The Impact of Gelatin and Fish Collagen on Alginate Hydrogel Properties: A Comparative Study
Abstract
1. Introduction
2. Results and Discussion
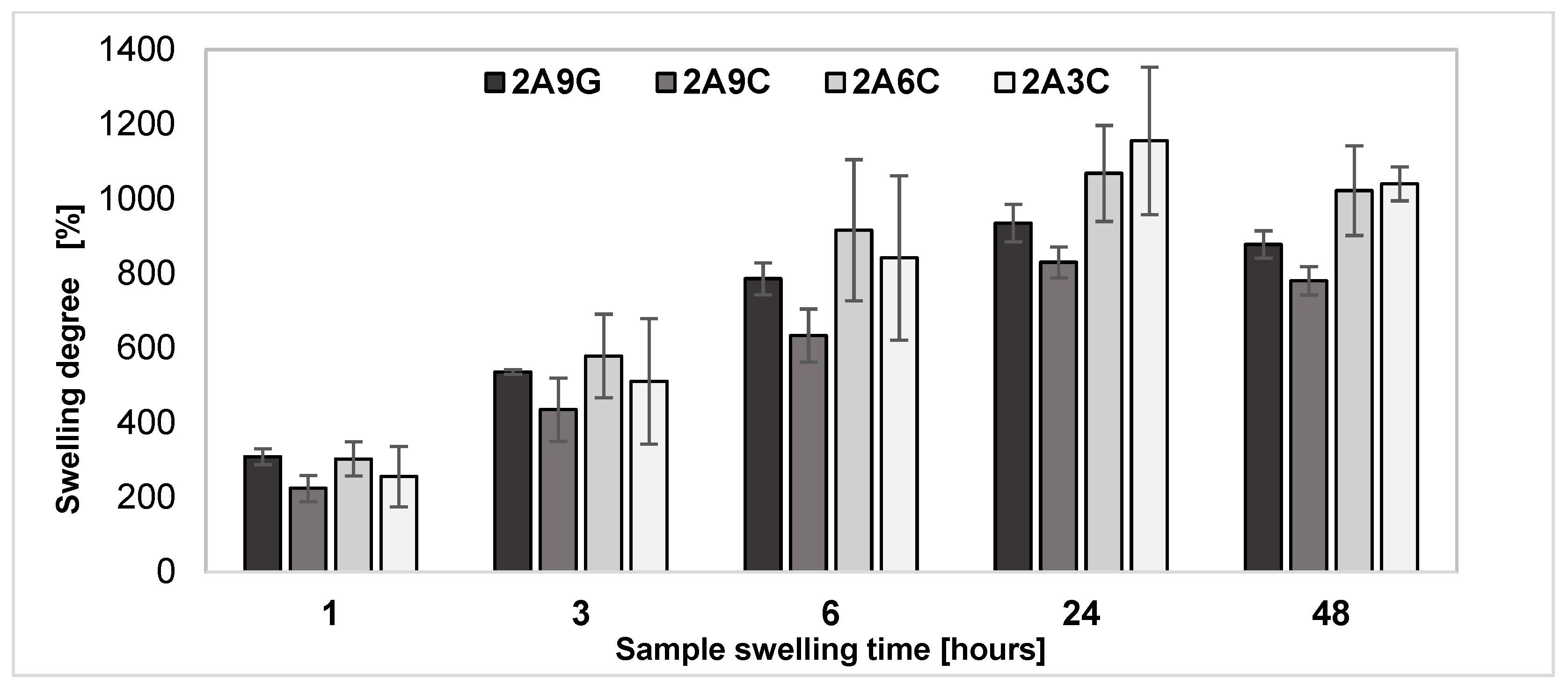
2.1. Swelling Degree of the Hydrogel Materials
2.2. Evaluation of Mechanical Properties of the Hydrogel Materials Using Static Compression Test
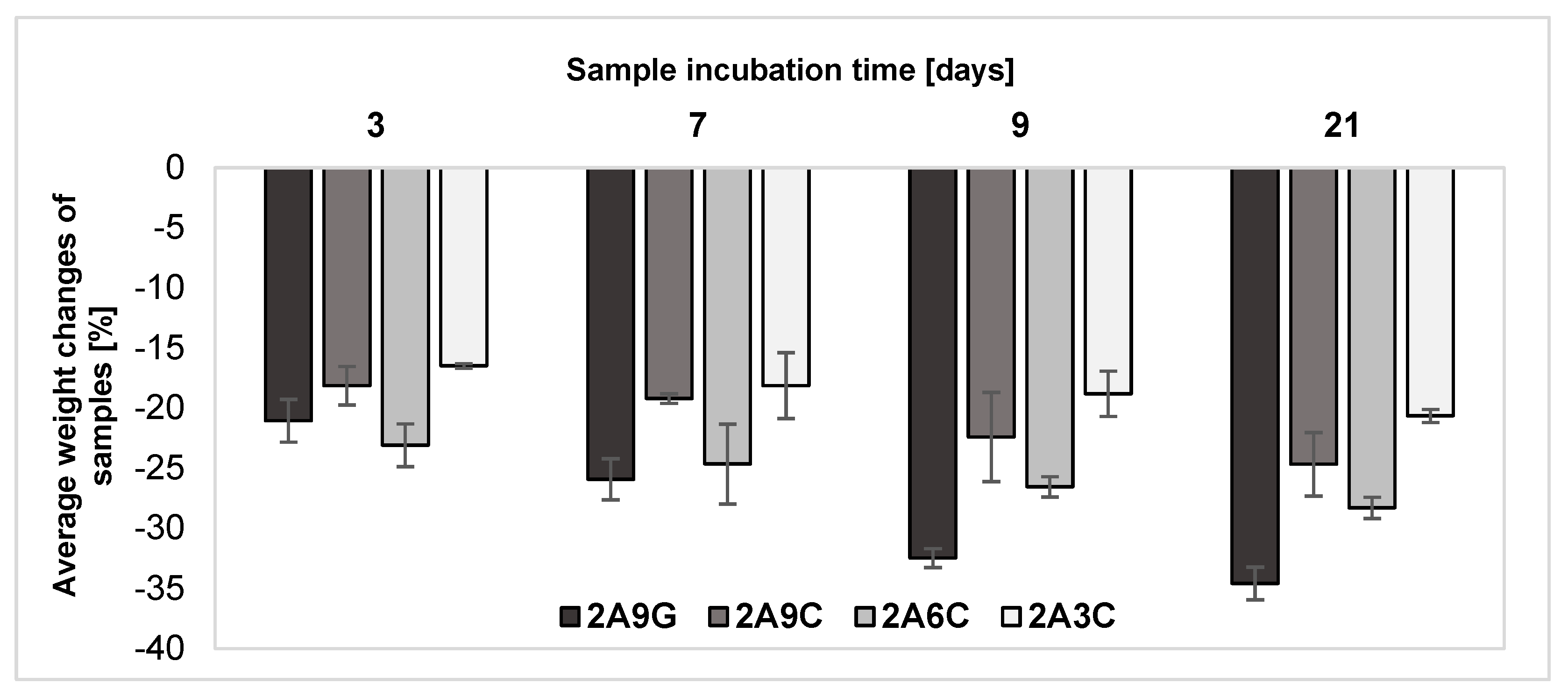
2.3. Evaluation of Changes in the Samples Weight after Individual Incubation Periods
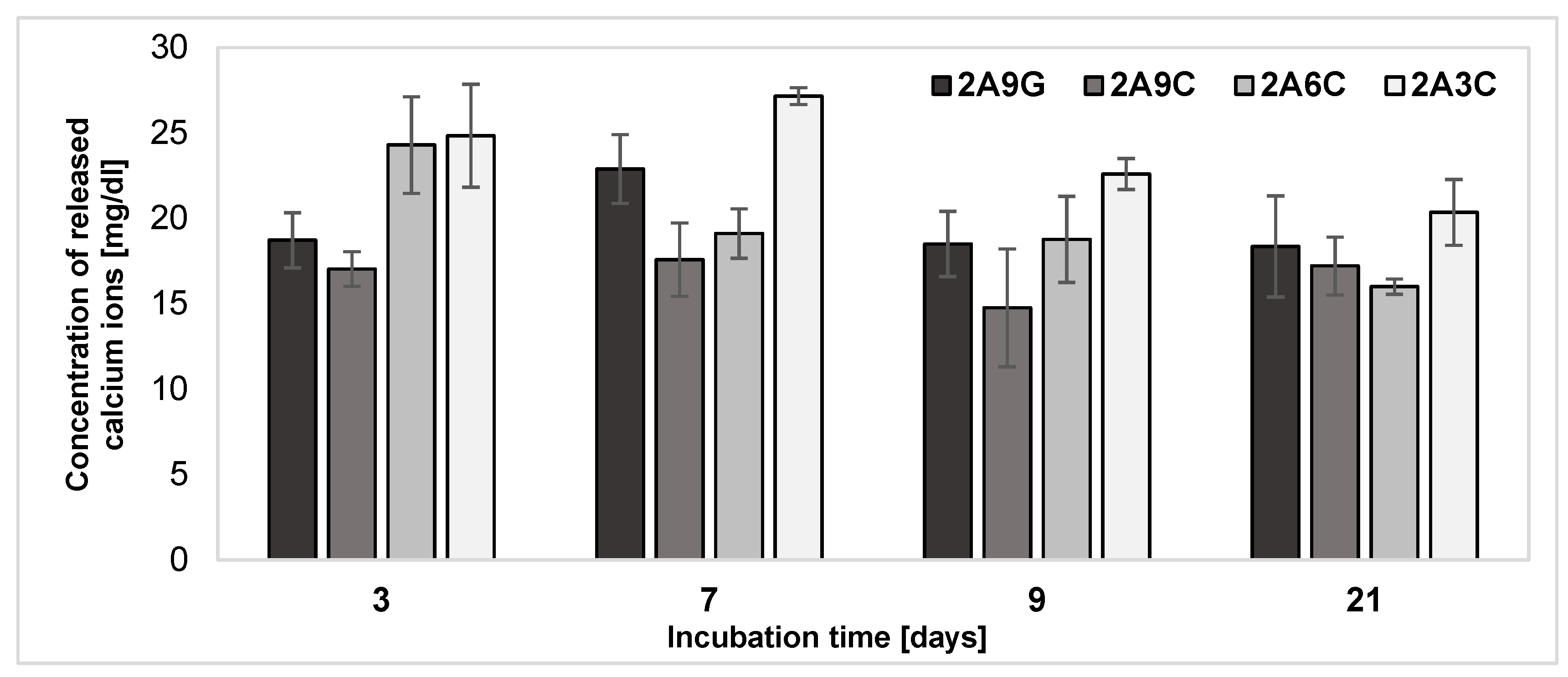
2.4. Release of Calcium Ions from the Hydrogel Materials after Individual Incubation Periods
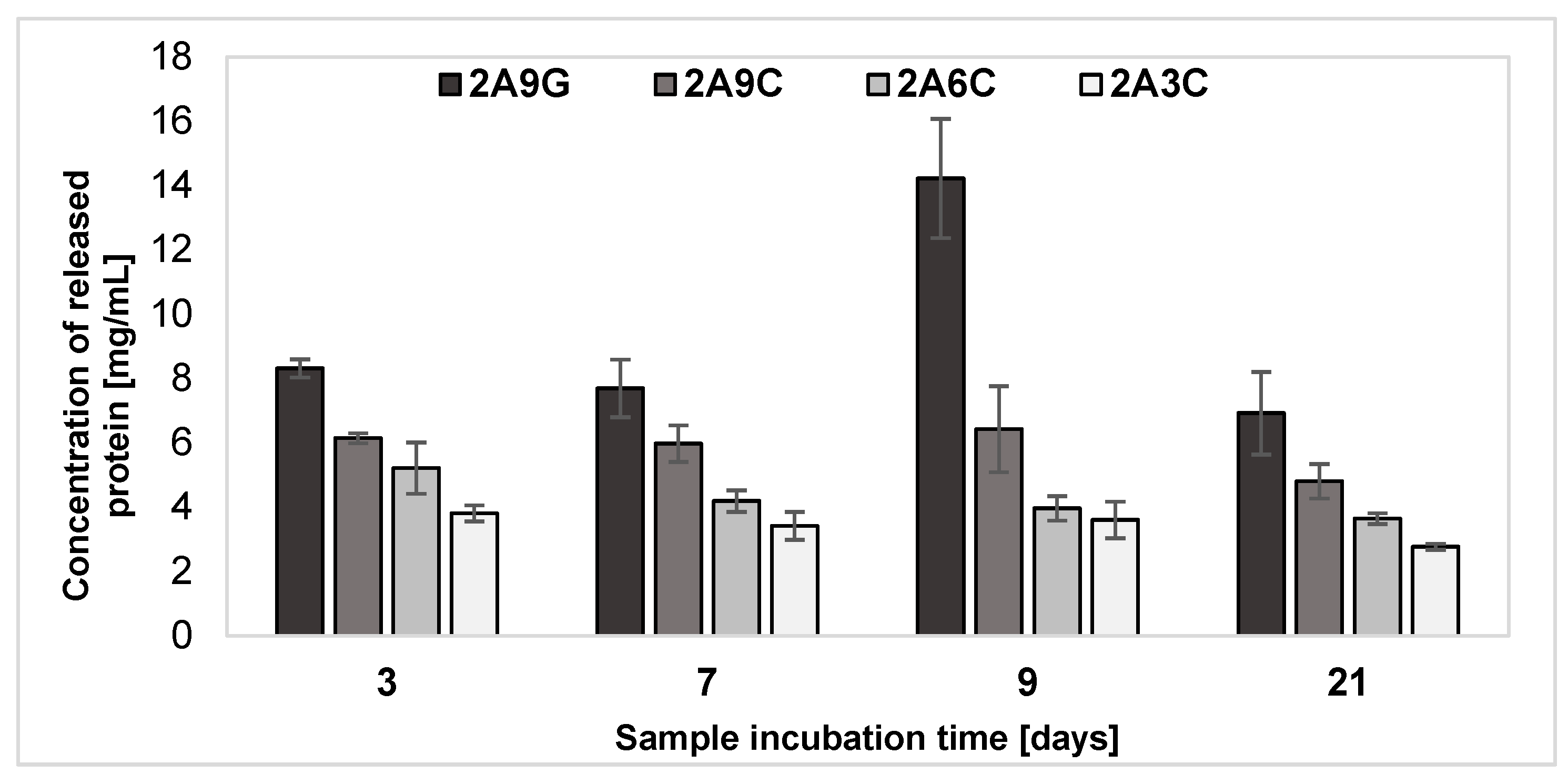
2.5. Release of Protein from the Hydrogel Materials after Individual Incubation Periods
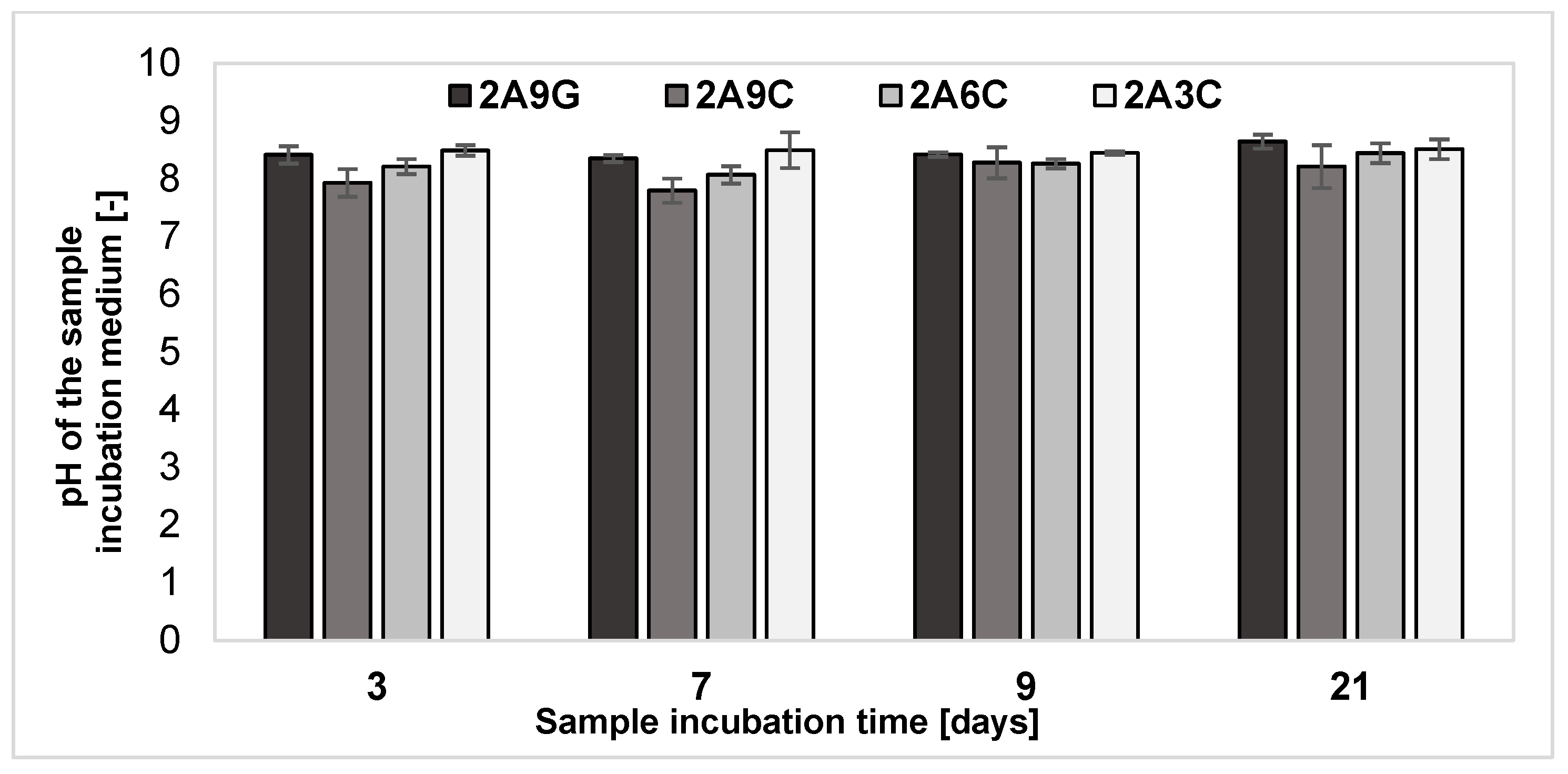
2.6. Analysis of pH Changes of the Degradation Medium
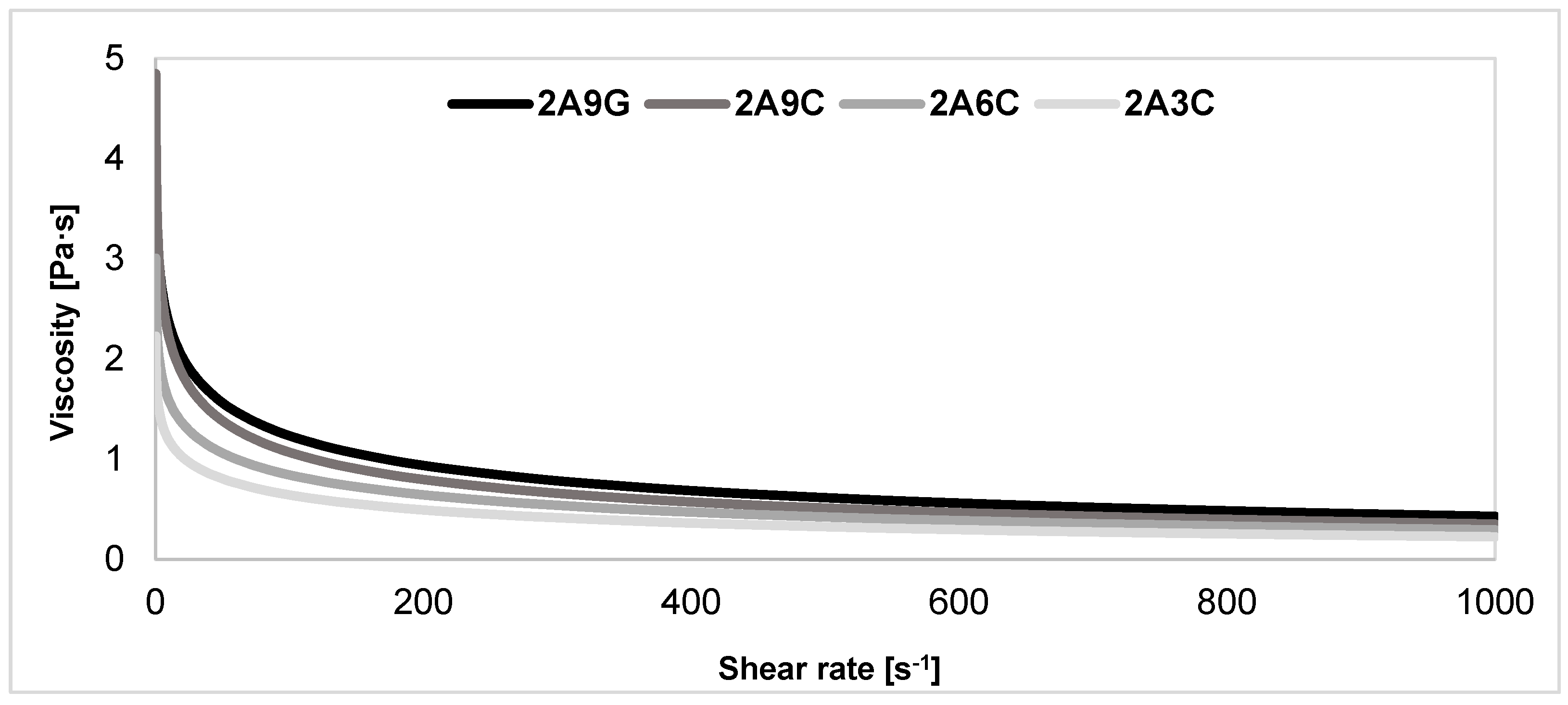
2.7. Evaluation of Rheological Properties of the Polymer Solutions
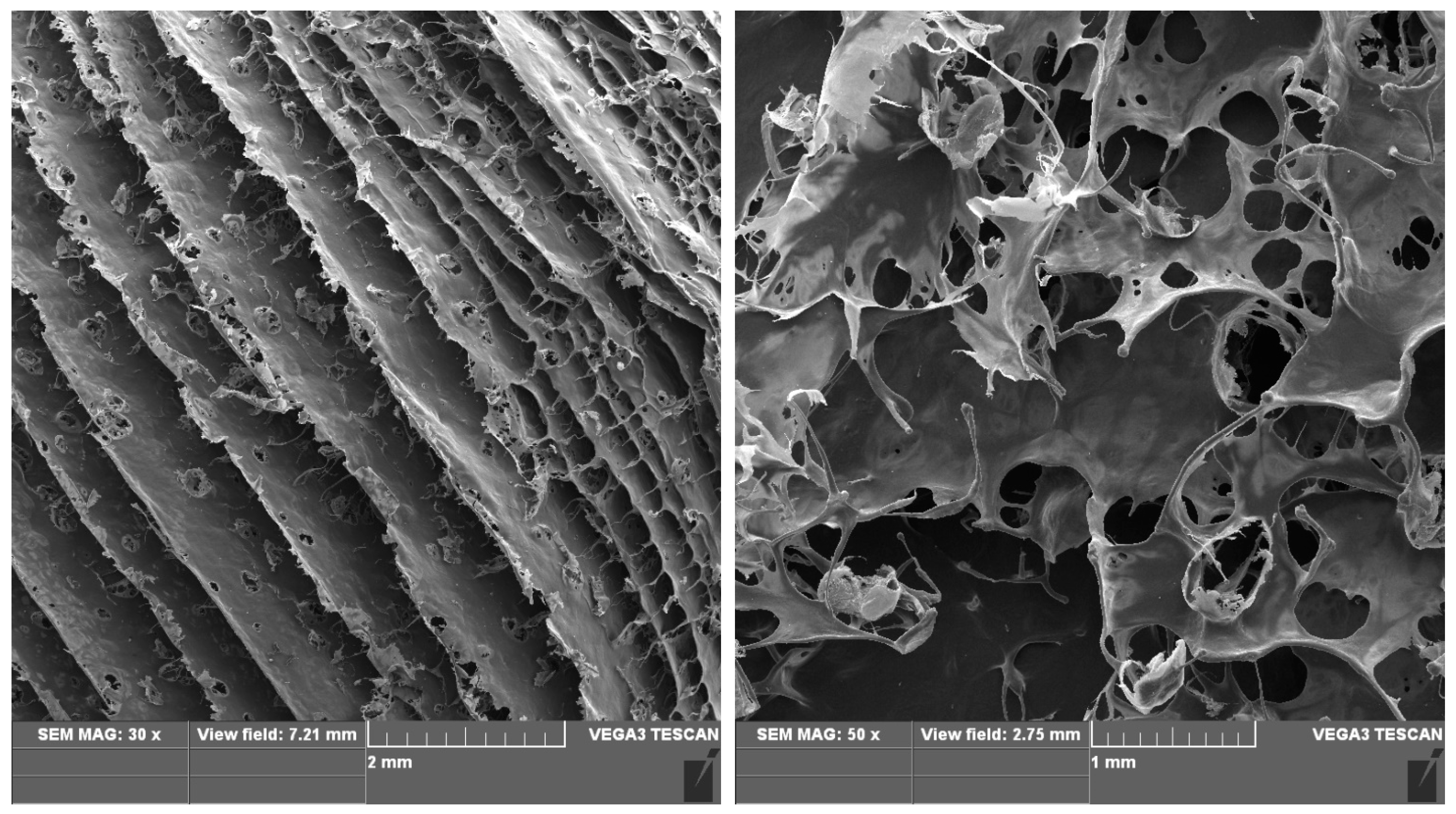
2.8. Three-Dimensional Printability of Prepared Polymer Compositions
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fish Collagen—Isolation and Obtaining the Test Material
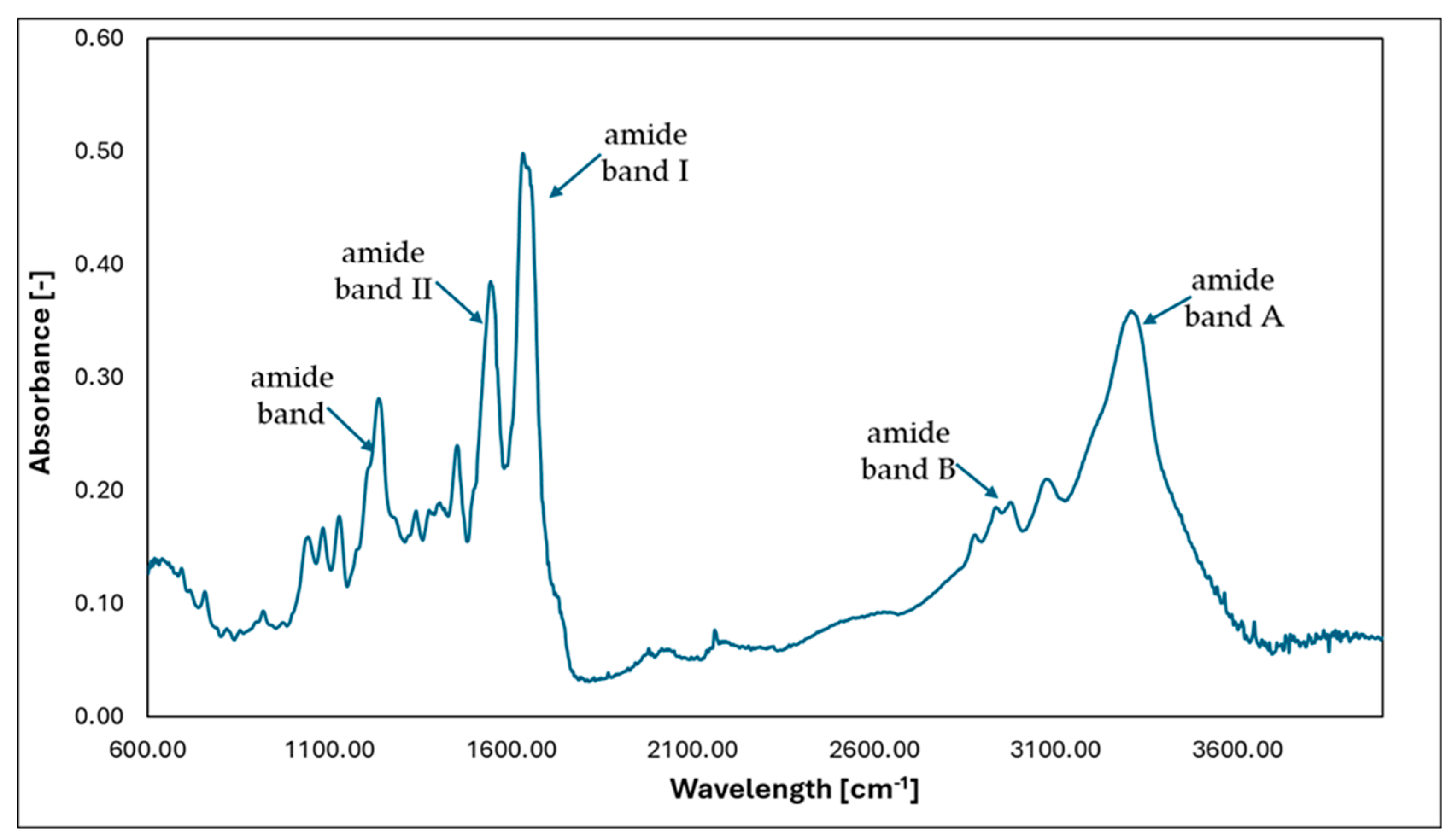
- Amide band A—3000–4000 cm−1—stretching vibrations from NH and NH2 groups;
- Amide band B—2880–2980 cm−1—asymmetric vibrations of CH2 groups;
- Amide band I—1600–1750 cm−1—stretching vibrations from amide C=O groups;
- Amide band II—1500–1600 cm−1—stretching vibrations of NH groups originating from CN groups;
- Amide band—1230–1245 cm−1—stretching vibrations of NH groups originating from CN groups.
4.3. Preparation of the Hydrogel Materials
