Gamma Radiation-Induced Synthesis of Carboxymethyl Cellulose-Acrylic Acid Hydrogels for Methylene Blue Dye Removal
Abstract
1. Introduction
2. Results and Discussion
2.1. Reaction Pathway in Gamma-Irradiated CMC/AAc Hydrogels
2.2. FTIR Spectra Analysis
2.3. Thermal Analysis of Gamma-Radiated CMC/AAc Hydrogels
2.4. Biodegradability of CMC/AAc Hydrogel Films
2.5. Impact of Radiation and AAc Content on Grafting in CMC/AAc Hydrogels
2.6. Impact of Acrylic Acid Concentration on CMC/AAc Hydrogel Properties
2.7. Effect of Standing Time on Water Absorption
2.8. Evaluation of CMC/AAc Hydrogels for Dye Removal from Wastewater
2.9. Adsorption Kinetics of MB on CMC/AAc Hydrogels
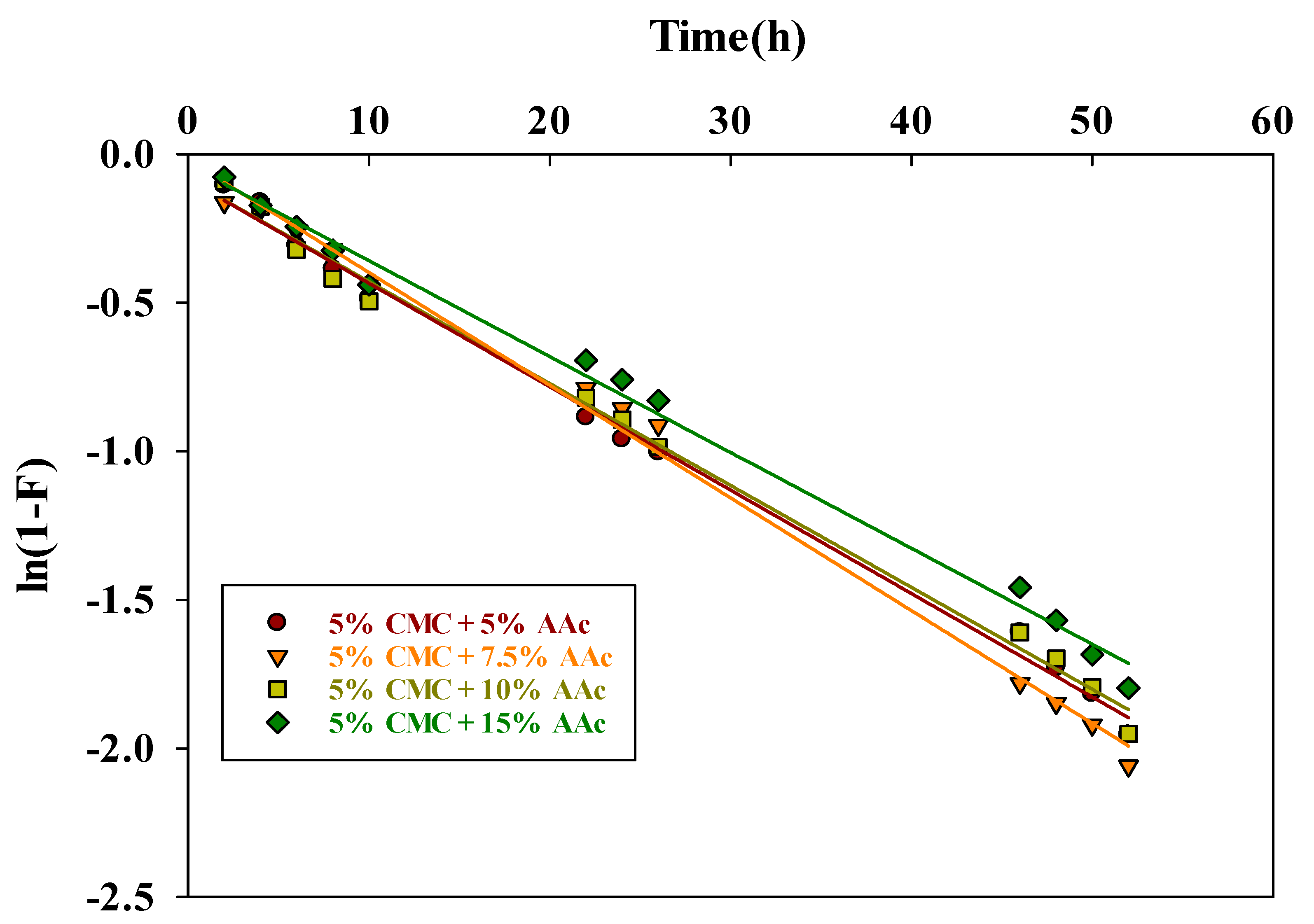
2.9.1. Pseudo-First Order Kinetics
2.9.2. Pseudo-Second Order Kinetics
2.9.3. Elovich Kinetics
2.10. Diffusion Models in Adsorption
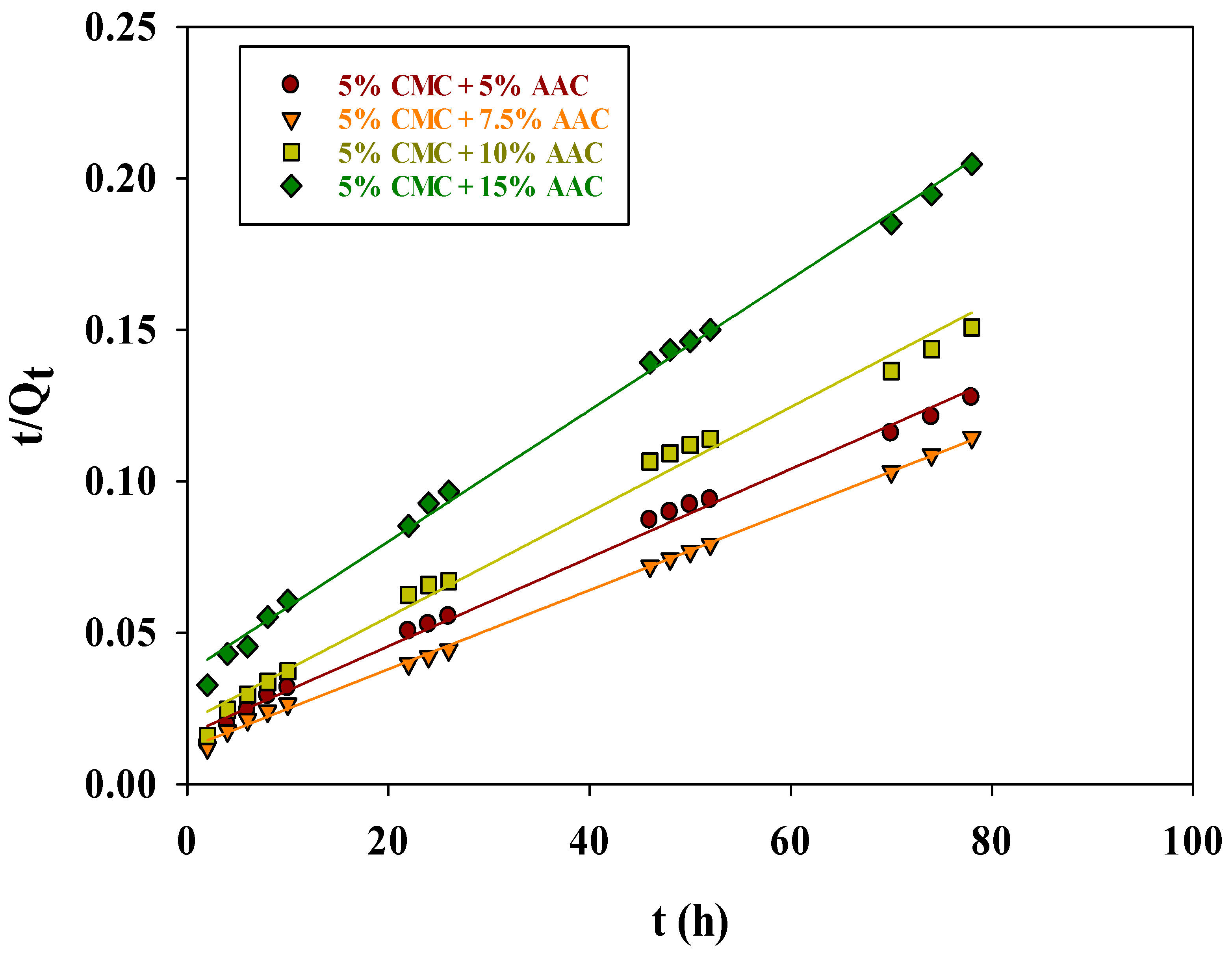
2.10.1. Liquid Film Diffusion Model
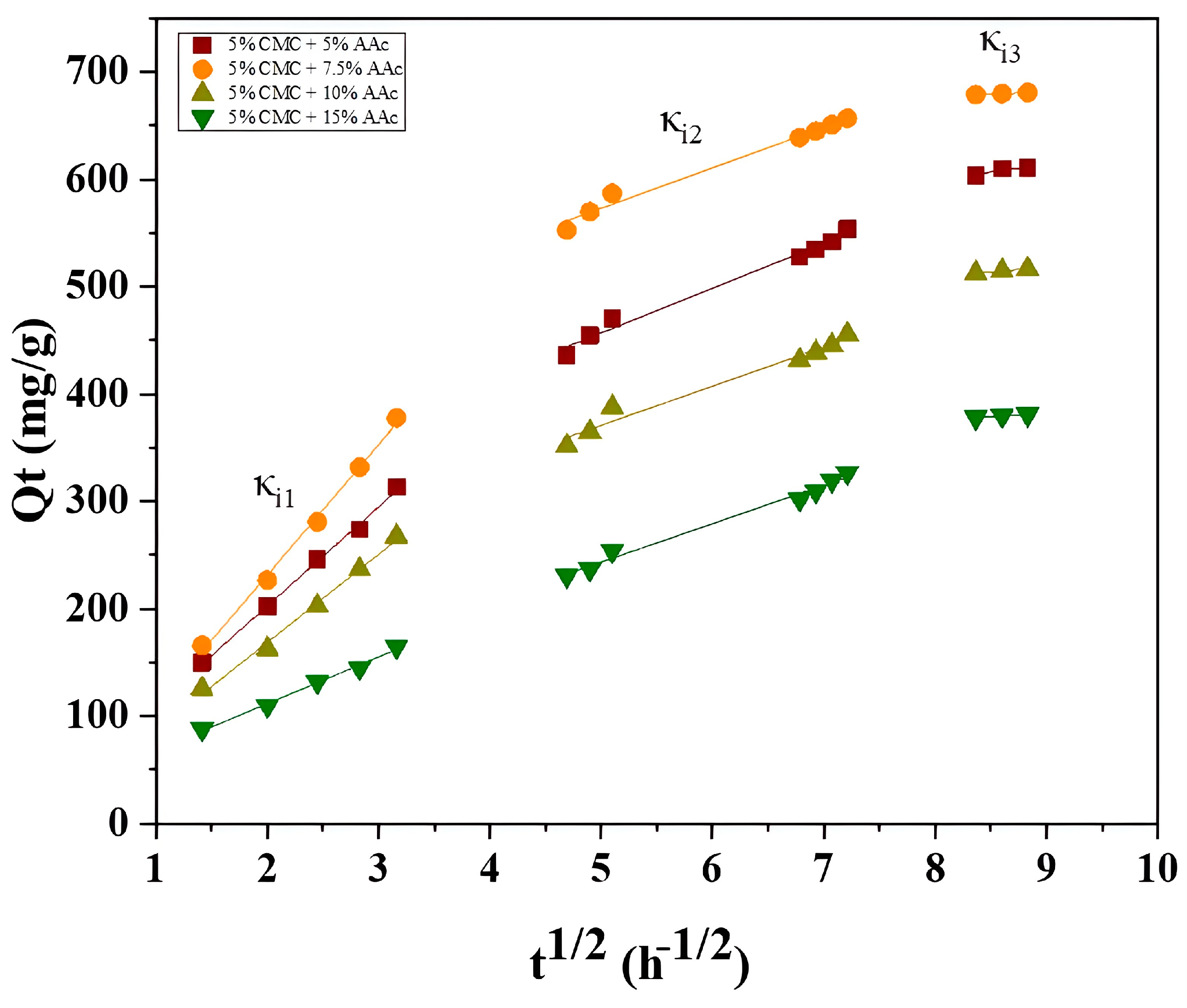
2.10.2. Intraparticle Diffusion Model
2.11. UV-Visible Spectroscopy of MB Adsorption
2.12. Mechanism of MB Adsorption
2.13. Comparative Study
2.14. Desorption Mechanism of MB from CMC/AAc Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of CMC/AAc Hydrogels
4.3. Degree of Grafting Determination
4.4. Gel Fraction Determination
4.5. Measurement of Water Absorbency and Equivalent Water Content
Degree of grafting (%) = (WiW1) × 100
4.6. Characterization
4.7. Biodegradability
4.8. Dye Adsorption Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Contreras, M.; Grande-Tovar, C.D.; Vallejo, W.; Chaves-López, C. Bio-Removal of Methylene Blue from Aqueous Solution by Galactomyces Geotrichum KL20A. Water 2019, 11, 282. [Google Scholar] [CrossRef]
- Sun, L.; Hu, D.; Zhang, Z.; Deng, X. Oxidative Degradation of Methylene Blue via PDS-Based Advanced Oxidation Process Using Natural Pyrite. Int. J. Environ. Res. Public. Health 2019, 16, 4773. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.S. Highly Efficient Removal of Methylene Blue Dye from an Aqueous Solution Using Cellulose Acetate Nanofibrous Membranes Modified by Polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- Mary Ealias, A.; Meda, G.; Tanzil, K. Recent Progress in Sustainable Treatment Technologies for the Removal of Emerging Contaminants from Wastewater: A Review on Occurrence, Global Status and Impact on Biota. Rev. Environ. Contam. Toxicol. 2024, 262, 16. [Google Scholar] [CrossRef]
