biosynthesis of TiO2 nanoparticles by Using Natural Extract of Citrus Sinensis. Mater. Today Proc. 2019, 36, 349–356. [Google Scholar] [CrossRef]
- Salomatina, E.V.; Loginova, A.S.; Ignatov, S.; Knyazev, A.V.; Spirina, I.V.; Smirnova, L.A. Structure and Catalytic Activity of Poly(Titanium Oxide) Doped by Gold Nanoparticles in Organic Polymeric Matrix. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1280–1291. [Google Scholar] [CrossRef]
- Chen, J.; Bai, F.Q.; Wang, J.; Hao, L.; Xie, Z.F.; Pan, Q.J.; Zhang, H.X. Theoretical Studies on Spectroscopic Properties of Ruthenium Sensitizers Absorbed to TiO2 Film Surface with Connection Mode for DSSC. Dye. Pigment. 2012, 94, 459–468. [Google Scholar] [CrossRef]
- Carbó Vela, P.; Rocha Rangel, E. Electrochemical Process in Solar Cells Sensibilized with a Natural Dye. Investig. Cienc. Univ. Autónoma Aguascalientes 2012, 8, 3–10. [Google Scholar]
- Rajendhiran, R.; Atchudan, R.; Palanisamy, J.; Balasankar, A.; Oh, T.H.; Deivasigamani, V.; Ramasundaram, S. Prickly Pear Fruit Extract: Capping Agent for the Sol–Gel Synthesis of Discrete Titanium Dioxide Nanoparticles and Sensitizer for Dye-Sensitized Solar Cell. Coatings 2023, 13, 579. [Google Scholar] [CrossRef]
- Qaid, S.M.H.; Ghaithan, H.M.; Bawazir, H.S.; Bin Ajaj, A.F.; AlHarbi, K.K.; Aldwayyan, A.S. Successful Growth of TiO2 Nanocrystals with {001} Facets for Solar Cells. Nanomaterials 2023, 13, 928. [Google Scholar] [CrossRef]
- Kumar, P.N.; Kolay, A.; Kumar, S.K.; Patra, P.; Aphale, A.; Srivastava, A.K.; Deepa, M. Counter Electrode Impact on Quantum Dot Solar Cell Efficiencies. ACS Appl. Mater. Interfaces 2016, 8, 27688–27700. [Google Scholar] [CrossRef] [PubMed]
- Tvrdy, K.; Frantsuzov, P.A.; Kamat, P.V. Photoinduced Electron Transfer from Semiconductor Quantum Dots to Metal Oxide Nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.J.; Paik, T.; Edley, M.E.; Baxter, J.B.; Murray, C.B. Enhanced Charge Transfer Kinetics of CdSe Quantum Dot-Sensitized Solar Cell by Inorganic Ligand Exchange Treatments. ACS Appl. Mater. Interfaces 2014, 6, 3721–3728. [Google Scholar] [CrossRef]
- Lokman, M.Q.; Shaban, S.; Shafie, S.; Ahmad, F.; Yahaya, H.; Harun, A.M.; Rahman, M.A.A. Improving Dye-Sensitized Solar Cells Photocurrent Performance Based on Titanium Dioxide Photoanode Thickness. J. Adv. Res. Appl. Sci. Eng. Technol. 2024, 34, 177–186. [Google Scholar] [CrossRef]
