3D Bioprintable Self-Healing Hyaluronic Acid Hydrogel with Cysteamine Grafting for Tissue Engineering
Abstract
1. Introduction
2. Results and Discussion
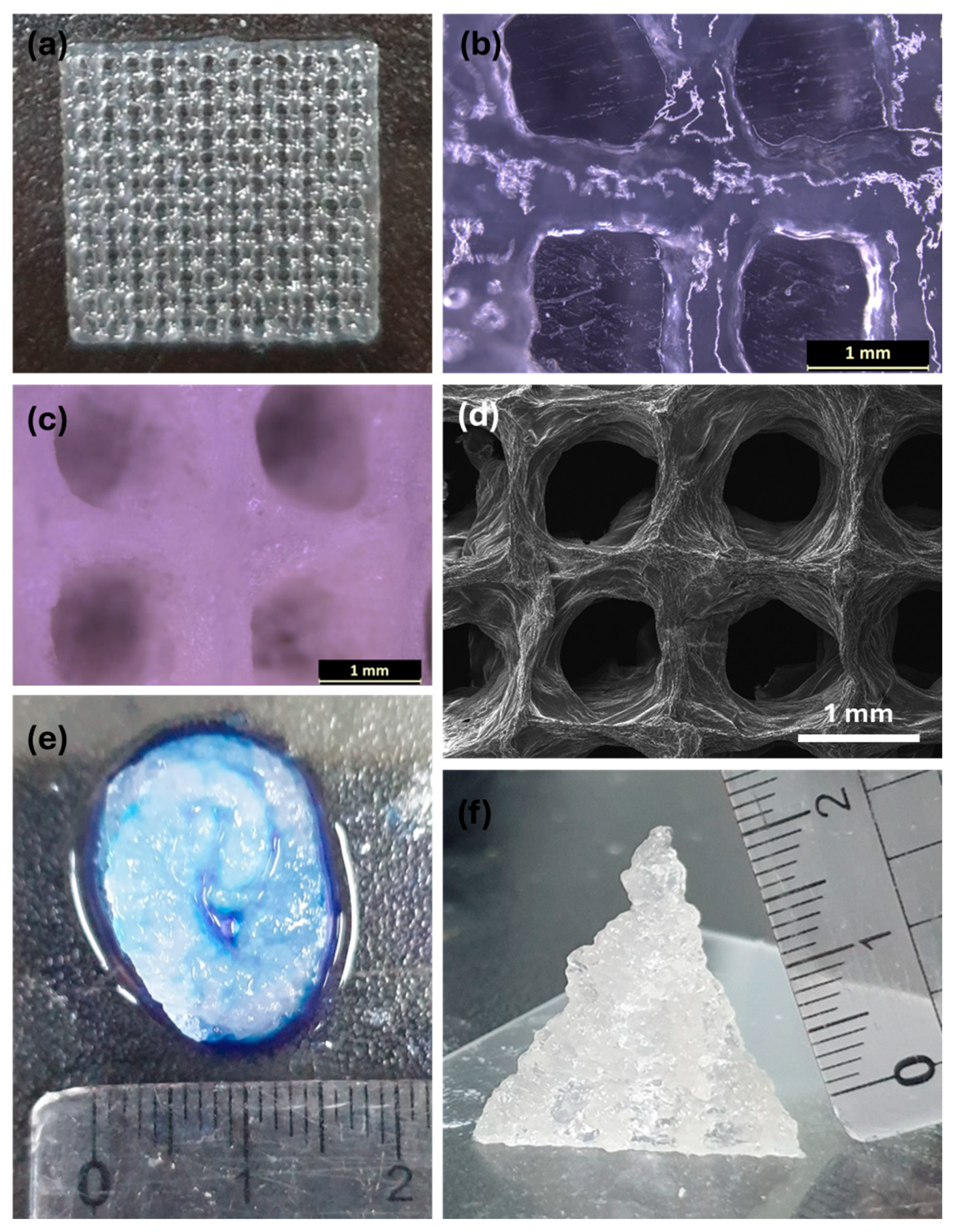
2.1. Functional Groups and Surface Morphology Analysis
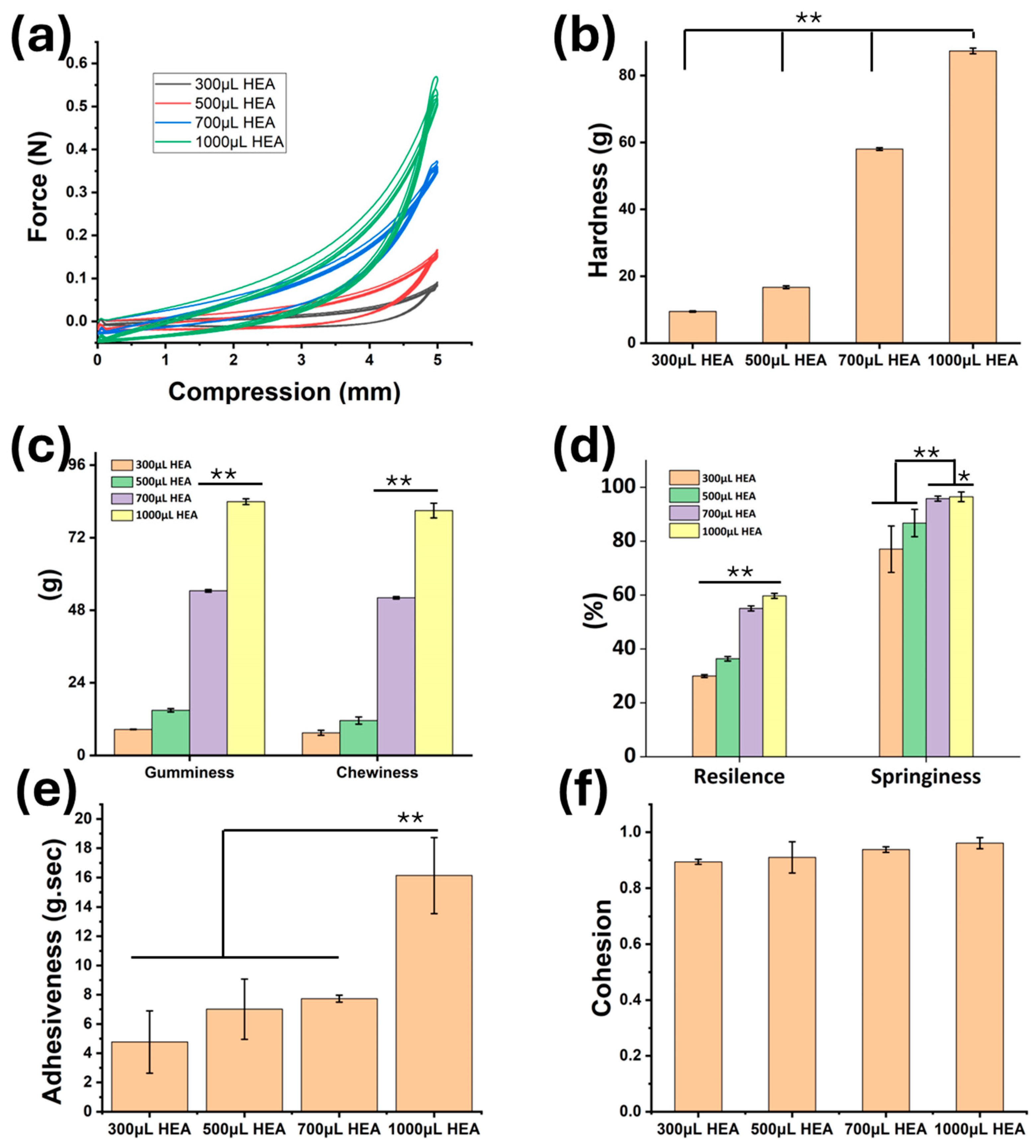
2.2. Mechanical Properties Analysis
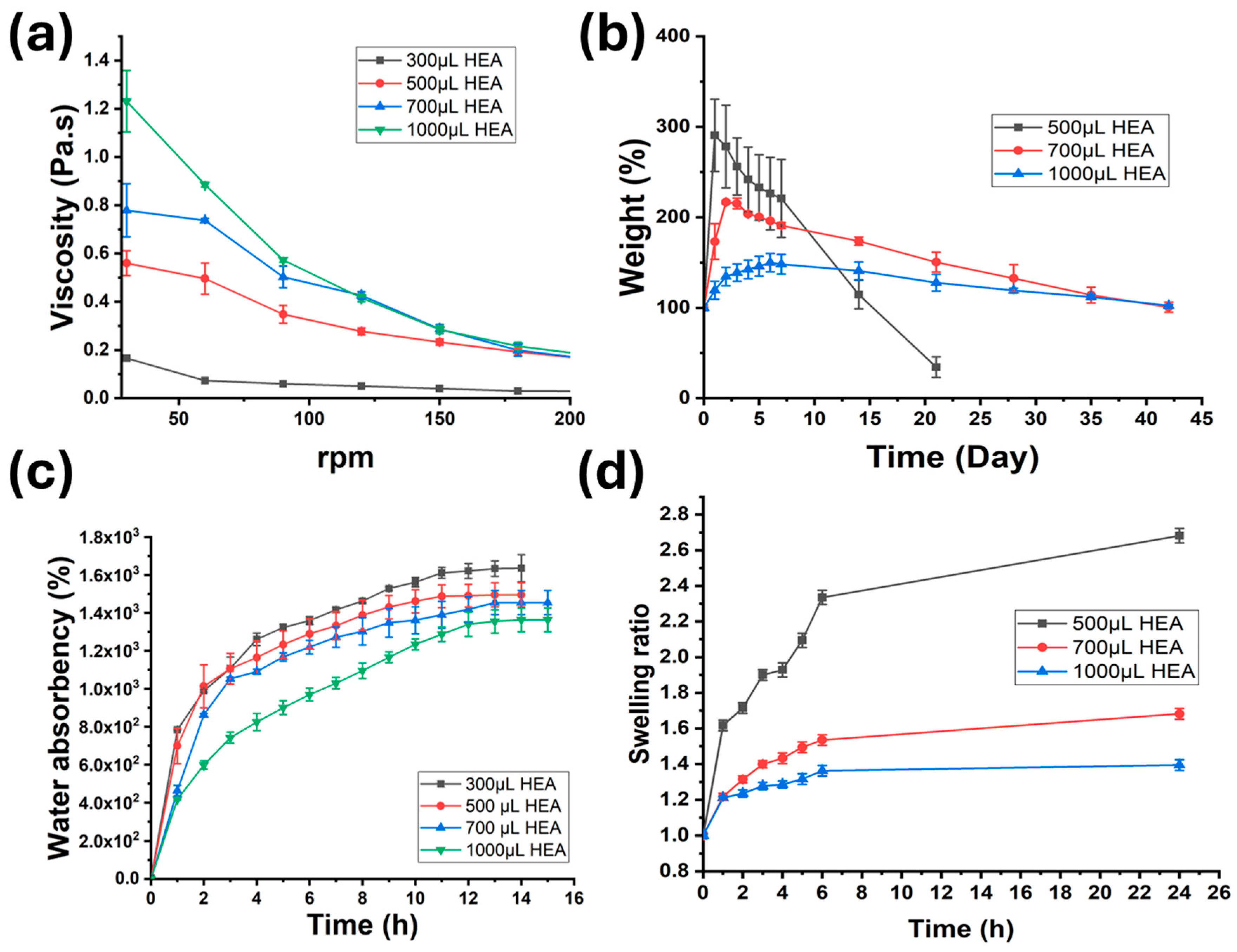
2.3. Physical Properties of Gels (Rheology, Degradation, Water Absorbency, and Swelling)
2.4. 3D Printability
2.5. Self-Healing and Adhesive Properties
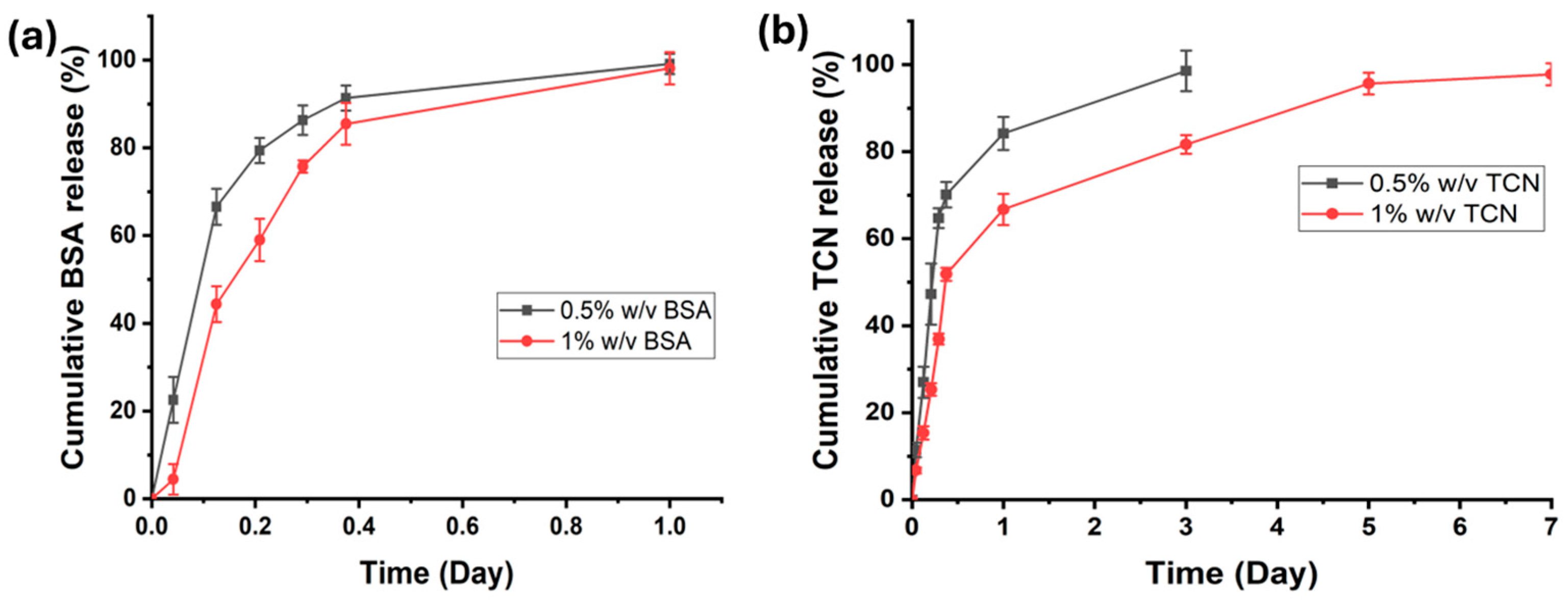
2.6. In Vitro Drug/Biomolecule Release Study
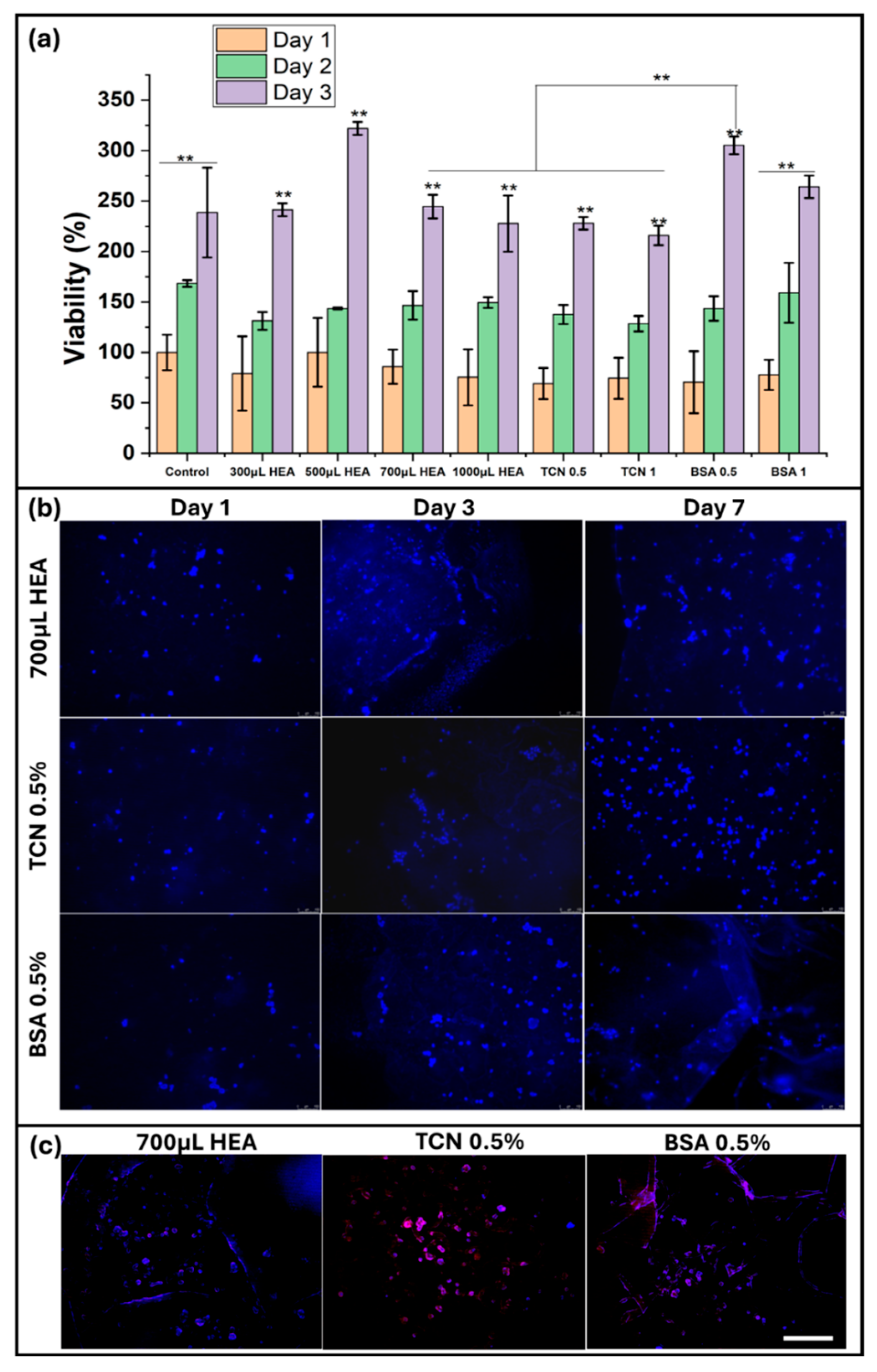
2.7. In Vitro Cell Culture Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogels
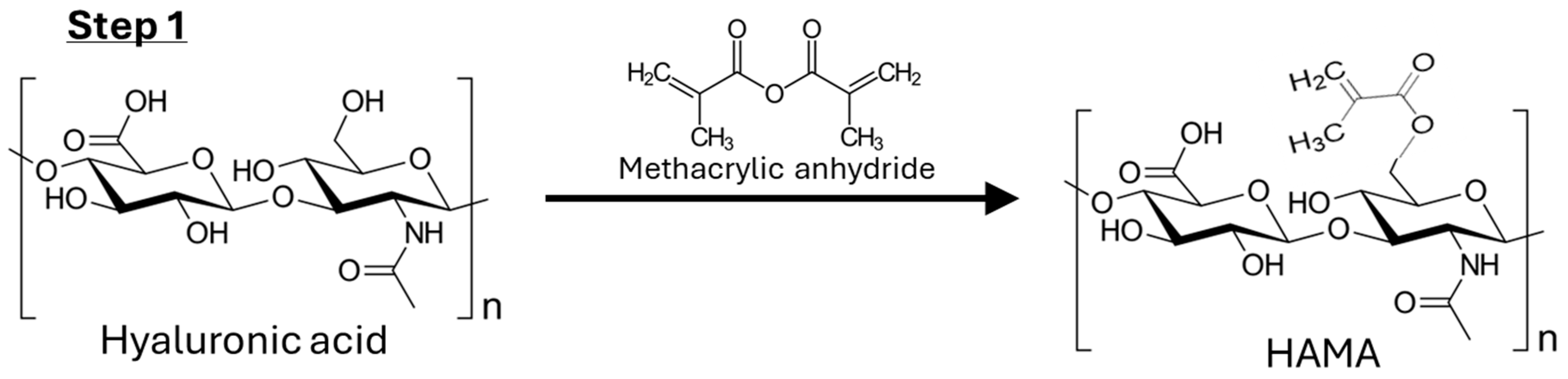
4.2.1. Step 1: Synthesis of HAMA
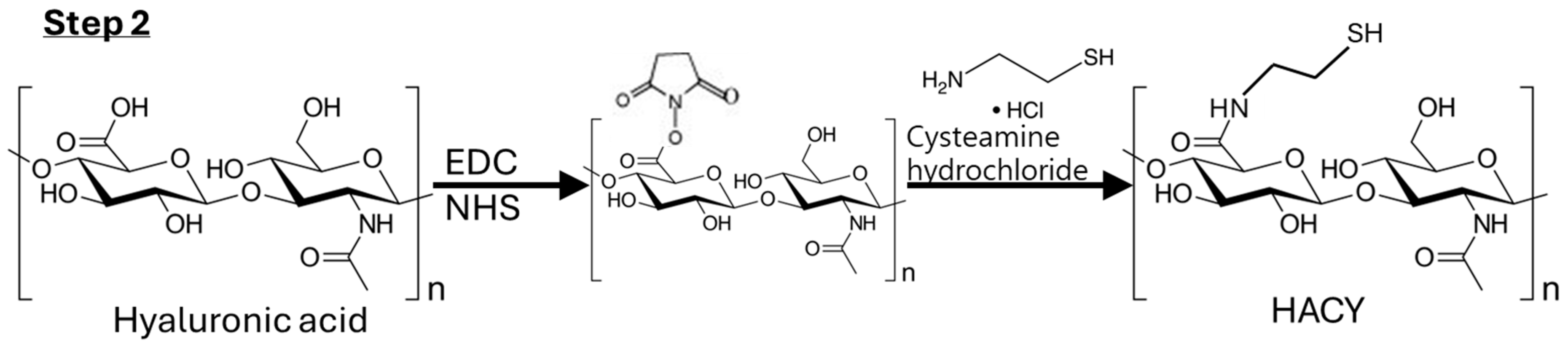
4.2.2. Step 2: Synthesis of HACY
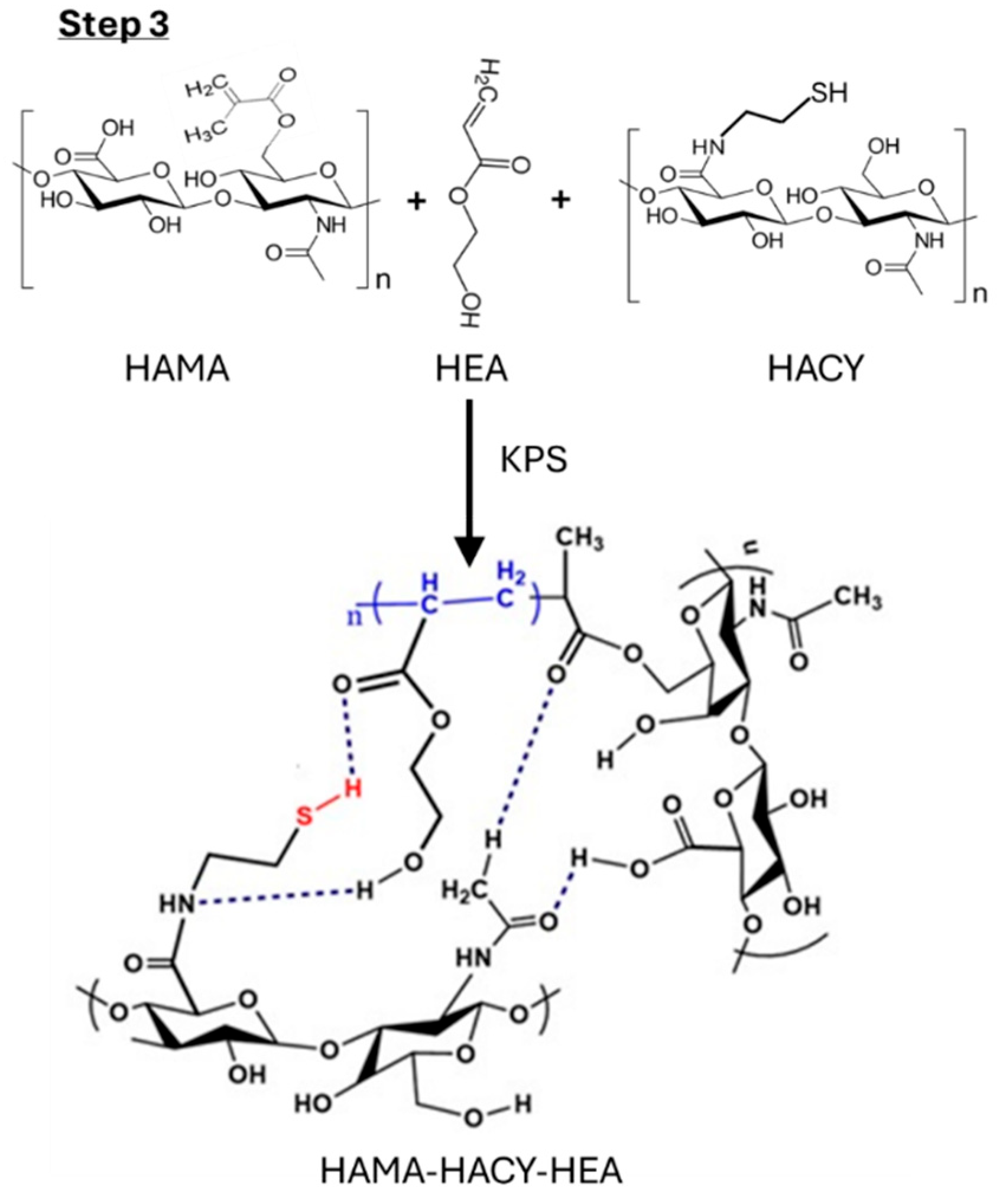
4.2.3. Step 3: Preparation of HACY-HAMA-HEA Hydrogel
4.3. Characterization
4.3.1. NMR Analysis
4.3.2. FTIR Analysis
4.3.3. Scanning Electron Microscopic Analysis
4.3.4. Mechanical Properties
4.3.5. Rheology Analysis
4.3.6. Degradation Test
4.3.7. Water Absorbency Test
V2 = 1/(1 + Q)
4.3.8. Swelling Test
4.3.9. 3D Printing
4.3.10. Self-Healing and Adhesive Property Test of the Optimized Gel
