Effect of Carrot Callus Cells on the Mechanical, Rheological, and Sensory Properties of Hydrogels Based on Xanthan and Konjac Gums
Abstract
1. Introduction
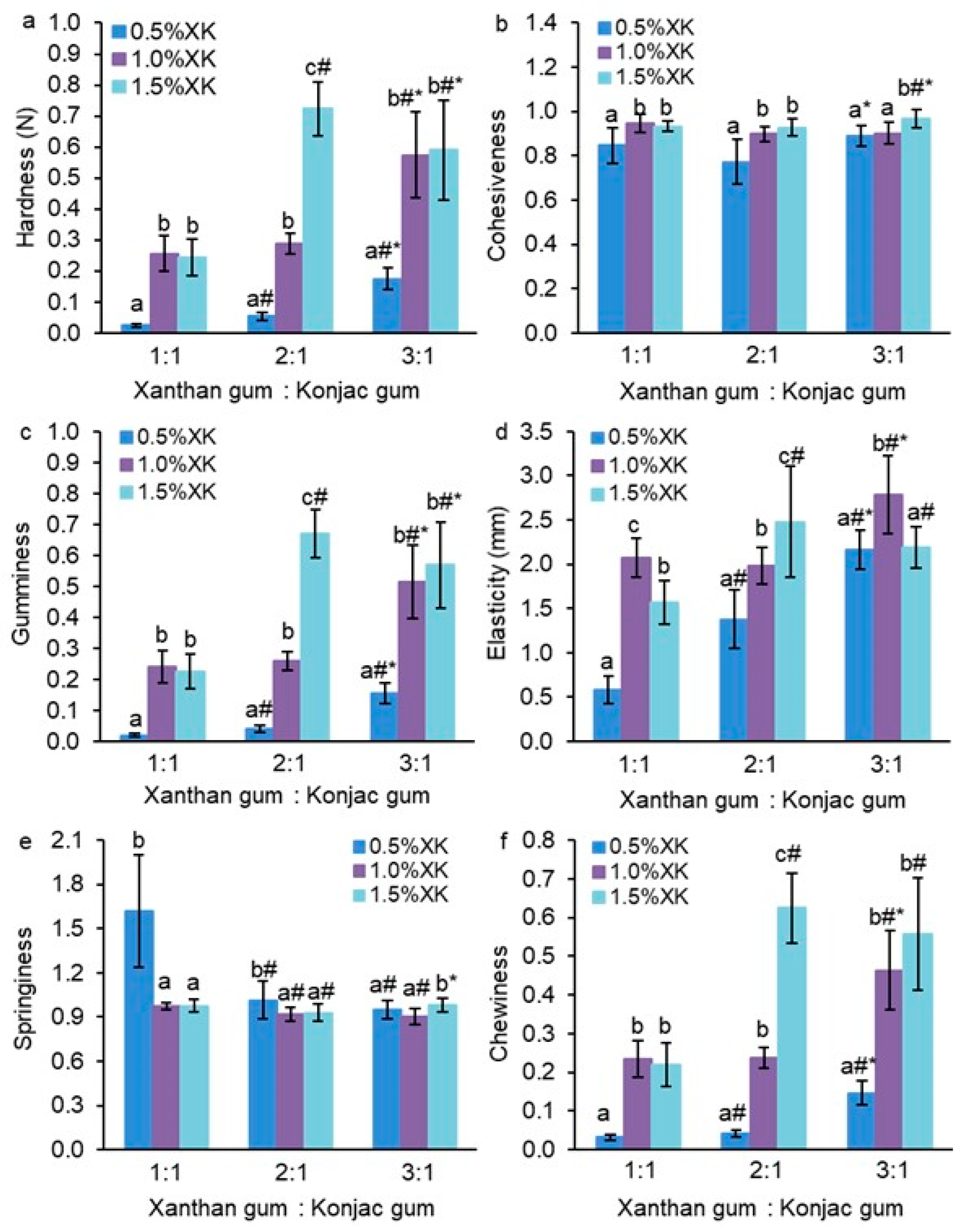
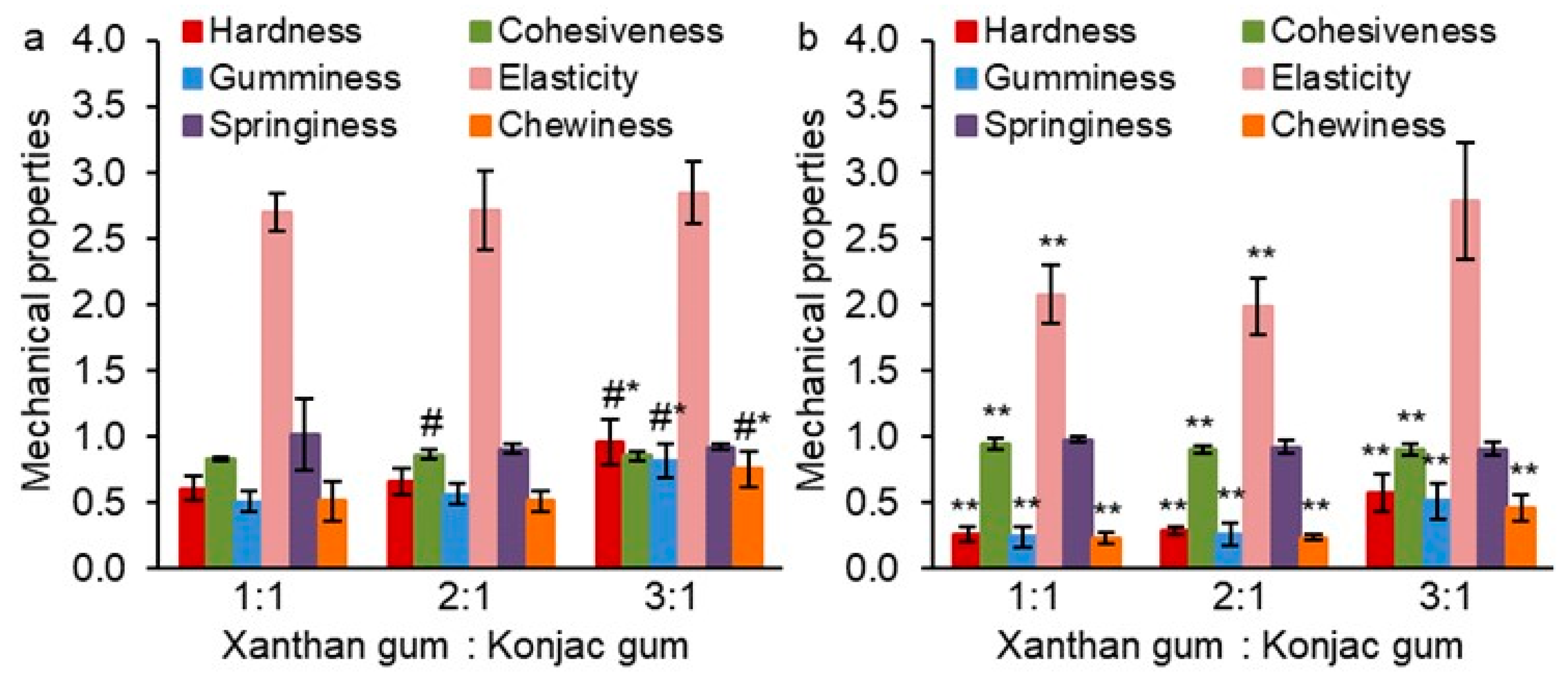
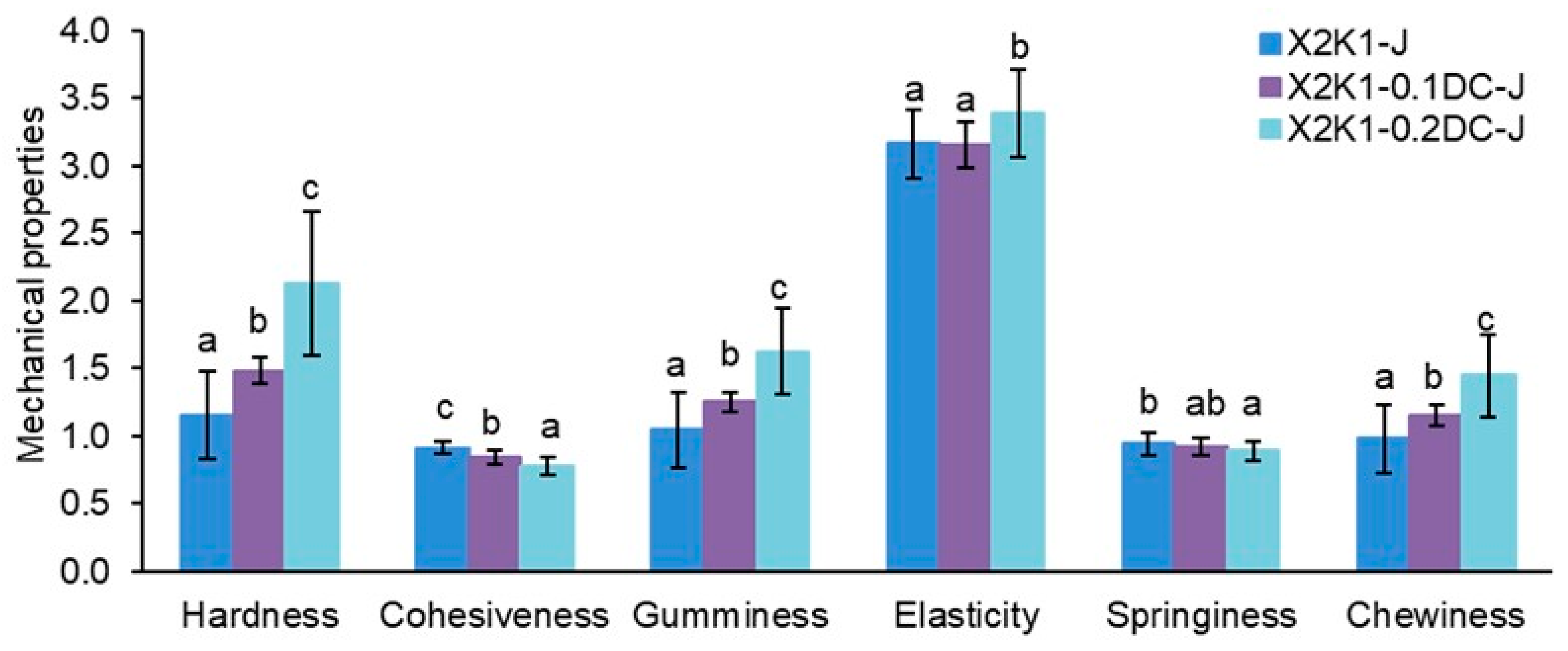
2. Results and Discussion
2.1. Mechanical Properties of Gum Hydrogels
2.2. Rheological Properties of Gum/Callus Hydrogels
2.3. Sensory Properties of Gum/Callus Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Callus Culture of Daucus Carota
4.3. Preparation of Gum/Callus Hydrogels
4.4. Mechanical Properties of Gum/Callus Hydrogels
4.5. Rheological Properties of Hydrogels
4.6. Sensory Properties of Gum/Callus Hydrogels
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yousefi, A.; Ako, K.; Hosseinzadeh, G.; Khodabakhshaghdam, S. Rheological properties of binary mixtures of Lepidium perfoliatum seed gum and xanthan gum. Chem. Biol. Technol. Agric. 2023, 10, 10–24. [Google Scholar] [CrossRef]
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in xanthan gum-based systems for the delivery of therapeutic agents. Pharmaceutics 2023, 15, 402. [Google Scholar] [CrossRef]
- Tu, W.; Shi, W.; Li, H.; Wang, Y.; Qiao, D.; Jiang, F.; Zhang, B. Xanthan gum inclusion optimizes the sol-gel and mechanical properties of agar/konjac glucomannan system for designing core-shell structural capsules. Food Hydrocoll. 2022, 122, 107101. [Google Scholar] [CrossRef]
- Paradossi, G.; Chiessi, E.; Barbiroli, A.; Fessas, D. Xanthan and glucomannan mixtures: Synergistic interactions and gelation. Biomacromolecules 2002, 3, 498–504. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Wang, Y.; Qi, C.; Ma, X.; Che, W.; Ma, K. Wetting–drying effects on the mechanical performance of xanthan gum biopolymer-stabilized soil. Environ. Earth Sci. 2024, 83, 197. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; MacNaughtan, W.; Sworn, G.; Foster, T.J. New insights into xanthan synergistic interactions with konjac glucomannan: A novel interaction mechanism proposal. Carbohydr. Polym. 2016, 144, 168–177. [Google Scholar] [CrossRef]
- Mirzaei, M.; Alimi, M.; Shokoohi, S.; Golchoobi, L. Synergistic interactions between konjac-mannan and xanthan/tragacanth gums in tomato ketchup: Physical, rheological and textural properties. J. Texture Stud. 2018, 49, 586–594. [Google Scholar] [CrossRef]
- Alves, A.; Miguel, S.P.; Araujo, A.R.; de Jesús Valle, M.J.; Sánchez Navarro, A.; Correia, I.J.; Ribeiro, M.P.; Coutinho, P. Xanthan gum–konjac glucomannan blend hydrogel for wound healing. Polymers 2020, 12, 99. [Google Scholar] [CrossRef]
- García-Segovia, P.; García-Alcaraz, V.; Balasch-Parisi, S.; Martínez-Monzó, J. 3D printing of gels based on xanthan/konjac gums. Innov. Food Sci. Emerg. Technol. 2020, 64, 102343. [Google Scholar] [CrossRef]
- Thakur, R.; Yadav, B.K.; Goyal, N. An insight into recent advancement in plant- and algae-based functional ingredients in 3D food printing ink formulations. Food Bioprocess Technol. 2023, 16, 1919–1942. [Google Scholar] [CrossRef]
- Xu, W.; Jia, Y.; Li, P.; Yue, M.; Miu, Z.; Yin, Y.; Luo, D. Sodium caseinate active film loaded clove essential oil based on konjac glucomannan and xanthan gum: Physical and structural properties. J. Food Meas. Charact. 2024, 18, 2065–2075. [Google Scholar] [CrossRef]
- Nordlund, E.; Lille, M.; Silventoinen, P.; Nygren, H.; Seppänen-Laakso, T.; Mikkelson, A.; Aura, A.-M.; Heiniö, R.-L.; Nohynek, L.; Puupponen-Pimiä, R.; et al. Plant cells as food—A concept taking shape. Food Res. Int. 2018, 107, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Seidel, J.; Ahlfeld, T.; Adolph, M.; Kümmritz, S.; Steingroewer, J.; Krujatz, F.; Bley, T.; Gelinsky, M.; Lode, A. Green bioprinting: Extrusion-based fabrication of plant cell-laden biopolymer hydrogel scaffolds. Biofabrication 2017, 9, 045011–045023. [Google Scholar] [CrossRef]