4.4. Swelling Degree of the Hydrogel Materials
4.5. Evaluation of Mechanical Properties of the Hydrogel Materials Using Static Compression Test
4.6. Preparation of the Extracts
4.7. Evaluation of Changes in the Samples Weight after Individual Incubation Periods
4.8. Release of Calcium Ions from the Hydrogel Materials after Individual Incubation Periods
4.9. Release of Protein from the Hydrogel Materials after Individual Incubation Periods
4.10. Analysis of Changes in the pH of Degradation Medium
4.11. Evaluation of Rheological Properties of the Polymer Solutions
4.12. Three-Dimensional Printing Process
- The ability of the hydrogel to flow through the printer nozzle without clogging or excessive spillage of the material was tested. Tests for this feature include flow analysis at various pressures and printing speeds.
- The behavior of the material was assessed and analyzed immediately after the printing process, whether it maintained its required printed structure or flowed and dripped from the working roller.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.M.; Olanrele, O.S.; Zhang, X.; Hsu, C.C. Fabrication of Hydrogel Materials for Biomedical Applications. In Novel Biomaterials for Regenerative Medicine; Chun, H., Park, K., Kim, C.H., Khang, G., Eds.; Advances in Experimental Medicine and Biology Series; Springer: Singapore, 2018; pp. 197–224. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Wheeler, J.C.; Woods, J.A.; Cox, M.J.; Cantrell, R.W.; Watkins, F.H.; Edlich, R.F. Evolution of hydrogel polymers as contact lenses, surface coatings, dressings, and drug delivery systems. J. Long. Term. Eff. Med. Implant. 