4.3.11. In Vitro Drug/Biomolecule Release Study
4.3.12. In Vitro Cell Culture Study
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sekar, M.P.; Suresh, S.; Zennifer, A.; Sethuraman, S.; Sundaramurthi, D. Hyaluronic Acid as Bioink and Hydrogel Scaffolds for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2023, 9, 3134–3159. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.R.; Bhattacharyya, A.; Gunbayar, M.; Jung, M.; Noh, I. Study on Bioresponsive Gelatin-Hyaluronic Acid-Genipin Hydrogel for High Cell-Density 3D Bioprinting. Gels 2023, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I.; Vu, T.T.; Jo, S.-H.; Park, S.-H.; Lim, K.T. Exploring the Progress of Hyaluronic Acid Hydrogels: Synthesis, Characteristics, and Wide-Ranging Applications. Materials 2024, 17, 2439. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Z.; Guo, Y.; Xu, P. Designing functional hyaluronic acid-based hydrogels for cartilage tissue engineering. Mater. Today Bio 2022, 17, 100495. [Google Scholar] [CrossRef]
- Hong, B.M.; Park, S.A.; Park, W.H. Effect of photoinitiator on chain degradation of hyaluronic acid. Biomater. Res. 2019, 23, 21. [Google Scholar] [CrossRef]