- Ritter, F.; Waqas Ahmad, H.; Aiman Bibi, H.; Chandrasekaran, M.; Ahmad, S.; Kyriakopoulos, G.L. Citation: Sustainable Wastewater Treatment Strategies in Effective Abatement of Emerging Pollutants. Water 2024, 16, 2893. [Google Scholar]
- Santal, A.R.; Rani, R.; Kumar, A.; Sharma, J.K.; Singh, N.P. Biodegradation and Detoxification of Textile Dyes Using a Novel Bacterium Bacillus Sp. AS2 for Sustainable Environmental Cleanup. Biocatal. Biotransform. 2024, 42, 41–55. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Chakraborty, N.; Jeong, J.-H. Easy fabrication of PVA-CaO-CuO composite films for efficient photocatalyst: Towards distinct luminescence property, morphology and thermal stability. Inorg. Chem. Commun. 2024, 170, 113287. [Google Scholar] [CrossRef]
- Ashraf, M.; Rehman, R.; Hatshan, M.R.; Bathula, C.; Dar, A.; Akram, M. Process Modeling of Methylene Blue Dye Adsorptive Removal by Physio-Chemically Treated Cicer Arietinum Husk for Effective Wastewater Treatment by Green Chemistry. Water Air Soil. Pollut. 2024, 235, 267. [Google Scholar] [CrossRef]
- Tan, T.V.; Nguyen, H.T.T. Activated Carbon Based Rice Husk for Highly Efficient Adsorption of Methylene Blue: Kinetic and Isotherm. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012078. [Google Scholar] [CrossRef]
- Ahmad, W.; Amin, Z.; Ur Rehman, T.; Hussain, F.; Ilyas, M. Treatment of Dyes Contaminated Water Using Surfactants Modifieactivated Carbon Derived from Rice Husk. Desalination Water Treat. 2022, 248, 288–299. [Google Scholar] [CrossRef]
- Wazir, A.H.; Ullah, I.; Yaqoob, K. Chemically Activated Carbon Synthesized from Rice Husk for Adsorption of Methylene Blue in Polluted Water. Environ. Eng. Sci. 2023, 40, 307–317. [Google Scholar] [CrossRef]
- Pourramezan, E.; Omidvar, M.; Motavalizadehkakhky, A.; Zhiani, R.; Darzi, H.H. Enhanced Adsorptive Removal of Methylene Blue Using Ternary Nanometal Oxides in an Aqueous Solution. Biomass Convers. Biorefin. 2024, 14, 1–3. [Google Scholar] [CrossRef]