1996, 6, 207–2017. [Google Scholar]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Cattelan, G.; Guerrero Gerbolés, A.; Foresti, R.; Pramstaller, P.P.; Rossini, A.; Miragoli, M.; Caffarra Malvezzi, C. Alginate Formulations: Current Developments in the Race for Hydrogel-Based Cardiac Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li HXu, H.; Wang, L.; Zhang, M.; Liu, J.; Tan, F. Alginate hydrogels crosslinked with different strontium-calcium ratios as injectable scaffolds for bone tissue engineering. J. Mater. Sci. 2021, 56, 17221–17234. [Google Scholar] [CrossRef]
- Rosińska, K.; Bartniak, M.; Wierzbicka, A.; Sobczyk-Guzenda, A.; Bociaga, D. Solvent types used for the preparation of hydrogels determine their mechanical properties and influence cell viability through gelatine and calcium ions release. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 314–330. [Google Scholar] [CrossRef]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.W.; Takeda, K.; Alayoubi, A.; Mirdamadi, E.; Zidan, A.; Bauer, S.R.; Degheidy, H. 3D bioprinting optimization of human mesenchymal stromal cell laden gelatin-alginate-collagen bioink. Biomed. Mater. 2023, 18, 015016. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen-vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; IntechOpen: London, UK, 2011; pp. 17–52. Available online: https://www.intechopen.com (accessed on 16 November 2011).