- Sorouri, F.; Hosseini, P.; Sharifzadeh, M.; Kiani, S.; Khoobi, M. In Situ Cross-Linkable Hyaluronic–Ferulic Acid Conjugate Containing Bucladesine Nanoparticles Promotes Neural Regeneration after Spinal Cord Injury. ACS Appl. Mater. Interfaces 2023, 15, 42251–42270. [Google Scholar] [CrossRef]
- Goodarzi, K.; Rao, S.S. Hyaluronic acid-based hydrogels to study cancer cell behaviors. J. Mater. Chem. B 2021, 9, 6103–6115. [Google Scholar] [CrossRef]
- Zhou, K.; Feng, M.; Mao, H.; Gu, Z. Photoclick polysaccharide-based bioinks with an extended biofabrication window for 3D embedded bioprinting. Biomater. Sci. 2022, 10, 4479–4491. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef]
- Ghorbani, F.; Ghalandari, B.; Khajehmohammadi, M.; Bakhtiary, N.; Tolabi, H.; Sahranavard, M.; Fathi-Karkan, S.; Nazar, V.; Niar, S.H.N.; Armoon, A.; et al. Photo-cross-linkable hyaluronic acid bioinks for bone and cartilage tissue engineering applications. Int. Mater. Rev. 2023, 68, 901–942. [Google Scholar] [CrossRef]
- Gao, Z.; Golland, B.; Tronci, G.; Thornton, P.D. A redox-responsive hyaluronic acid-based hydrogel for chronic wound management. J. Mater. Chem. B 2019, 7, 7494–7501. [Google Scholar] [CrossRef] [PubMed]
- Kikani, T.; Dave, S.; Thakore, S. Functionalization of hyaluronic acid for development of self-healing hydrogels for biomedical applications: A review. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124950. [Google Scholar] [CrossRef] [PubMed]
