Lithium-Ion-Sieve Hydrogel Based on Aluminum Doping with High Stretchability, Strong Adsorption Capacity and Low Dissolution Loss
Abstract
1. Introduction
2. Results and Discussion
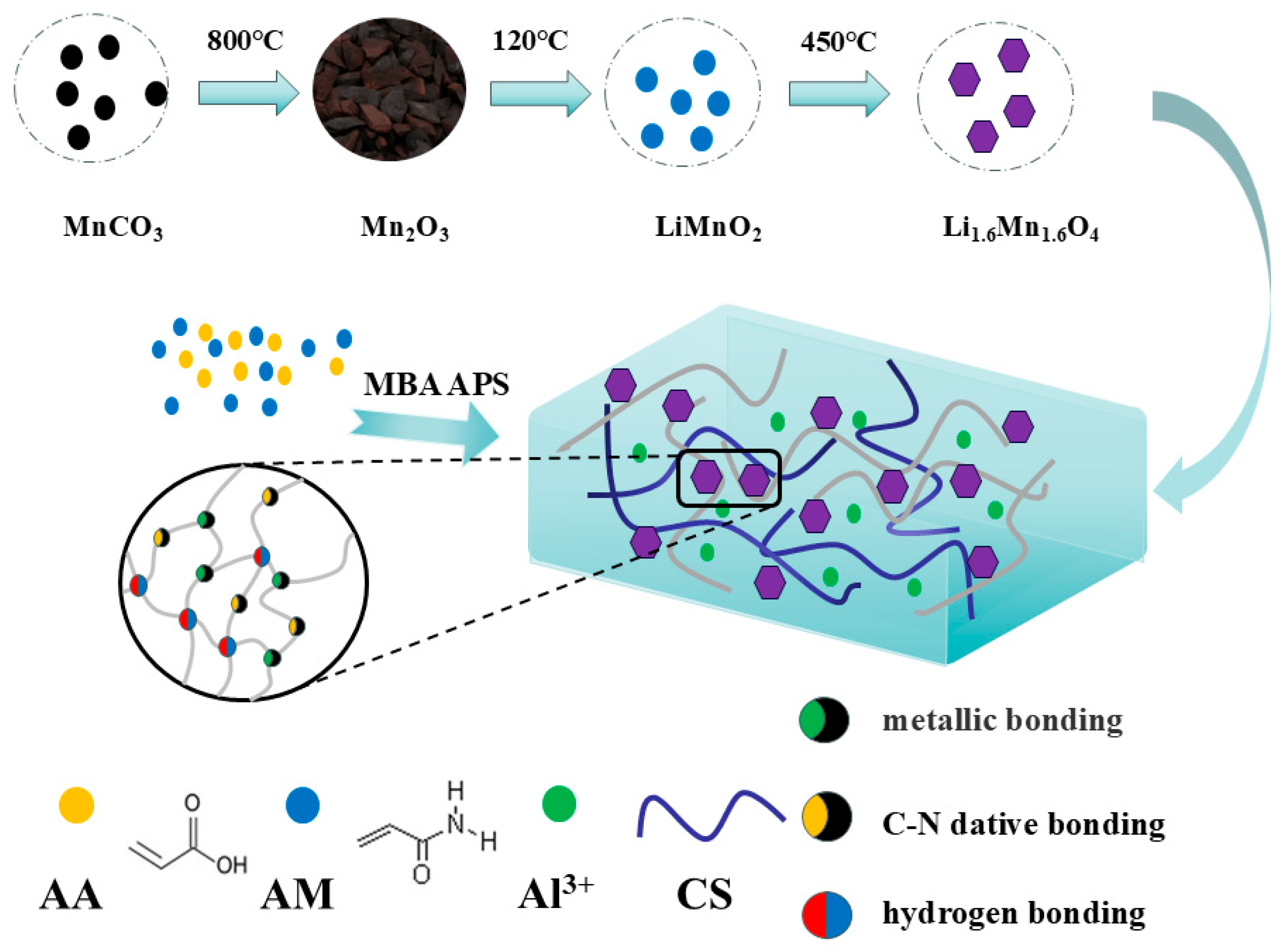
2.1. Material Characterization Results
2.2. Mechanical Properties of Hydrogel
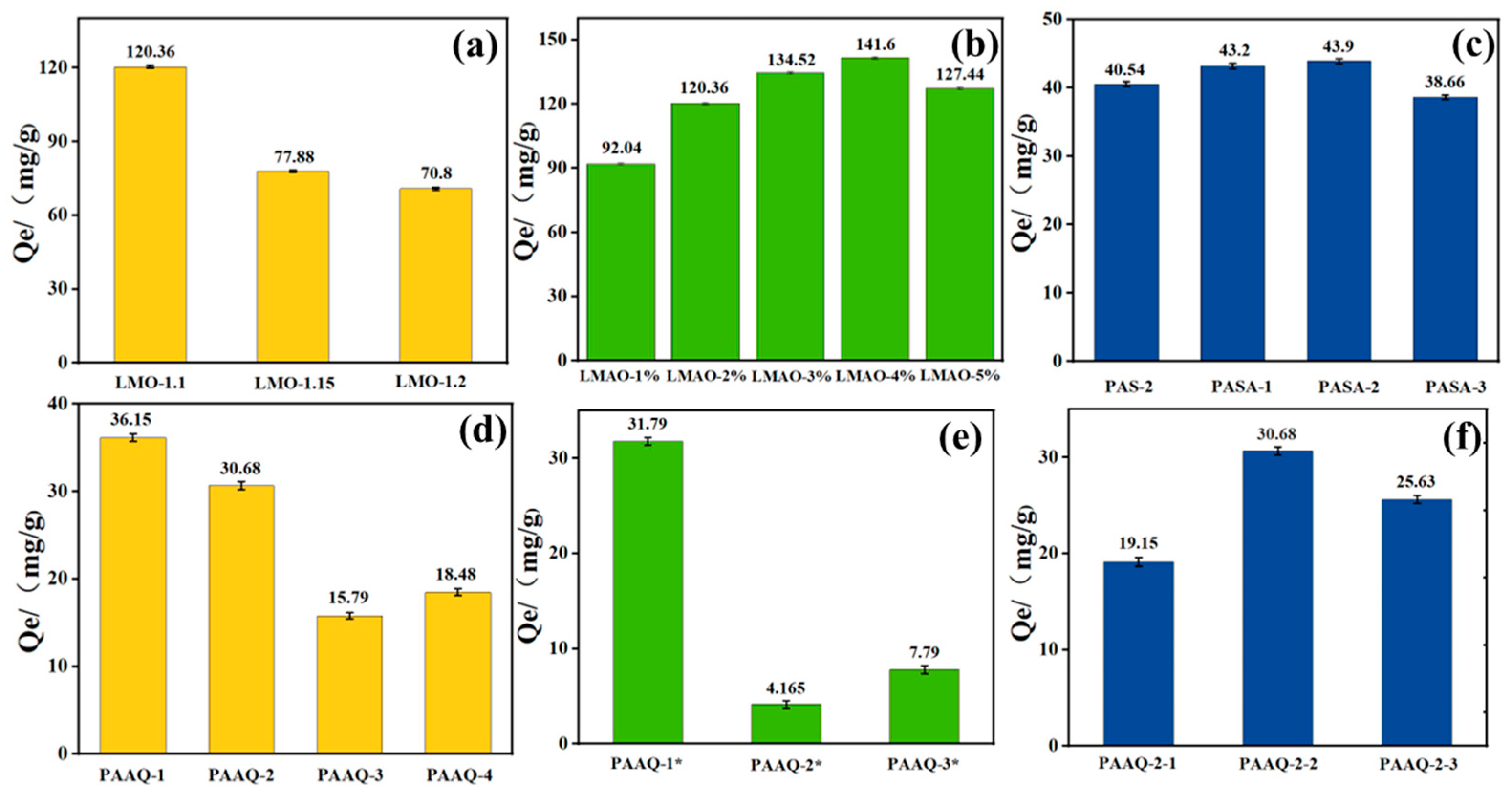
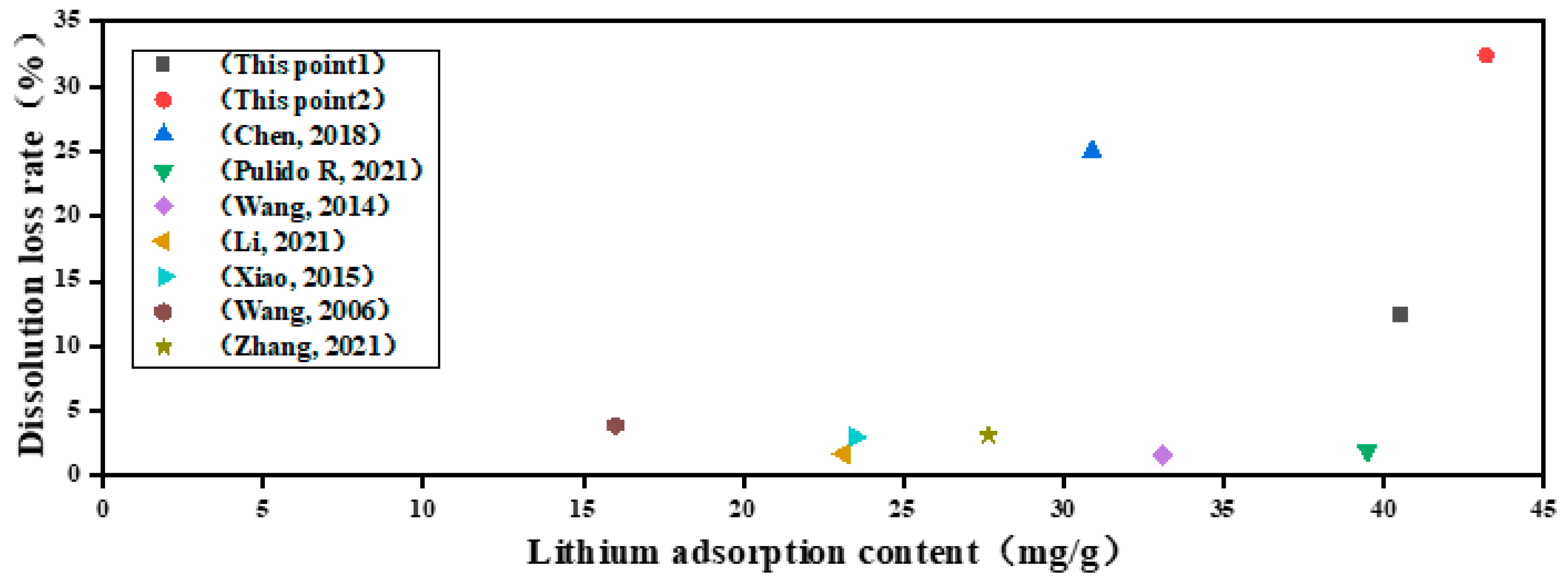
2.3. Adsorption Performance of Lithium Ions
3. Conclusions
4. Material and Methods
4.1. Material
4.2. Preparation of Lithium-Ion Sieve
4.2.1. Synthesis of Li1.6Mn1.6O4 Precursor
4.2.2. LIS Synthesis
4.3. Preparation of Hydrogel
4.4. Characterization of Material Characteristics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Swain, B. Recovery and Recycling of Lithium: A Review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Hamzaoui, A.H.; M’nif, A.; Hammi, H.; Rokbani, R. Contribution to the Lithium Recovery from Brine. Desalination Environ. Fresh Water All. 2003, 158, 221–224. [Google Scholar] [CrossRef]
- Kentish, S.E.; Stevens, G.W. Innovations in Separations Technology for the Recycling and Re-Use of Liquid Waste Streams. Environ. Chem. Eng. 2001, 84, 149–159. [Google Scholar] [CrossRef]
- Hien-Dinh, T.T.; Luong, V.T.; Gieré, R.; Tran, T. Extraction of Lithium from Lepidolite via Iron Sulphide Roasting and Water Leaching. Hydrometallurgy 2015, 153, 154–159. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, F.; Qin, W.; Fei, W. Coupled Reaction and Solvent Extraction Process to Form Li2CO3: Mechanism and Product Characterization. AIChE J. 2014, 60, 282–288. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-Based Technologies for Lithium Recovery from Water Lithium Resources: A Review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Qian, F.; Guo, M.; Qian, Z.; Li, Q.; Wu, Z.; Liu, Z. Highly Lithium Adsorption Capacities of H1.6Mn1.6O4 Ion-Sieve by Ordered Array Structure. ChemistrySelect 2019, 4, 10157–10163. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, M.; Chen, Y.; Zhang, T.; Yang, D.; Qiu, F. Li4Mn5O12 Doped Cellulose Acetate Membrane with Low Mn Loss and High Stability for Enhancing Lithium Extraction from Seawater. Desalination 2021, 506, 115003. [Google Scholar] [CrossRef]
- Alves, D.C.S.; Gonçalves, J.O.; Coseglio, B.B.; Burgo, T.A.L.; Dotto, G.L.; Pinto, L.A.A.; Cadaval, T.R.S. Adsorption of Phenol onto Chitosan Hydrogel Scaffold Modified with Carbon Nanotubes. J. Environ. Chem. Eng. 2019, 7, 103460. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; da Silva, K.A.; Rios, E.C.; Crispim, M.M.; Dotto, G.L.; de Almeida Pinto, L.A. Chitosan Hydrogel Scaffold Modified with Carbon Nanotubes and Its Application for Food Dyes Removal in Single and Binary Aqueous Systems. Int. J. Biol. Macromol. 2020, 142, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tao, B.; Jia, Q.; Guo, R. Preparation and Adsorption Performance of Multi-Morphology H1.6Mn1.6O4 for Lithium Extraction. Chin. J. Chem. Eng. 2021, 34, 68–76. [Google Scholar] [CrossRef]
- Zhang, G.; Hai, C.; Zhou, Y.; Tang, W.; Zhang, J.; Zeng, J.; Liu, Y.; Dong, S.; Peng, G. Al and F Ions Co-Modified Li1.6Mn1.6O4 with Obviously Enhanced Li+ Adsorption Performances. Chem. Eng. J. 2022, 450, 137912. [Google Scholar] [CrossRef]
- Luo, Q.; Dong, M.; Nie, G.; Liu, Z.; Wu, Z.; Li, J. Extraction of Lithium from Salt Lake Brines by Granulated Adsorbents. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127256. [Google Scholar] [CrossRef]