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Mousavi, S.; Khoshfetrat, A.B.; Khatami, N.; Ahmadian, M.; Rahbarghazi, R. Comparative study of collagen and gelatin in chitosan-based hydrogels for effective wound dressing: Physical properties and fibroblastic cell behavior. Biochem. Biophys. Res. Commun. 2019, 518, 625–631. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz-Robak, B.; Swiderski, F. Hydrokoloidy pochodzenia roślinnego jako zamienniki żelatyny. Bezpieczna Żywność 2001, 1, 31–37. [Google Scholar]
- Thakur, G.; Mitra, A.; Basak, A. Genipin crosslinked drug-gelatin composite for drug transport and cytocompatibility. Biomed. Eng. 2011, 23, 113–118. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Gentile, P.; Saracino, S.; Chiono, V.; Nandagiri, V.K.; Muzio, G.; Canuto, R.A.; Ciardelli, G. Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int. J. Biol. Macromol. 2011, 49, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef] [PubMed]
- Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen bioinks for bioprinting: A systematic review of hydrogel properties, bioprinting parameters, protocols, and bioprinted structure characteristics. Biomedicines 2021, 9, 1137. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lo, A.C.Y. Collagen–alginate composite hydrogel: Application in tissue engineering and biomedical sciences. Polymers 2021, 13, 1852. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed. Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef] [PubMed]
- Panwar, A.; Tan, L.P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Jiao, T.; Lian, Q.; Lian, W.; Wang, Y.; Li, D.; Reis, R.L.; Oliveira, J.M. Properties of Collagen/Sodium Alginate Hydrogels for Bioprinting of Skin Models. J. Bionic Eng. 2023, 20, 105–118. [Google Scholar] [CrossRef]