- Ajala, O.A.; Akinnawo, S.O.; Bamisaye, A.; Adedipe, D.T.; Adesina, M.O.; Okon-Akan, O.A.; Adebusuyi, T.A.; Ojedokun, A.T.; Adegoke, K.A.; Bello, O.S. Adsorptive Removal of Antibiotic Pollutants from Wastewater Using Biomass/Biochar-Based Adsorbents. RSC Adv. 2023, 13, 4678–4712. [Google Scholar] [CrossRef] [PubMed]
- Kolya, H.; Kang, C.W. Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers 2023, 15, 3421. [Google Scholar] [CrossRef]
- Harshan, K.; Rajan, A.P.; Kingsley, D.; Sheikh, R.A.; Aashmi, J.; Rajan, A.P. Plant-Based Biopolymers for Wastewater Pollutants Mitigation. Phys. Sci. Rev. 2024, 9, 1973–1989. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; El-Kamash, A.M.; Hung, Y.T. Applications of Nano-Zeolite in Wastewater Treatment: An Overview. Water 2022, 14, 137. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhu, Y.; Fang, W.; Tan, Y.; He, Z.; Liao, H. Research Status, Trends, and Mechanisms of Biochar Adsorption for Wastewater Treatment: A Scientometric Review. Environ. Sci. Eur. 2024, 36, 25. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural Waste Peels as Versatile Biomass for Water Purification—A Review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Bendaoudi, A.A.; Boudouaia, N.; Jellali, S.; Benhafsa, F.M.; Bengharez, Z.; Papamichael, I.; Jeguirim, M. Facile Synthesis of Double-Cross-Linked Alginate-Based Hydrogel: Characterization and Use in a Context of Circular Economy for Cationic Dye Removal. Waste Manag. Res. 2024, 42, 495–507. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Kumar, R.; Kaushal, D.; Chauhan, V.; Thakur, S.; Shandilya, P.; Sharma, P.P. Gum Acacia Based Hydrogels and Their Composite for Wastewater Treatment: A Review. Int. J. Biol. Macromol. 2024, 262, 129914. [Google Scholar] [CrossRef] [PubMed]
- Dardeer, H.M.; Gad, A.N.; Mahgoub, M.Y. Promising Superabsorbent Hydrogel Based on Carboxymethyl Cellulose and Polyacrylic Acid: Synthesis, Characterization, and Applications in Fertilizer Engineering. BMC Chem. 2024, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Radoor, S.; Karayil, J.; Parameswaranpillai, J.; Siengchin, S. Adsorption of Methylene Blue Dye from Aqueous Solution by a Novel PVA/CMC/Halloysite Nanoclay