- Luaces-Rodríguez, A.; Díaz-Tomé, V.; González-Barcia, M.; Silva-Rodríguez, J.; Herranz, M.; Gil-Martínez, M.; Rodríguez-Ares, M.T.; García-Mazás, C.; Blanco-Mendez, J.; Lamas, M.J.; et al. Cysteamine polysaccharide hydrogels: Study of extended ocular delivery and biopermanence time by PET imaging. Int. J. Pharm. 2017, 528, 714–722. [Google Scholar] [CrossRef]
- Cao, H.; Chen, M.; Cui, X.; Liu, Y.; Deng, S.; Yuan, T.; Fan, Y.; Wang, Q.; Zhang, X. Cell-Free Osteoarthritis Treatment with Sustained-Release of Chondrocyte-Targeting Exosomes from Umbilical Cord-Derived Mesenchymal Stem Cells to Rejuvenate Aging Chondrocytes. ACS Nano 2023, 17, 13358–13376. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Sui, J.; Ma, M.; Xu, Y.; Lin, W.; Chen, Y.; Man, Y.; Sun, Y.; Fan, Y.; Zhang, X. The preparation and biocompatible evaluation of injectable dual crosslinking hyaluronic acid hydrogels as cytoprotective agents. J. Mater. Chem. B 2019, 7, 4413–4423. [Google Scholar] [CrossRef]
- Das, D.; Cho, H.; Kim, N.; Pham, T.T.H.; Kim, I.G.; Chung, E.-J.; Noh, I. A terpolymeric hydrogel of hyaluronate-hydroxyethyl acrylate-gelatin methacryloyl with tunable properties as biomaterial. Carbohydr. Polym. 2019, 207, 628–639. [Google Scholar] [CrossRef]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef]