- Xue, F.; Zhang, X.; Niu, Y.; Yi, C.; Ju, S.; Xing, W. Preparation and Evaluation of α-Al2O3 Supported Lithium Ion Sieve Membranes for Li+ Extraction. Chin. J. Chem. Eng. 2020, 28, 2312–2318. [Google Scholar] [CrossRef]
- Nisola, G.M.; Limjuco, L.A.; Vivas, E.L.; Lawagon, C.P.; Park, M.J.; Shon, H.K.; Mittal, N.; Nah, I.W.; Kim, H.; Chung, W.-J. Macroporous Flexible Polyvinyl Alcohol Lithium Adsorbent Foam Composite Prepared via Surfactant Blending and Cryo-Desiccation. Chem. Eng. J. 2015, 280, 536–548. [Google Scholar] [CrossRef]
- Xue, F.; Wang, B.; Chen, M.; Yi, C.; Ju, S.; Xing, W. Fe3O4-Doped Lithium Ion-Sieves for Lithium Adsorption and Magnetic Separation. Sep. Purif. Technol. 2019, 228, 115750. [Google Scholar] [CrossRef]
- Cheng, M.; Yao, C.; Su, Y.; Liu, J.; Xu, L.; Hou, S. Synthesis of Membrane-Type Graphene Oxide Immobilized Manganese Dioxide Adsorbent and Its Adsorption Behavior for Lithium Ion. Chemosphere 2021, 279, 130487. [Google Scholar] [CrossRef]
- Xu, L.; Sun, P.; Jiang, X.; Chen, J.; Wang, J.; Zhang, H.; Zhu, W. Hierarchical Quasi Waxberry-like Ba5Si8O21 Microspheres: Facile Green Rotating Hydrothermal Synthesis, Formation Mechanism and High Adsorption Performance for Congo Red. Chem. Eng. J. 2020, 384, 123387. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Zhang, Y.; Zhang, Y.; Zheng, S. Lithium Adsorption from Brine by Iron-Doped Titanium Lithium Ion Sieves. Particuology 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A Promising Approach for Directly Extracting Lithium from α-Spodumene by Alkaline Digestion and Precipitation as Phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Ji, L.; Hu, Y.; Li, L.; Shi, D.; Li, J.; Nie, F.; Song, F.; Zeng, Z.; Sun, W.; Liu, Z. Lithium Extraction with a Synergistic System of Dioctyl Phthalate and Tributyl Phosphate in Kerosene and FeCl3. Hydrometallurgy 2016, 162, 71–78. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G.; Wang, J.; Cui, L.; Chen, Y. Recovery of Lithium from Alkaline Brine by Solvent Extraction with Functionalized Ionic Liquid. Fluid. Phase Equilibria 2019, 493, 129–136. [Google Scholar] [CrossRef]
- Halcrow, M.A. Jahn-Teller Distortions in Transition Metal Compounds, and Their Importance in Functional Molecular and Inorganic Materials. Chem. Soc. Rev. 2013, 42, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, R.; Ju, S.; Zhang, X.; Xue, F.; Xing, W. Improved Performance of Al-Doped LiMn2O4 Ion-Sieves for Li+ Adsorption. Microporous Mesoporous Mater. 2018, 261, 29–34. [Google Scholar] [CrossRef]
- Pulido, R.; Naveas, N.; Graber, T.A.; Martín-Palma, R.J.; Agulló-Rueda, F.; Brito, I.; Morales, C.; Soriano, L.; Pascual, L.; Marini, C.; et al. Hydrothermal Control of the Lithium-Rich Li2MnO3 Phase in Lithium Manganese Oxide Nanocomposites and Their Application as Precursors for Lithium Adsorbents. Dalton Trans. 2021, 50, 10765–10778. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Liu, R.; Li, H.Y.; Meng, C.G. Correlation between Li+ Adsorption Capacity and the Preparation Conditions of Spinel Lithium Manganese Precursor. Solid. State Ion. 2006, 177, 1421–1428. [Google Scholar] [CrossRef]
- Li, L.; Deshmane, V.G.; Paranthaman, M.P.; Bhave, R.; Moyer, B.A.; Harrison, S. Lithium Recovery from Aqueous Resources and Batteries: A Brief Review: A Review of the Methods to Produce Lithium and Approaches to Recycling from End-of-Life Lithium-Ion Batteries. Johns. Matthey Technol. Rev. 2018, 62, 161–176. [Google Scholar] [CrossRef]