Bio Composite: Characterization, Kinetics, Isotherm, and Antibacterial Properties. J. Environ. Health Sci. Eng. 2020, 18, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Salama, A.; Zahran, F.; Abdelfattah, M.S.; Alsalme, A.; Bechelany, M.; Barhoum, A. Fabrication of Cellulose Nanocrystals/Carboxymethyl Cellulose/Zeolite Membranes for Methylene Blue Dye Removal: Understanding Factors, Adsorption Kinetics, and Thermodynamic Isotherms. Front. Chem. 2024, 12, 1330810. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pan, Y.; Cai, P. Sugarcane Cellulose-Based Composite Hydrogel Enhanced by g-C3N4 Nanosheet for Selective Removal of Organic Dyes from Water. Int. J. Biol. Macromol. 2022, 205, 37–48. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, W.; Ge, W.; Xia, L.; Li, H.; Song, S. Preparation of Carboxymethyl Cellulose-Based Hydrogel Supported by Two-Dimensional Montmorillonite Nanosheets for Methylene Blue Removal. J. Polym. Environ. 2021, 29, 3918–3931. [Google Scholar] [CrossRef]
- Shi, L.; Liu, W.; Zhang, X.; Hu, J. Adsorption of Methylene Blue from Aqueous Solution by Crosslinked Carboxymethyl Cellulose/Organo-Montmorillonite Composite Hydrogels. J. Polym. Res. 2023, 30, 305. [Google Scholar] [CrossRef]
- Alver, E.; Doğan, D.; Mert, H.; Metin, A.Ü. Environmentally Friendly Pathway Applying Sustainable Resources to Remove Anionic Dye from Aqueous Solutions: Encapsulation in Carboxymethyl Cellulose Nanoneedles. Chem. Pap. 2023, 77, 6365–6376. [Google Scholar] [CrossRef]
- Xia, N.N.; Wu, Q.; Bi, S.L. Ultra Efficient Removal of Heavy-Metal Ions and Dyes Using a Novel Cellulose-Based Three-Dimensional Network. Cellulose 2024, 31, 3747–3761. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, T.; Cheng, Y.; Zhao, K.; Meng, Z.; Huang, J.; Cai, W.; Lai, Y. Recent Advances in Functional Cellulose-Based Materials: Classification, Properties, and Applications. Adv. Fiber Mater. 2024, 6, 1343–1368. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, G.D.; Im, J.N.; Doh, S.J. Optimization of Water-Insoluble Carboxymethyl Cellulose Foam for Wound Dressing Materials. Fibers Polym. 2024, 25, 1975–1983. [Google Scholar] [CrossRef]