- Rosaming, P.; Jirayupapong, J.; Thamnium, S.; Win, Y.Y.; Limprasutr, V.; Rodsiri, R.; Pavasant, P.; Luckanagul, J.A. Interpenetrating Low-Molecular Weight Hyaluronic Acid in Hyaluronic Acid-Based In Situ Hydrogel Scaffold for Periodontal and Oral Wound Applications. Polymers 2022, 14, 4986. [Google Scholar] [CrossRef]
- Arunprasert, K.; Pornpitchanarong, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Patrojanasophon, P. Mussel-inspired poly(hydroxyethyl acrylate-co-itaconic acid)-catechol/hyaluronic acid drug-in-adhesive patches for transdermal delivery of ketoprofen. Int. J. Pharm. 2022, 629, 122362. [Google Scholar] [CrossRef]
- Buckley, C.; Montgomery, T.R.; Szank, T.; Murray, B.A.; Quigley, C.; Major, I. Modification of hyaluronic acid to enable click chemistry photo-crosslinking of hydrogels with tailorable degradation profiles. Int. J. Biol. Macromol. 2023, 240, 124459. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, M.; Yaraki, M.T.; Ahmadieh, M.; Tahriri, M.; Vashaee, D.; Tayebi, L. Determination of total aflatoxin using cysteamine-capped CdS quantum dots as a fluorescence probe. Colloid Polym. Sci. 2016, 294, 1453–1462. [Google Scholar] [CrossRef]