- Devasvaran, K.; Lim, V. Green Synthesis of Metallic Nanoparticles Using Pectin as a Reducing Agent: A Systematic Review of the Biological Activities. Pharm. Biol. 2021, 59, 494–503. [Google Scholar] [CrossRef]
- Miu, B.A.; Stan, M.S.; Mernea, M.; Dinischiotu, A.; Voinea, I.C. Pure Epigallocatechin-3-Gallate-Assisted Green Synthesis of Highly Stable Titanium Dioxide Nanoparticles. Materials 2024, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Kashale, A.A.; Gattu, K.P.; Ghule, K.; Ingole, V.H.; Dhanayat, S.; Sharma, R.; Chang, J.Y.; Ghule, A.V. Biomediated Green Synthesis of TiO2 Nanoparticles for Lithium Ion Battery Application. Compos. Part B Eng. 2016, 99, 297–304. [Google Scholar] [CrossRef]
- Rao, K.G.; Ashok, C.H.; Rao, K.V.; Chakra, C.H.S.; Akshaykranth, A. Eco-Friendly Synthesis of MgO Nanoparticles from Orange Fruit Waste. Int. J. Adv. Res. Phys. 2015, 2, 1–6. [Google Scholar]
- Gemeiner, P.; Mikula, M. Efficiency of Dye Sensitized Solar Cells with Various Compositions of TiO2 Based Screen Printed Photoactive Electrodes. Acta Chim. Slovaca 2013, 6, 29–34. [Google Scholar] [CrossRef]
- Opara Krašovec, U.; Berginc, M.; Hočevar, M.; Topič, M. Unique TiO2 Paste for High Efficiency Dye-Sensitized Solar Cells. Sol. Energy Mater. Sol. Cells 2009, 93, 379–381. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Mohammad, K.N.; Liska, P.; Péchy, P.; Grätzel, M. Fabrication of Screen-Printing Pastes from TiO2 Powders for Dye-Sensitised Solar Cells. Prog. Photovolt. Res. Appl. 2007, 15, 603–612. [Google Scholar] [CrossRef]
- Ito, S.; Murakami, T.N.; Comte, P.; Liska, P.; Grätzel, C.; Nazeeruddin, M.K.; Grätzel, M. Fabrication of Thin Film Dye Sensitized Solar Cells with Solar to Electric Power Conversion Efficiency over 10%. Thin Solid Films 2008, 516, 4613–4619. [Google Scholar] [CrossRef]
- Hočevar, M.; Opara Krašovec, U.; Berginc, M.; Dražič, G.; Hauptman, N.; Topič, M. Development of TiO2 Pastes Modified with Pechini Sol-Gel Method for High Efficiency Dye-Sensitized Solar Cell. J. Sol-Gel Sci. Technol. 2008, 48, 156–162. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xia, X.H.; Tu, J.P.; Mai, Y.J.; Shi, S.J.; Wang, X.L.; Gu, C.D. Self-Assembled Synthesis of Hierarchically Porous NiO Film and Its Application for Electrochemical Capacitors. J. Power Sources 2012, 199, 413–417. [Google Scholar] [CrossRef]