- Xiao, J.; Nie, X.; Sun, S.; Song, X.; Li, P.; Yu, J. Lithium Ion Adsorption–Desorption Properties on Spinel Li4Mn5O12 and pH-Dependent Ion-Exchange Model. Adv. Powder Technol. 2015, 26, 589–594. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, Y.; Wang, X.; Zeng, M. Preparation and Characterization of Lithium λ-MnO2 Ion-Sieves. Front. Chem. Sci. Eng. 2014, 8, 471–477. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhou, Y.; Qi, G.; Zeng, J.; Sun, Y.; Shen, Y.; Li, X.; Ren, X.; Dong, S.; et al. Practical Synthesis of Manganese Oxide MnO2·0.5H2O for an Advanced and Applicable Lithium Ion-Sieve. J. Solid. State Chem. 2021, 293, 121768. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Guo, L.; Yan, C.; Li, L.; Cui, S.; Wang, Y. Lithium-Ion-Sieve Hydrogel Based on Aluminum Doping with High Stretchability, Strong Adsorption Capacity and Low Dissolution Loss. Gels 2024, 10, 710. https://doi.org/10.3390/gels10110710
Zhang Y, Wang Y, Guo L, Yan C, Li L, Cui S, Wang Y. Lithium-Ion-Sieve Hydrogel Based on Aluminum Doping with High Stretchability, Strong Adsorption Capacity and Low Dissolution Loss. Gels. 2024; 10(11):710. https://doi.org/10.3390/gels10110710
Chicago/Turabian StyleZhang, Yujie, Yang Wang, Le Guo, Chenzhengzhe Yan, Long Li, Shuyun Cui, and Yujie Wang. 2024. "Lithium-Ion-Sieve Hydrogel Based on Aluminum Doping with High Stretchability, Strong Adsorption Capacity and Low Dissolution Loss" Gels 10, no. 11: 710. https://doi.org/10.3390/gels10110710
APA StyleZhang, Y., Wang, Y., Guo, L., Yan, C., Li, L., Cui, S., & Wang, Y. (2024). Lithium-Ion-Sieve Hydrogel Based on Aluminum Doping with High Stretchability, Strong Adsorption Capacity and Low Dissolution Loss. Gels, 10(11), 710. https://doi.org/10.3390/gels10110710