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019, 95, 176–187. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Ham, H.-W.; Sonh, J.; Gunbayar, M.; Jeffy, R.; Nagarajan, R.; Khatun, M.R.; Noh, I. 3D bioprinting of complex tissue scaffolds with in situ homogeneously mixed alginate-chitosan-kaolin bioink using advanced portable biopen. Carbohydr. Polym. 2023, 317, 121046. [Google Scholar] [CrossRef]
- Wan, T.; Fan, P.; Zhang, M.; Shi, K.; Chen, X.; Yang, H.; Liu, X.; Xu, W.; Zhou, Y. Multiple Crosslinking Hyaluronic Acid Hydrogels with Improved Strength and 3D Printability. ACS Appl. Bio Mater. 2022, 5, 334–343. [Google Scholar] [CrossRef]
- Grosjean, M.; Gangolphe, L.; Nottelet, B. Degradable Self-Healable Networks for Use in Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2205315. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Abdiryim, T.; Liu, X. Self-Healing Hydrogels: From Synthesis to Multiple Applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Khatun, M.R.; Bhattacharyya, A.; Taheri, S.; Ham, H.; Kim, H.; Chang, S.H.; Noh, I. High Molecular Weight Fucoidan Loading into and Release from Hyaluronate-Based Prefabricated Hydrogel and its Nanogel Particles Controlled by Variable Pitch and Differential Extensional Shear Technology of Advanced Twin Screw-Based System. Adv. Mater. Technol. 2022, 8, 2201478. [Google Scholar] [CrossRef]