- Karami, A.; Tebyanian, H.; Sayyad Soufdoost, R.; Motavallian, E.; Barkhordari, A.; Nourani, M.R. Extraction and Characterization of Collagen with Cost-Effective Method from Human Placenta for Biomedical Applications. World J. Plast. Surg. 2019, 8, 352–358. [Google Scholar] [CrossRef]
- Matinong AM, E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Shahidi, F. Isolation and Characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chem. 2010, 119, 1519–1526. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Maher, M.; Glattauer, V.; Onofrillo, C.; Duchi, S.; Yue, Z.; Hughes, T.C.; Ramshaw JA, M.; Wallace, G.G. Suitability of Marine-and Porcine-Derived Collagen Type I Hydrogels for Bioprinting and Tissue Engineering Scaffolds. Mar. Drugs 2022, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Benjakul, S.; Nalinanon, S.; Shahidi, F. Fish Collagen. In Food Biochemistry and Food Processing, 2nd ed.; Wiley Online Library: Hoboken, NJ, USA, 2012; pp. 365–387. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Furtado, M.; Chen, L.; Chen, Z.; Chen, A.; Cui, W. Development of fish collagen in tissue regeneration and drug delivery. Eng. Regen. 2022, 3, 217–231. [Google Scholar] [CrossRef]
- Hackett, C.; Grim, B.J. The Global Religious Landscape A Report on the Size and Distribution of the World’s Major Religious Groups as of 2010; Pew Research Center: Washington, DC, USA, 2012; Available online: https://www.researchgate.net/publication/264782431_The_Global_Religious_Landscape_A_Report_on_the_Size_and_Distribution_of_the_World's_Major_Religious_Groups_as_of_2010 (accessed on 28 June 2024).