- Vancauwenberghe, V.; Baiye Mfortaw Mbong, V.; Vanstreels, E.; Verboven, P.; Lammertyn, J.; Nicolai, B. 3D printing of plant tissue for innovative food manufacturing: Encapsulation of alive plant cells into pectin based bio-ink. J. Food Eng. 2019, 263, 454–464. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, H.W.; Park, H.J. Callus-based 3D printing for food exemplified with carrot tissues and its potential for innovative food production. J. Food Eng. 2020, 271, 109781–109788. [Google Scholar] [CrossRef]
- Varma, A.; Gemeda, H.B.; McNulty, M.J.; McDonald, K.A.; Nandi, S.; Knipe, J.M. Immobilization of transgenic plant cells towards bioprinting for production of a recombinant biodefense agent. Biotechnol. J. 2021, 16, 2100133. [Google Scholar] [CrossRef]
- Landerneau, S.; Lemarié, L.; Marquette, C.; Petiot, E. Green 3D bioprinting of plant cells: A new scope for 3D bioprinting. Bioprinting 2022, 27, e00216. [Google Scholar] [CrossRef]
- Belova, K.; Dushina, E.; Popov, S.; Zlobin, A.; Martinson, E.; Vityazev, F.; Litvinets, S. Enrichment of 3D-printed κ-carrageenan food gel with callus tissue of narrow-leaved lupin Lupinus angustifolius. Gels 2023, 9, 45. [Google Scholar] [CrossRef]
- Dushina, E.; Popov, S.; Zlobin, A.; Martinson, E.; Paderin, N.; Vityazev, F.; Belova, K.; Litvinets, S. Effect of homogenized callus tissue on the rheological and mechanical properties of 3D-printed food. Gels 2024, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Smirnov, V.; Khramova, D.; Paderin, N.; Chistiakova, E.; Ptashkin, D.; Vityazev, F. Effect of Hogweed Pectin on Rheological, Mechanical, and Sensory Properties of Apple Pectin Hydrogel. Gels 2023, 9, 225. [Google Scholar] [CrossRef]