- Eltayeb, M.; Stride, E.; Edirisinghe, M.; Harker, A. Electrosprayed nanoparticle delivery system for controlled release. Mater. Biol. Appl. 2016, 66, 138–146. [Google Scholar] [CrossRef]
- Ajzashokouhi, A.H.; Razavi, B.M.; Sadeghian, H.; Hosseinzadeh, H. In vivo and in vitro effects of crocetin and its amide derivative on acrylamide-induced neurotoxicity. Avicenna J. Phytomed. 2024, 14, 78–89. [Google Scholar]
- Huchthausen, J.; Escher, B.I.; Grasse, N.; König, M.; Beil, S.; Henneberger, L. Reactivity of Acrylamides Causes Cytotoxicity and Activates Oxidative Stress Response. Chem. Res. Toxicol. 2023, 36, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Priya, V.N.K.; Kim, J.-H.; Khatun, M.R.; Nagarajan, R.; Noh, I. Nanodiamond enhanced mechanical and biological properties of extrudable gelatin hydrogel cross-linked with tannic acid and ferrous sulphate. Biomater. Res. 2022, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. On the synthesis and characterization of biofunctional hyaluronic acid based injectable hydrogels for the repair of cartilage lesions. Eur. Polym. J. 2019, 114, 47–56. [Google Scholar] [CrossRef]


| SI No. | Sample Code | HACY (w/v) | HAMA (w/v) | 2-HEA | KPS (5%, w/v) |
|---|---|---|---|---|---|