- Zukalova, M.; Zukal, A.; Kavan, L.; Nazeeruddin, M.K.; Liska, P.; Grätzel, M. Organized Mesoporous TiO2 Films Exhibiting Greatly Enhanced Performance in Dye-Sensitized Solar Cells. Nano Lett. 2005, 5, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Sosa, R. Preparación y Caracterización de Películas de TiO2 Sensibilizadas Con CdS Para Aplicaciones en Celdas Solares; Instituto Politecnico Nacional: Mexico City, Mexico, 2014. [Google Scholar]
- Lewis, N.S. Toward Solar Cost-Effective Energy Use. Am. Assoc. Adv. Sci. 2007, 315, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Sankapal, B.R.; Mane, R.S.; Lokhande, C.D. Successive Ionic Layer Adsorption and Reaction (SILAR) Method for the Deposition of Large Area (Approximately 10 cm2) Tin Disulfide (SnS2) Thin Films. Mater. Res. Bull. 2000, 35, 2027–2035. [Google Scholar] [CrossRef]
- Hernadez-Enríquez, J.; García-Alamilla, R.; Zereño-Resendiz, B.; Del Angel-Sánchez, T.; Cuento-Hérnandez, A. Síntesis y Caracterización de Nanopartículas de N-TiO2—Anatasa. Superf. Vacío 2008, 21, 1–5. [Google Scholar]
- Ito, S.; Takahashi, K.; Yusa, S.I.; Imamura, T.; Tanimoto, K. Effects of Homogenization Scheme of TiO2 Screen-Printing Paste for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2012, 2012, 405642. [Google Scholar] [CrossRef]
- Hu, Y.; Tsai, H.L.; Huang, C.L. Phase Transformation of Precipitated TiO2 Nanoparticles. Mater. Sci. Eng. 2003, 344, 209–214. [Google Scholar] [CrossRef]
- Wagemaker, M.; Borghols, W.J.H.; Mulder, F.M. Large Impact of Particle Size on Insertion Reactions. A Case for Anatase LixTiO2. J. Am. Chem. Soc. 2007, 129, 4323–4327. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.O.V.; Cruz, O.E.T.; Rojas, J.M.P.; Tarmeño, E.I.C.; Kiyán, J.A.A.; Gutierrez, H.O.C.; Gutierrez, M.M.C. Caracterización Estructural y Óptica de Películas Delgadas de Dióxido de Titanio Depositadas Por Rociado Pirolítico. S. Florida J. Dev. 2021, 2, 2141–2155. [Google Scholar] [CrossRef]
- Kuriechen, S.K.; Murugesan, S.; Paul Raj, S. Mineralization of Azo Dye Using Combined Photo-Fenton and Photocatalytic Processes under Visible Light. J. Catal. 2013, 2013, 104019. [Google Scholar] [CrossRef]