- Günter, E.; Popeyko, O.; Vityazev, F.; Popov, S. Effect of callus cell immobilization on the textural and rheological properties, loading and releasing of grape seed extract from pectin hydrogels. Gels 2024, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Nakauma, M. Instrumental food texture evaluation in relation to human perception. Food Hydrocoll. 2022, 124, 107253. [Google Scholar] [CrossRef]
- Appleton, K.M.; Newbury, A.; Almiron-Roig, E.; Yeomans, M.R.; Brunstrom, J.M.; de Graaf, K.; Geurts, L.; Kildegaard, H.; Vinoy, S. Sensory and physical characteristics of foods that impact food intake without affecting acceptability: Systematic review and meta-analyses. Obes. Rev. 2021, 8, e13234. [Google Scholar] [CrossRef]
- Hayakawa, F. Vocabularies and terminologies of food texture description and characterization. In Modifying Food Texture Volume 2: Sensory Analysis, Consumer Requirements and Preferences; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2015; pp. 3–18. [Google Scholar]
- Wen, M.; Ni, X.; Xiao, W.; Li, Y.; Gao, Z. Influence of dispersion media on the rheology and oral tribology of the konjac glucomannan/xanthan gum thickener. J. Food Meas. Charact. 2024, 18, 4472–4483. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, D.; Guo, Q.; Liu, C. Textural and structural properties of a κ-carrageenan–konjac gum mixed gel: Effects of κ-carrageenan concentration, mixing ratio, sucrose and Ca2+ concentrations and its application in milk pudding. J. Sci. Food Agric. 2021, 101, 3021–3029. [Google Scholar] [CrossRef]
- Shomer, I.; Novacky, A.J.; Pike, S.M.; Yermiyahu, U.; Kinraide, T.B. Electrical potentials of plant cell walls in response to the ionic environment. Plant Physiol. 2003, 133, 411–422. [Google Scholar] [CrossRef]