- Sayed, A.; Hany, F.; Abdel-Raouf, M.E.-S.; Mahmoud, G.A. Gamma Irradiation Synthesis of Pectin- Based Biohydrogels for Removal of Lead Cations from Simulated Solutions. J. Polym. Res. 2022, 29, 372. [Google Scholar] [CrossRef]
- More, A.P.; Chapekar, S. Irradiation Assisted Synthesis of Hydrogel: A Review. Polym. Bull. 2024, 81, 5839–5908. [Google Scholar] [CrossRef]
- Betraoui, A.; Seddiki, N.; Souag, R.; Guerfi, N.; Semlali, A.; Aouak, T.; Aliouche, D. Synthesis of New Hydrogels Involving Acrylic Acid and Acrylamide Grafted Agar-Agar and Their Application in the Removal of Cationic Dyes from Wastewater. Gels 2023, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Liu, C.; Zeng, H.; Li, J.H. Intramolecular Crosslinking of Polyvinylidene Fluoride by Homogeneous Solution Irradiation. High. Energy Chem. 2021, 55, 436–441. [Google Scholar] [CrossRef]
- Dafader, N.C.; Adnan, M.N.; Haque, M.E.; Huq, D.; Akhtar, F. Study on the Properties of Copolymer Hydrogel Obtained from Acrylamide/2-Hydroxyethyl Methacrylate (HEMA) by the Application of Gamma Radiation. Afr. J. Pure Appl. Chem. 2011, 5, 111–118. [Google Scholar]
- Said, H.M.; Alla, S.G.A.; El-Naggar, A.W.M. Synthesis and Characterization of Novel Gels Based on Carboxymethyl Cellulose/Acrylic Acid Prepared by Electron Beam Irradiation. React. Funct. Polym. 2004, 61, 397–404. [Google Scholar] [CrossRef]
- Hullar, T.; Anastasio, C. Yields of Hydrogen Peroxide from the Reaction of Hydroxyl Radical with Organic Compounds in Solution and Ice. Atmos. Chem. Phys. 2011, 11, 7209–7222. [Google Scholar] [CrossRef]
- Gibas, I.; Janik, H. Review: Synthetic polymer hydrogels for biomedical. Chem. Technol. 2010, 4, 297–304. [Google Scholar]
- Devine, D.M.; Higginbotham, C.L. The Synthesis of a Physically Crosslinked NVP Based Hydrogel. Polymer 2003, 44, 7851–7860. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; He, Y.G.; Ren, F.X.; Wang, G.X. Synthesis, Characterization, and Applied Properties of Carboxymethyl Cellulose and Polyacrylamide Graft Copolymer. Carbohydr. Polym. 2009, 78, 95–99. [Google Scholar] [CrossRef]