- Allende, P.; Barrientos, L.; Orera, A.; Laguna-Bercero, M.A.; Salazar, N.; Valenzuela, M.L.; Diaz, C. TiO2/SiO2 Composite for Efficient Protection of UVA and UVB Rays Through of a Solvent-Less Synthesis. J. Clust. Sci. 2019, 30, 1511–1517. [Google Scholar] [CrossRef]
- Alsaad, A.; Al Dairy, A.R.; Ahmad, A.; Qattan, I.A.; Al Fawares, S.; Al-Bataineh, Q. Synthesis and Characterization of Polymeric (PMMA-PVA) Hybrid Thin Films Doped with TiO2 Nanoparticles Using Dip-Coating Technique. Crystals 2021, 11, 99. [Google Scholar] [CrossRef]
- Jubu, P.R.; Obaseki, O.S.; Yam, F.K.; Stephen, S.M.; Avaa, A.A.; McAsule, A.A.; Yusof, Y.; Otor, D.A. Influence of the Secondary Absorption and the Vertical Axis Scale of the Tauc’s Plot on Optical Bandgap Energy. J. Opt. 2023, 52, 1426–1435. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yu, J.; Fan, J.; Lv, K. Anatase TiO2 Nanosheets with Exposed (001) Facets: Improved Photoelectric Conversion Efficiency in Dye-Sensitized Solar Cells. Nanoscale 2010, 2, 2144–2149. [Google Scholar] [CrossRef]
- Ramachari, D.; Esparza, D.; López-Luke, T.; Romero, V.H.; Perez-Mayen, L.; De la Rosa, E.; Jayasankar, C.K. Synthesis of Co-Doped Yb3+-Er3+:ZrO2 upconversion Nanoparticles and Their Applications in Enhanced Photovoltaic Properties of Quantum Dot Sensitized Solar Cells. J. Alloys Compd. 2017, 698, 433–441. [Google Scholar] [CrossRef]
- Esparza, D.; Zarazúa, I.; López-Luke, T.; Carriles, R.; Torres-Castro, A.; Rosa, E.D. La Photovoltaic Properties of Bi2S3 and CdS Quantum Dot Sensitized TiO2 Solar Cells. Electrochim. Acta 2015, 180, 486–492. [Google Scholar] [CrossRef]
- Aboulouard, A.; Gultekin, B.; Can, M.; Erol, M.; Jouaiti, A.; Elhadadi, B.; Zafer, C.; Demic, S. Dye Sensitized Solar Cells Based on Titanium Dioxide Nanoparticles Synthesized by Flame Spray Pyrolysis and Hydrothermal Sol-Gel Methods: A Comparative Study on Photovoltaic Performances. J. Mater. Res. Technol. 2020, 9, 1569–1577. [Google Scholar] [CrossRef]
- Park, N.G.; Kim, K.M.; Kang, M.G.; Ryu, K.S.; Chang, S.H.; Shin, Y.J. Chemical Sintering of Nanoparticles: A Methodology for Low-Temperature Fabrication of Dye-Sensitized TiO2 Films. Adv. Mater. 2005, 17, 2349–2353. [Google Scholar] [CrossRef]