- Harding, S.E.; Smith, I.H.; Lawson, C.J.; Gahler, R.J.; Wood, S. Studies on macromolecular interactions in ternary mixtures of konjac glucomannan, xanthan gum and sodium alginate. Carbohydr. Polym. 2011, 83, 329–338. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Puttipipatkhachorn, S. Xanthan–alginate composite gel beads: Molecular interaction and in vitro characterization. Int. J. Pharm. 2007, 331, 61–71. [Google Scholar] [CrossRef]
- Saroglu, O.; Karadag, A.; Cakmak, Z.H.T.; Karasu, S. The formulation and microstructural, rheological, and textural characterization of salep-xanthan gum-based liposomal gels. Polym. Bull. 2023, 80, 9941–9962. [Google Scholar] [CrossRef]
- Adib, M.; Yaich, H.; Hidouri, H.; Ayadi, M.A. Effect of substituted gelling from pomegranate peel on colour, textural and sensory properties of pomegranate Jam. Food Chem. 2018, 239, 1047–1054. [Google Scholar]
- Alghooneh, A.; Razavi, S.M.A.; Kasapis, S. Classification of hydrocolloids based on small amplitude oscillatory shear, large amplitude oscillatory shear, and textural properties. J. Texture Stud. 2019, 50, 520–538. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Nakauma, M.; Funami, T.; Tanaka, T.; Nishihari, K.; Kohyama, K. Electromyograthy during oral processing in relation to mechanical and sensory properties of soft gels. J. Texture Stud. 2011, 42, 254–267. [Google Scholar] [CrossRef]
- Marques, C.; Correia, E.; Dinis, L.-T.; Vilela, A. An overview of sensory characterization techniques: From classical descriptive analysis to the emergence of novel profiling methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef]