- Okieimen, F.E. Preparation, Characterization, and Properties of Cellulose-Polyacrylamide Graft Copolymers. J. Appl. Polym. Sci. 2003, 89, 913–923. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent Crosslinked Carboxymethyl Cellulose-PEG Hydrogels for Potential Wound Dressing Applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef] [PubMed]
- Priya, G.; Narendrakumar, U.; Manjubala, I. Thermal Behavior of Carboxymethyl Cellulose in the Presence of Polycarboxylic Acid Crosslinkers. J. Therm. Anal. Calorim. 2019, 138, 89–95. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent Advances in the Synthesis of Smart Hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Mahon, R.; Balogun, Y.; Oluyemi, G.; Njuguna, J. Correction to Swelling Performance of Sodium Polyacrylate and Poly(Acrylamide-co-acrylic Acid) Potassium Salt. SN Appl. Sci. 2020, 2, 117. [Google Scholar] [CrossRef]
- Sternik, D.; Szewczuk-Karpisz, K.; Siryk, O.; Samchenko, Y.; Derylo-Marczewska, A.; Kernosenko, L.; Pakhlov, E.; Goncharuk, O.; H Ch Ch, C.C. Structure and Thermal Properties of Acrylic Copolymer Gels: Effect of Composition and Cross-Linking Method Heating FTIR Analysis of Gaseous Products. J Therm Anal Calorim 2024, 149, 9057–9072. [Google Scholar] [CrossRef]
- Coleman, M.M.; Ho Lee, K.; Skrovanek, D.J.; Painter, P.C.; Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1986; Volume 19. [Google Scholar]
- Kiatkamjornwong, S.; Mongkolsawat, K.; Sonsuk, M. Synthesis and Property Characterization of Cassava Starch Grafted Poly [Acrylamide-Co-(Maleic Acid)] Superabsorbent via γ-Irradiation. Polymer 2002, 43, 3915–3924. [Google Scholar] [CrossRef]
- Dodda, J.M.; Deshmukh, K.; Bezuidenhout, D.; Yeh, Y.-C. Hydrogels: Definition, History, Classifications, Formation, Constitutive Characteristics, and Applications. In Multicomponent Hydrogels; Royal Society of Chemistry: London, UK, 2023; pp. 1–25. [Google Scholar]
- Wei, L.; Chen, C.; Hou, Z.; Wei, H. Poly (Acrylic Acid Sodium) Grafted Carboxymethyl Cellulose as a High-Performance Polymer Binder for Silicon Anode in Lithium-Ion Batteries. Sci. Rep. 2016, 6, 19583. [Google Scholar] [CrossRef]