| 1 | 300 μL HEA | 3% 3.0 mL | 3% 2.0 mL | 300 μL | 500 μL |
| 2 | 500 μL HEA | 3% 3.0 mL | 3% 2.0 mL | 500 μL | 500 μL |
| 3 | 700 μL HEA | 3% 3.0 mL | 3% 2.0 mL | 700 μL | 500 μL |
| 4 | 1000 μL HEA | 3% 3.0 mL | 3% 2.0 mL | 1000 μL | 500 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraja, K.; Bhattacharyya, A.; Jung, M.; Kim, D.; Khatun, M.R.; Noh, I. 3D Bioprintable Self-Healing Hyaluronic Acid Hydrogel with Cysteamine Grafting for Tissue Engineering. Gels 2024, 10, 780. https://doi.org/10.3390/gels10120780
Nagaraja K, Bhattacharyya A, Jung M, Kim D, Khatun MR, Noh I. 3D Bioprintable Self-Healing Hyaluronic Acid Hydrogel with Cysteamine Grafting for Tissue Engineering. Gels. 2024; 10(12):780. https://doi.org/10.3390/gels10120780
Chicago/Turabian StyleNagaraja, Kasula, Amitava Bhattacharyya, Minsik Jung, Dajeong Kim, Mst Rita Khatun, and Insup Noh. 2024. "3D Bioprintable Self-Healing Hyaluronic Acid Hydrogel with Cysteamine Grafting for Tissue Engineering" Gels 10, no. 12: 780. https://doi.org/10.3390/gels10120780
APA StyleNagaraja, K., Bhattacharyya, A., Jung, M., Kim, D., Khatun, M. R., & Noh, I. (2024). 3D Bioprintable Self-Healing Hyaluronic Acid Hydrogel with Cysteamine Grafting for Tissue Engineering. Gels, 10(12), 780. https://doi.org/10.3390/gels10120780