- Liu, J.; Yu, S.; Xu, Y.; Li, J.; Liu, B.; Liu, S.; Ning, H.; Xu, D.; Low, S.S. In situ quantitative assessment of food oral processing parameters: A review of feasible techniques and devices. J. Texture Stud. 2023, 1, 3–20. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, S.A. Revised medium for rapid growth and bioassays with tobaco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Wang, H.; Sha, X.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef]
- Holdsworth, S.D. Applicability of rheological models to the interpretation of flow properties and processing of liquid foods. J. Textured Stud. 1971, 2, 393–418. [Google Scholar] [CrossRef]
- Ramkumar, D.H.S.; Bhattacharya, M. Relaxation behavior and the application of integral constitutive equations to wheat dough. J. Texture Stud. 1996, 27, 517–544. [Google Scholar] [CrossRef]
- Gabriele, D.G.; Migliori, M.; Sanzo, M.D.; Rossi, C.; Ruffolo, S.A.; Sindio, B. Characterisation of dairy emulsions by NMR and rheological techniques. Food Hydrocoll. 2009, 23, 619–628. [Google Scholar] [CrossRef]
- Chang, K.L.B.; Lin, J. Swelling behavior and the release of protein from chitosan-pectin composite particles. Carbohydr. Polym. 2000, 43, 163–169. [Google Scholar] [CrossRef]
- Chang, W.-H.; Chen, M.-H.; Fang, J.-F.; Chung, W.-L.; Chiu, L.-L.; Huang, Y.-F. Surface electromyography for evaluating the effect of aging on the coordination of swallowing muscles. Dysphagia 2023, 38, 1430–1439. [Google Scholar] [CrossRef] [PubMed]



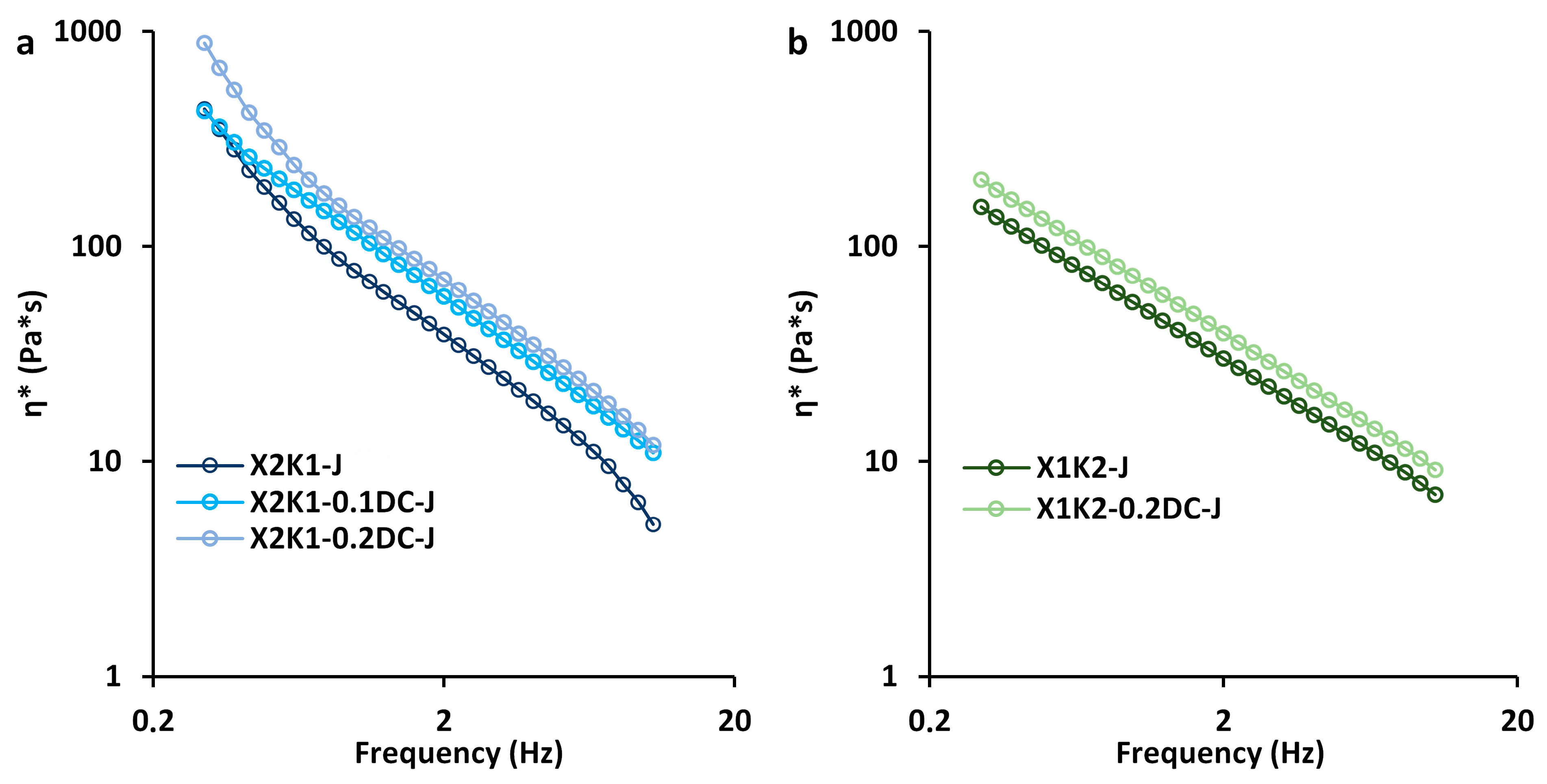
| Parameters | X2K1-J (Control) (n = 8) | X2K1-0.1DC-J (n = 8) | X2K1-0.2DC-J (n = 8) | X1K2-J (Control) (n = 8) | X1K2-0.2DC-J (n = 8) |
|---|---|---|---|---|---|
| Strength of linkage | |||||
| G′LVE (Pa) | 344.5 ± 1.8 a* | 613.7 ± 28.5 b | 773.4 ± 17.4 cα | 417.1 ± 7.0 1# | 806.6 ± 27.0 2β |
| G*FP (Pa) | 353.9 ± 24.5 a* | 550.9 ± 44.4 b | 548.2 ± 75.3 bα | 405.3 ± 11.1 1* | 810.6 ± 473.1 2α |