- Khutoryanskaya, O.V.; Morrison, P.W.J.; Seilkhanov, S.K.; Mussin, M.N.; Ozhmukhametova, E.K.; Rakhypbekov, T.K.; Khutoryanskiy, V.V. Hydrogen-Bonded Complexes and Blends of Poly(Acrylic Acid) and Methylcellulose: Nanoparticles and Mucoadhesive Films for Ocular Delivery of Riboflavin. Macromol Biosci 2014, 14, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Khatooni, H.; Peighambardoust, S.J.; Foroutan, R.; Mohammadi, R.; Ramavandi, B. Adsorption of Methylene Blue Using Sodium Carboxymethyl Cellulose-g-Poly (Acrylamide-Co-Methacrylic Acid)/Cloisite 30B Nanocomposite Hydrogel. J. Polym. Environ. 2023, 31, 297–311. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 147–156. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zeldowitsch, J. Über Den Mechanismus Der Katalytischen Oxydation von CO an MnO2. Acta Physicochim. URSS 1934, 1, 364–449. [Google Scholar]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The Kinetics of Sorption of Basic Dyes from Aqueous Solution by Sphagnum Moss Peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Yi, X.; Xu, Z.; Liu, Y.; Guo, X.; Ou, M.; Xu, X. Highly Efficient Removal of Uranium (VI) from Wastewater by Polyacrylic Acid Hydrogels. RSC Adv. 2017, 7, 6278–6287. [Google Scholar] [CrossRef]
- Morales, A.; Bordallo, E.; Leon, V.; Rieumont, J. Adsorption and Releasing Properties of Bead Cellulose. Chin. J. Polym. Sci. 2004, 22, 417. [Google Scholar]
- Salunkhe, B.; Schuman, T.P. Super-Adsorbent Hydrogels for Removal of Methylene Blue from Aqueous Solution: Dye Adsorption Isotherms, Kinetics, and Thermodynamic Properties. Macromol 2021, 1, 256–275. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Amar, A.; Loulidi, I.; Jabri, M.; Hadey, C.; Lgaz, H.; Alrashdi, A.A.; Boukhlifi, F. Characterization and Adsorption Capacity of Four Low-Cost Adsorbents Based on Coconut, Almond, Walnut, and Peanut Shells for Copper Removal. Biomass Convers. Biorefinery 2024, 14, 3655–3666. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Wang, L.; Tuo, Y.; Yan, S.; Ma, J.; Zhang, X.; Shen, Y.; Guo, H.; Han, L. Experimental and DFT Insights into the Adsorption Mechanism of Methylene Blue by Alkali-Modified Corn Straw Biochar. RSC Adv. 2024, 14, 1854–4865. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, F.; Golshan, M.; Haddadi-Asl, V.; Salami-Kalajahi, M. Adsorption Kinetics of Methylene Blue from Wastewater Using PH-Sensitive Starch-Based Hydrogels. Sci. Rep. 2023, 13, 11900. [Google Scholar] [CrossRef]