- Tang, Y.; Jin, S.; Li, X.; Li, X.; Hu, X.; Chen, Y.; Huang, F.; Yang, Z.; Yu, F.; Ding, G. Physicochemical properties and biocompatibility evaluation of collagen from the skin of giant croaker (Nibea japonica). Mar. Drugs 2018, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zong, C.; Jin, S.; Zheng, J.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.; Yang, Z.; Tang, Y.; et al. Optimization of extraction conditions and characterization of pepsin-solubilised collagen from skin of giant croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, S.; Tang, Y. Marine collagen peptides promote cell proliferation of NIH-3T3 fibroblasts via NF-κB signaling pathway. Molecules 2019, 24, 4201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Han, Y.; Huang, Y.; Wei, P.; Yin, J.; Jiang, J. The application of 3D bioprinting in urological diseases. Mater. Today Bio 2022, 16, 100388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Dai, Y.; Yang, L.; Chen, G. Application of 3D Bioprinting in Urology. Micromachines 2022, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Basu, B. An Overview of Hydrogel-Based Bioinks for 3D Bioprinting of Soft Tissues. J. Indian. Inst. Sci. 2019, 99, 405–428. [Google Scholar] [CrossRef]
- Bejoy, A.M.; Makkithaya, K.N.; Hunakunti, B.B.; Hegde, A.; Krishnamurthy, K.; Sarkar, A.; Lobo, C.F.; Keshav, D.V.S.; Dharshini, G.; Dhivya Dharshini, S.; et al. An insight on advances and applications of 3d bioprinting: A review. Bioprinting 2021, 24, e00176. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Bartniak, M.; Rosińska, K.; Bociąga, D. Optimization of the Preparation Process Stages of the Bioink Compositions Based on Sodium Alginate and Gelatin to Improve the Viability of Biological Material Contained in Hydrogel 3D Printouts. Eng. Biomater. 2022, 165, 7–16. [Google Scholar] [CrossRef]
- Sionkowska, A.; Musiał, K.; Gadomska, M.; Adamiak, K. Fish Collagen and Chitosan Mixtures as a Promising Biomaterial for Potential Use in Medicine and Cosmetic Industry. Eng. Biomater. 2022, 164, 16–24. [Google Scholar] [CrossRef]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E.; Ferreira, A.M. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater. Sci. Eng. C 2018, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Ratanavaraporn, J.; Damrongsakkul, S.; Sanchavanakit, N.; Banaprasert, T.; Kanokpanont, S. Comparison of Gelatin and Collagen Scaffolds for Fibroblast Cell Culture. J. Met. Mater. Miner. 2006, 16, 31–36. Available online: https://api.semanticscholar.org/CorpusID:35933980 (accessed on 1 July 2024).
- Li, Y.; Tanaka, T. Kinetics of swelling and shrinking of gels. J. Chem. Phys. 1990, 92, 1365–1371. [Google Scholar] [CrossRef]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and printability of Sodium alginate -Gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 2019, 9, 19914. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.D.A.S.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef] [PubMed]
- Evingür, G.A.; Sağlam, N.A.; Çimen, B.; Uysal, B.Ö.; Pekcan, Ö. The WS2 dependence on the elasticity and optical band gap energies of swollen PAAm composites. J. Compos. Mater. 2021, 55, 71–76. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A review on the adaption of alginate-gelatin hydrogels for 3D cultures and bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Shoichet, M.S.; Li, R.H.; White, M.L.; Winn, S.R. Stability of hydrogels used in cell encapsulation: An in vitro comparison of alginate and agarose. Biotechnol. Bioeng. 1996, 50, 374–381. [Google Scholar] [CrossRef]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bohidar, H.B. Effect of cationic size on gelation temperature and properties of gelatin hydrogels. Int. J. Biol. Macromol. 2005, 35, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cranck, J. Diffusion with Moving Boundary. In The Mathematics of Diffusion; Oxford at the Clarendon Press: London, UK, 1956; pp. 97–120. [Google Scholar]
- Chen, Y.; Zhou, Y.; Wang, C. Investigation of Collagen-Incorporated Sodium Alginate Bioprinting Hydrogel for Tissue Engineering. J. Compos. Sci. 2022, 6, 227. [Google Scholar] [CrossRef]
- Geevarghese, R.; Somasekharan, L.T.; Bhatt, A.; Kasoju, N.; Nair, R.P. Development and evaluation of a multicomponent bioink consisting of alginate, gelatin, diethylaminoethyl cellulose and collagen peptide for 3D bioprinting of tissue construct for drug screening application. Int. J. Biol. Macromol. 2022, 207, 278–288. [Google Scholar] [CrossRef]
- BioRender.com. Available online: https://www.biorender.com/ (accessed on 2 July 2024).
- ISO 10993-5:2009; European Standard EN ISO 10993-5 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. European Committee for Standardization: Brussels, Belgium, 2009.