| Tan [δ]AF | 0.015 ± 0.005 a* | 0.018 ± 0.004 a | 0.018 ± 0.009 aα | 0.006 ± 0.001 1* | 0.007 ± 0.0011α |
| τL (Pa) | 347.6 ± 20.4 a* | 599.1 ± 53.4 c | 563.3 ± 37.1 bα | 368.6 ± 104.0 1* | 629.3 ± 299.8 1α |
| k′ (Pa·s) | 512.2 ± 94.4 a* | 725.1 ± 85.6 b | 908.7 ± 157.2 cα | 366.1 ± 18.4 1# | 400.8 ± 25.4 1β |
| k″(Pa·s) | 39.9 ± 14.8 a* | 41.1 ± 14.6 a | 92.4 ± 14.9 bα | 73.7 ± 2.9 1# | 73.0 ± 3.9 1α |
| A (Pa·s) | 489.3 ± 100.3 a* | 716.3 ± 90.0 b | 960.5 ± 155.0 cα | 366.0 ± 18.2 1* | 400.7 ± 25.6 1β |
| Number of linkages | |||||
| G*max/G*LVE | 1.002 ± 0.008 a* | 1.027 ± 0.008 b | 1.063 ± 0.012 cα | 1.028 ± 0.014 1* | 1.013 ± 0.004 1β |
| τFr (Pa) | 177.7 ± 27.4 a* | 283.7 ± 31.8 b | 302.6 ± 52.3 bα | 215.6 ± 48.5 1* | 311.7 ± 130.2 1α |
| n′ | 0.096 ± 0.058 b* | 0.012 ± 0.008 a | 0.037 ± 0.024 aα | 0.149 ± 0.003 1* | 0.133 ± 0.008 1β |
| z | 41.5 ± 4.2 a* | 52.1 ± 25.6 a | 44.0 ± 34.0 aα | 7.2 ± 0.9 1# | 7.4 ± 0.4 1α |
| Timescale of junction zone | |||||
| Tan [δ]LVE | 0.077 ± 0.028 b* | 0.057 ± 0.006 a | 0.081 ± 0.015 bα | 0.220 ± 0.007 1# | 0.194 ± 0.020 1β |
| k″/k′ | 0.085 ± 0.011 a* | 0.048 ± 0.021 a | 0.094 ± 0.001 bα | 0.201 ± 0.0111# | 0.182 ± 0.003 1β |
| η*s (Pa·s) | 85.2 ± 15.7 a* | 115.9 ± 13.8 b | 156.6 ± 26.6 cα | 56.8 ± 3.5 1# | 64.3 ± 4.5 1β |
| γL (%) | 49.9 ± 7.6 b* | 11.6 ± 2.9 a | 43.1 ± 5.4 bα | 75.1 ± 6.9 1# | 36.1 ± 8.9 2α |
| γFr (%) | 100.1 ± 22.8 b* | 68.8 ± 9.3 a | 68.7 ± 9.3 aα | 149.9 ± 26.8 1# | 112.8 ± 8.2 2β |
| Distance of linkage | |||||
| n″ | 0.213 ± 0.103 b* | 0.026 ± 0.021 a | 0.154 ± 0.068 bα | 0.196 ± 0.005 1* | 0.167 ± 0.026 1α |
| Parameter | Hydrogel | |
|---|---|---|
| X2K1-J | X2K1-0.2DC-J | |
| Nine-point hedonic scale | ||
| Overall liking | 5.4 ± 1.7 a | 4.9 ± 1.6 a |
| Consistency liking | 5.1 ± 1.7 a | 4.9 ± 1.6 a |
| 100 mm visual analog scale | ||