- May, C.D. Industrial Pectins: Sources, Production and Applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Alaya, Y.; Souissi, R.; Toumi, M.; Madani, M.; El Mir, L.; Bouguila, N.; Alaya, S. Annealing Effect on the Physical Properties of TiO2 Thin Films Deposited by Spray Pyrolysis. RSC Adv. 2023, 13, 21852–21860. [Google Scholar] [CrossRef]

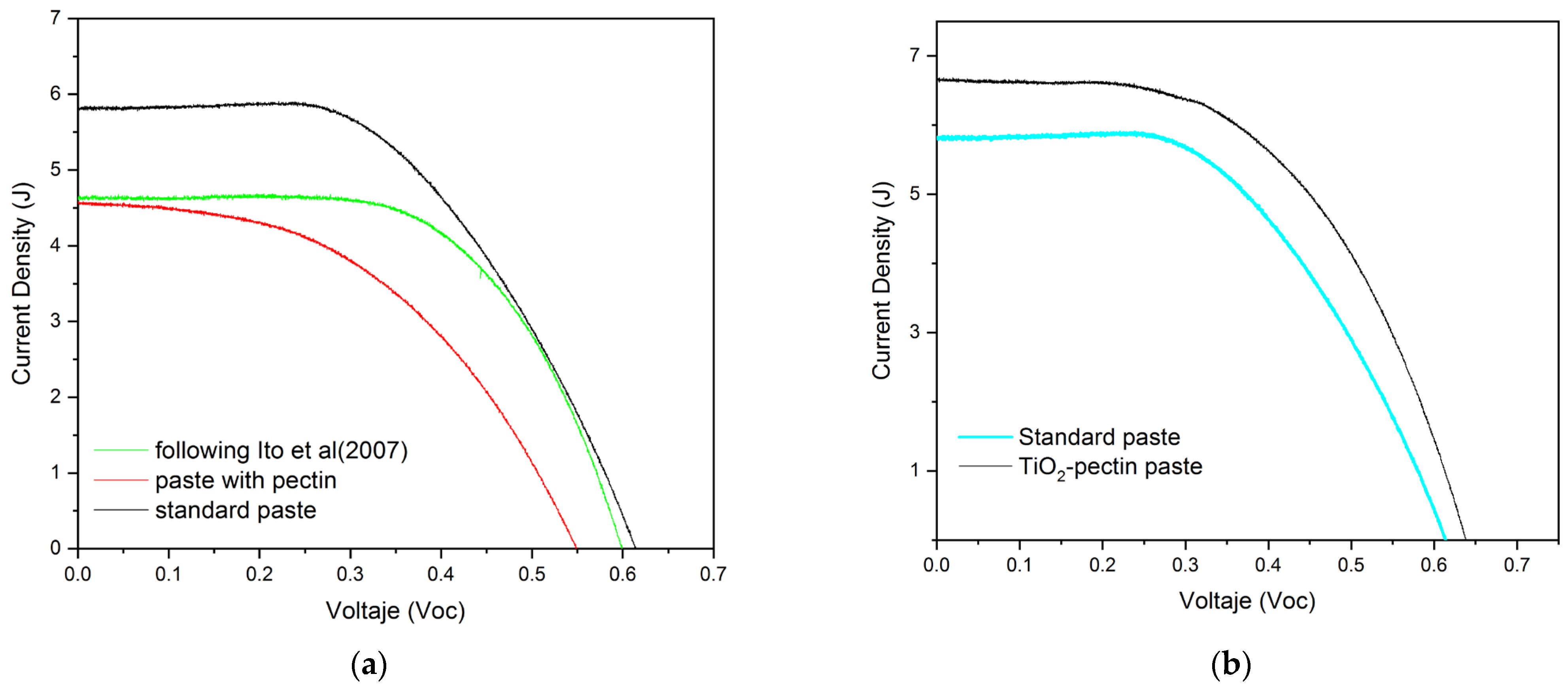
| Configuration | Voc (mV) | Jsc (mA/cm2) | FF (%) | (%) | Reference |
|---|---|---|---|---|---|
| TiO2/CdS/Cu2S | 8.3 | 474 | 41.3 | 1.56 | [53] |
| TiO2/CdS/ZnS/Cu2S | 7.2 | 504 | 52.9 | 1.94 | [53] |
| TiO2/CdS/ZnS/ZrO2 with Yb3þ/Er3þ/Cu2S | 7.1 | 593 | 68.7 | 3 | [53] |
| TiO2/Bi2s3/CdS/Zn/Cu2S | 9.3 | — | 53.7 | 2.52 | [54] |
| TiO2/DB/N719/CC | 10.96 | — | 55 | 3.81 | [27] |
| FSPTiO2/DB/N719/PT | 5.25 | 720 | 64 | 2.44 | [55] |
| HTTiO2/DB/N719/PT | 13.46 | 660 | 44 | 3.94 | [55] |
| TiO2/COM/N719/PT | 19.56 | 720 | 54 | 7.67 | [55] |
| GSHTiO2/PPE/RB/CC | 3.22 | 660 | 55 | 1.16 | [22] |
| GSHTiO2/PPE/EY/CC | 2.85 | 640 | 48 | 0.8 | [22] |
| GSHTiO2/PPE/PP/CC | 3.49 | 660 | 61 | 1.4 | [22] |
| TiO2/DB/CdS/Cu2S | 12.56 | 723 | 31.7 | 2.88 | [22] |
| TiO2/CdS/ZnS/Cu2S | 5.7 | 610 | 54.1 | 1.88 | This article |
| TiO2/PNB/CdS/ZnS/Cu2S | 4.5 | 560 | 48.9 | 1.23 | This article |
| SGPNTiO2/PNB/CdS/ZnS/Cu2S | 6.5 | 640 | 54.7 | 2.27 | This article |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Gómez, J.; Mota-Macías, S.; Guerrero-Jiménez, J.P.; Romero-Arellano, V.H.; Morales-Rivera, J. Sol–Gel Synthesis of TiO2 with Pectin and Their Efficiency in Solar Cells Sensitized by Quantum Dots. Gels 2024, 10, 470. https://doi.org/10.3390/gels10070470
Flores-Gómez J, Mota-Macías S, Guerrero-Jiménez JP, Romero-Arellano VH, Morales-Rivera J. Sol–Gel Synthesis of TiO2 with Pectin and Their Efficiency in Solar Cells Sensitized by Quantum Dots. Gels. 2024; 10(7):470. https://doi.org/10.3390/gels10070470
Chicago/Turabian StyleFlores-Gómez, Jean, Silvia Mota-Macías, Juan P. Guerrero-Jiménez, Victor Hugo Romero-Arellano, and Juan Morales-Rivera. 2024. "Sol–Gel Synthesis of TiO2 with Pectin and Their Efficiency in Solar Cells Sensitized by Quantum Dots" Gels 10, no. 7: 470. https://doi.org/10.3390/gels10070470