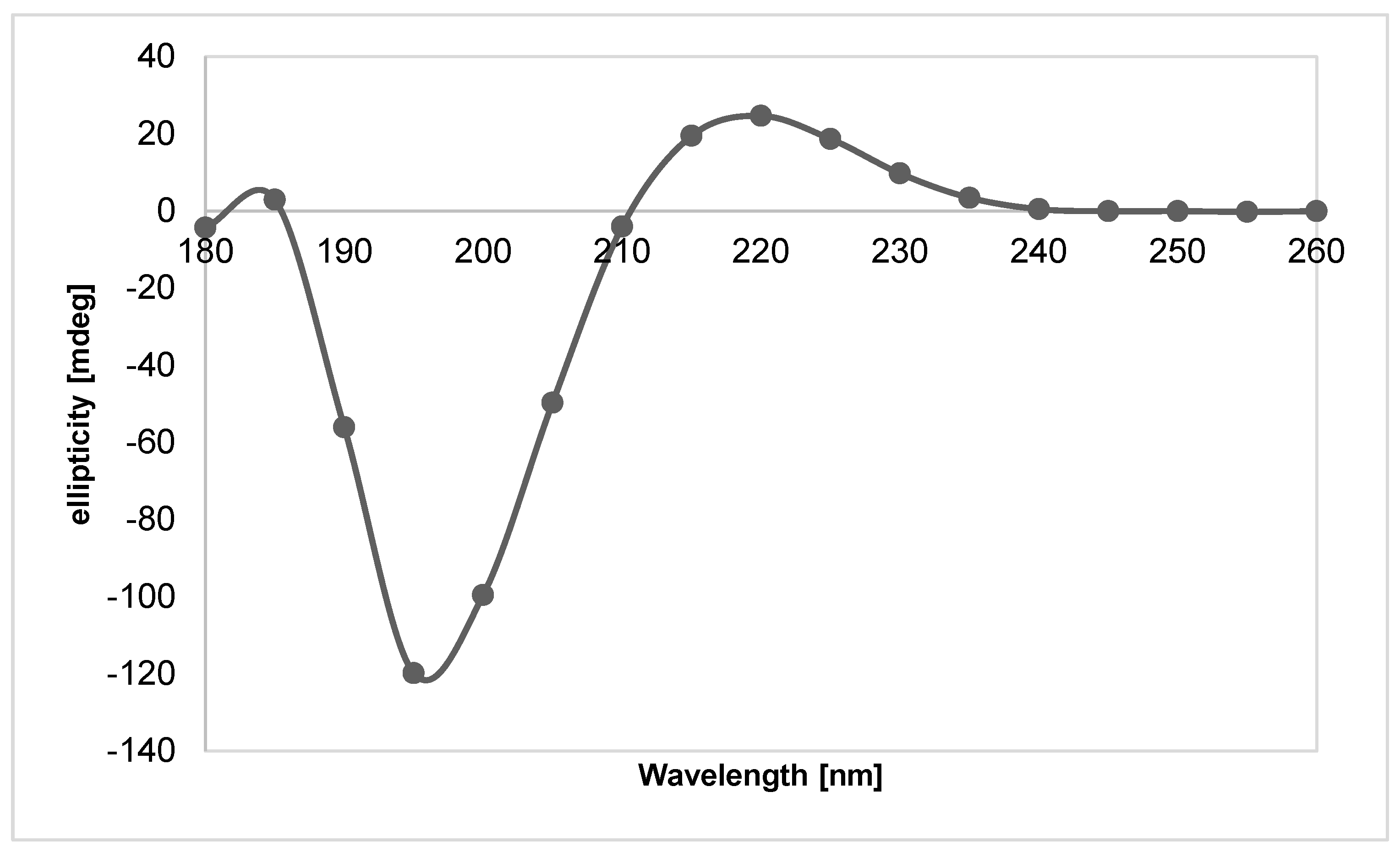
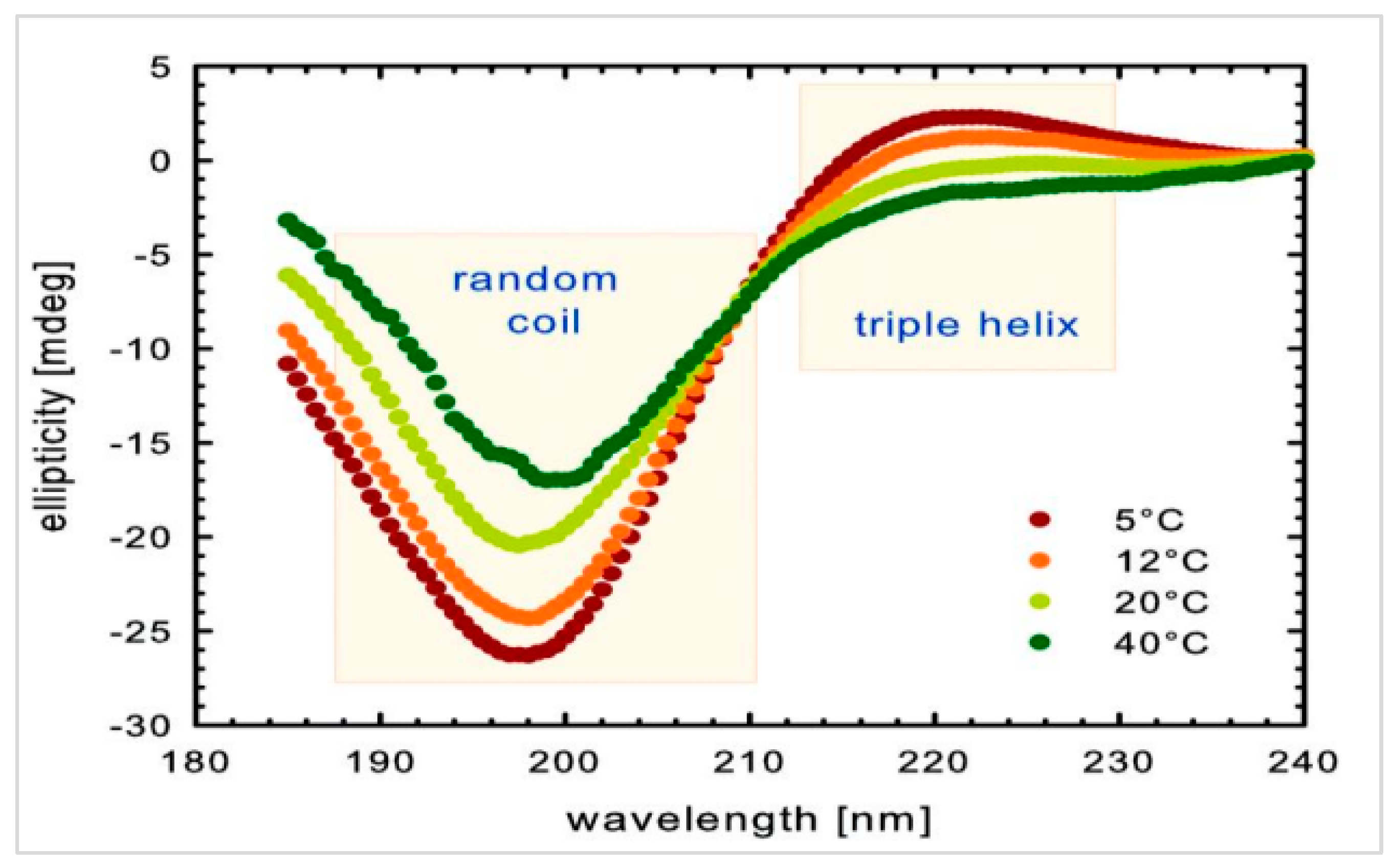
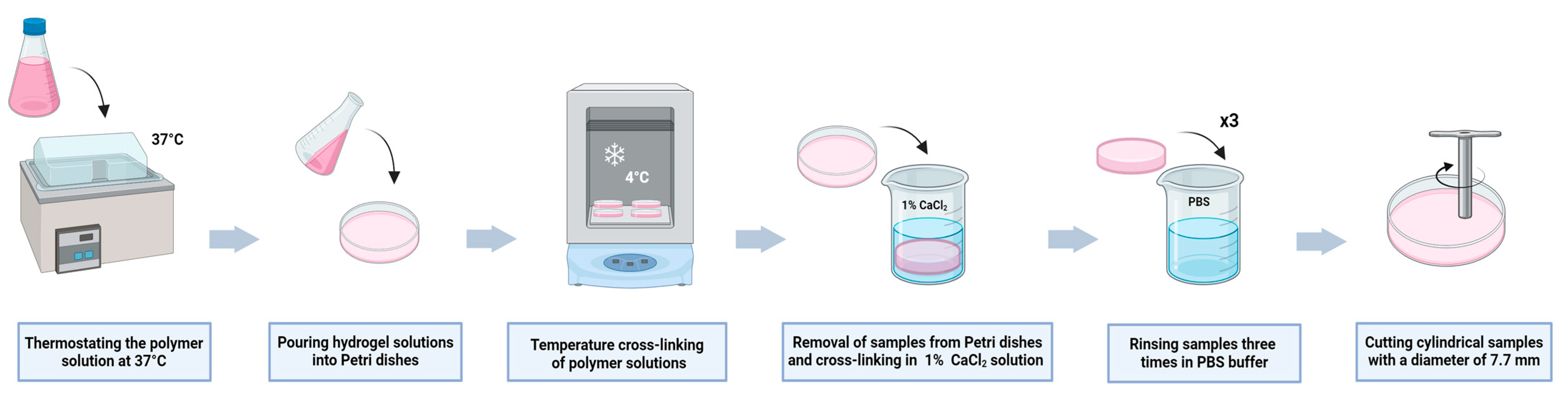
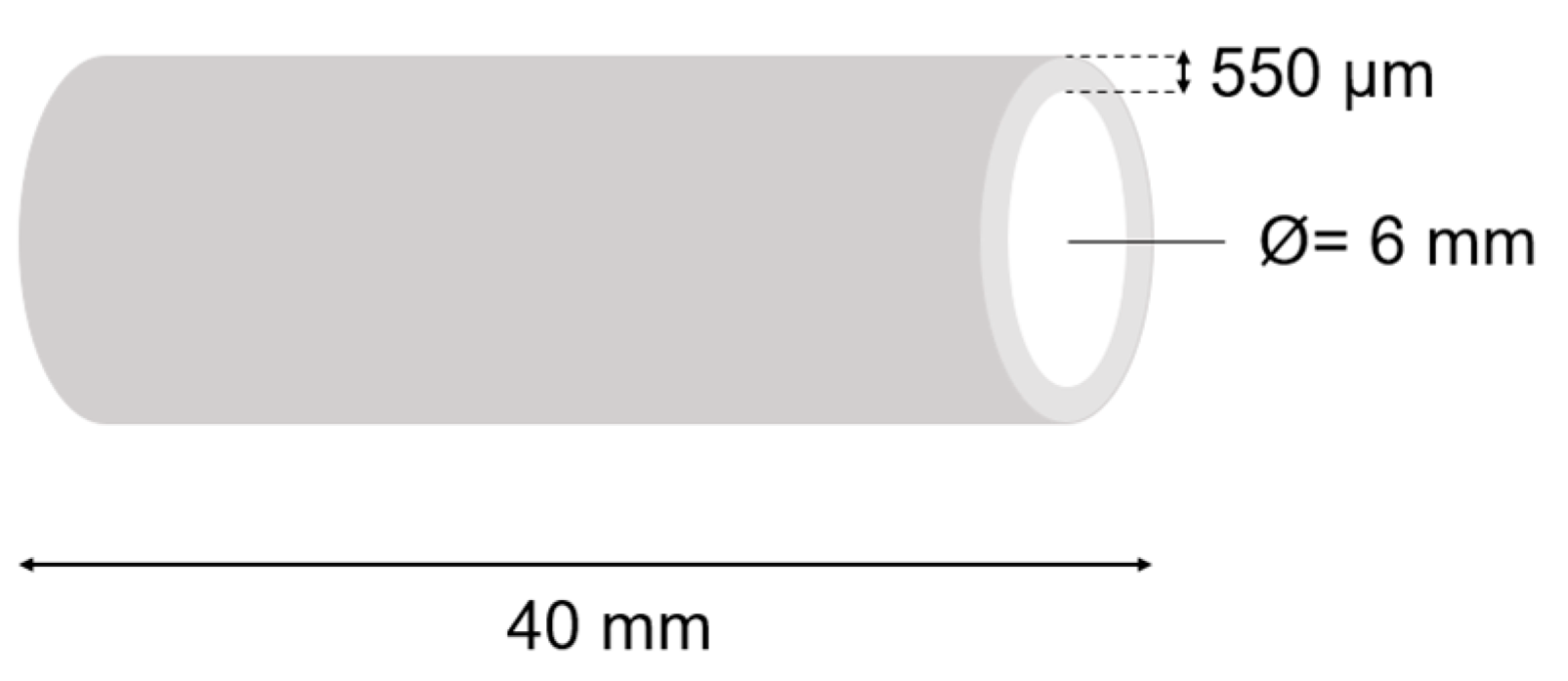
| The Analyzed Hydrogel Composition | 2A9G | 2A9C | 2A6C | 2A3C |
|---|---|---|---|---|
| 3D Printability | Possibility of 3D printing | Possibility of 3D printing | Possibility of 3D printing | Possibility of 3D printing |
| Symbol of the Hydrogel Composition | Concentration % (w/v) | ||
|---|---|---|---|
| Sodium Alginate (A) | Gelatin (G) | Fish Collagen (C) | |
| 2A3C | 2 | - | 3 |
| 2A6C | 2 | - | 6 |
| 2A9C | 2 | - | 9 |
| 2A9G | 2 | 9 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicka, A.; Bartniak, M.; Waśko, J.; Kolesińska, B.; Grabarczyk, J.; Bociaga, D. The Impact of Gelatin and Fish Collagen on Alginate Hydrogel Properties: A Comparative Study. Gels 2024, 10, 491. https://doi.org/10.3390/gels10080491
Wierzbicka A, Bartniak M, Waśko J, Kolesińska B, Grabarczyk J, Bociaga D. The Impact of Gelatin and Fish Collagen on Alginate Hydrogel Properties: A Comparative Study. Gels. 2024; 10(8):491. https://doi.org/10.3390/gels10080491
Chicago/Turabian StyleWierzbicka, Adrianna, Mateusz Bartniak, Joanna Waśko, Beata Kolesińska, Jacek Grabarczyk, and Dorota Bociaga. 2024. "The Impact of Gelatin and Fish Collagen on Alginate Hydrogel Properties: A Comparative Study" Gels 10, no. 8: 491. https://doi.org/10.3390/gels10080491
APA StyleWierzbicka, A., Bartniak, M., Waśko, J., Kolesińska, B., Grabarczyk, J., & Bociaga, D. (2024). The Impact of Gelatin and Fish Collagen on Alginate Hydrogel Properties: A Comparative Study. Gels, 10(8), 491. https://doi.org/10.3390/gels10080491