| Aroma | 42 ± 25 a | 41 ± 26 a |
| Taste | 56 ± 19 a | 51 ± 17 a |
| Hardness | 26 ± 21 a | 24 ± 18 a |
| Springiness | 58 ± 27 a | 50 ± 25 a |
| Adhesiveness | 20 ± 20 a | 23 ± 22 a |
| Gumminess | 28 ± 20 a | 26 ± 19 a |
| Moisture | 52 ± 23 a | 53 ± 28 a |
| Easy to swallow | 82 ± 17 a | 80 ± 19 a |
| Graininess | 31 ± 29 a | 57 ± 33 b |
| EMG parameters | ||
| Chewing time, s | 19 ± 9 a | 19 ± 8 a |
| Number of chews | 25 ± 11 a | 25 ± 11 a |
| Activity of Masseter muscle, mV × s | 0.19 ± 0.14 a | 0.19 ± 0.10 a |
| Activity of Temporalis muscle, mV × s | 0.35 ± 0.20 a | 0.19 ± 0.12 a |
| Activity of Suprahyoid muscles, mV × s | 0.59 ± 0.29 a | 0.40 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günter, E.; Popeyko, O.; Vityazev, F.; Zueva, N.; Velskaya, I.; Popov, S. Effect of Carrot Callus Cells on the Mechanical, Rheological, and Sensory Properties of Hydrogels Based on Xanthan and Konjac Gums. Gels 2024, 10, 771. https://doi.org/10.3390/gels10120771
Günter E, Popeyko O, Vityazev F, Zueva N, Velskaya I, Popov S. Effect of Carrot Callus Cells on the Mechanical, Rheological, and Sensory Properties of Hydrogels Based on Xanthan and Konjac Gums. Gels. 2024; 10(12):771. https://doi.org/10.3390/gels10120771
Chicago/Turabian StyleGünter, Elena, Oxana Popeyko, Fedor Vityazev, Natalia Zueva, Inga Velskaya, and Sergey Popov. 2024. "Effect of Carrot Callus Cells on the Mechanical, Rheological, and Sensory Properties of Hydrogels Based on Xanthan and Konjac Gums" Gels 10, no. 12: 771. https://doi.org/10.3390/gels10120771
APA StyleGünter, E., Popeyko, O., Vityazev, F., Zueva, N., Velskaya, I., & Popov, S. (2024). Effect of Carrot Callus Cells on the Mechanical, Rheological, and Sensory Properties of Hydrogels Based on Xanthan and Konjac Gums. Gels, 10(12), 771. https://doi.org/10.3390/gels10120771