- Abbasi, S.; Nezafat, Z.; Javanshir, S.; Aghabarari, B. Bionanocomposite MIL-100 (Fe)/Cellulose as a High-Performance Adsorbent for the Adsorption of Methylene Blue. Sci. Rep. 2024, 14, 14497. [Google Scholar] [CrossRef]
- Pourbaba, R.; Abdulkhani, A.; Rashidi, A.; Ashori, A. Lignin Nanoparticles as a Highly Efficient Adsorbent for the Removal of Methylene Blue from Aqueous Media. Sci. Rep. 2024, 14, 9039. [Google Scholar] [CrossRef]
- Rahman, N.; Dafader, N.C.; Marjub, M.M.; Sultana, S.; Miah, A.R.; Chowdhury, U. Efficiency of Biodegradable Acrylic Acid-Chitosan Hydrogel in Eliminating Methylene Blue from Wastewater. J. Polym. Sci. Technol. 2018, 3, 5–10. [Google Scholar]
- Bhuyan, M.M.; Dafader, N.C.; Hara, K.; Okabe, H.; Hidaka, Y.; Rahman, M.M.; Khan, M.M.R.; Rahman, N. Synthesis of Potato Starch-Acrylic-Acid Hydrogels by Gamma Radiation and Their Application in Dye Adsorption. Int. J. Polym. Sci. 2016, 2016, 11. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Peng, X.; Sun, J.; Li, C.; Zhang, X.; Zhang, H.; Chen, J.; Zhou, X.; Zeng, H.; et al. Hydrogels for the Removal of the Methylene Blue Dye from Wastewater: A Review. Environ. Chem. Lett. 2022, 20, 2665–2685. [Google Scholar] [CrossRef]
- Allouss, D.; Essamlali, Y.; Amadine, O.; Chakir, A.; Zahouily, M. Response Surface Methodology for Optimization of Methylene Blue Adsorption onto Carboxymethyl Cellulose-Based Hydrogel Beads: Adsorption Kinetics, Isotherm, Thermodynamics and Reusability Studies. RSC Adv. 2019, 9, 37858–37869. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, T.W.; Du, Y.; Yao, B.; Duan, S.; Yan, Y.; Hua, M.; Alsaid, Y.; Zhu, X.; He, X. Tough, Anti-Freezing and Conductive Ionic Hydrogels. NPG Asia Mater. 2022, 14, 7101–7144. [Google Scholar] [CrossRef]
- Sievers, J.; Sperlich, K.; Stahnke, T.; Kreiner, C.; Eickner, T.; Martin, H.; Guthoff, R.F.; Schünemann, M.; Bohn, S.; Stachs, O. Determination of Hydrogel Swelling Factors by Two Established and a Novel Non-Contact Continuous Method. J. Appl. Polym. Sci. 2021, 138, 50326. [Google Scholar] [CrossRef]
- Khamizov, R.K. A Pseudo-Second Order Kinetic Equation for Sorption Processes. Russ. J. Phys. Chem. A 2020, 94, 171–176. [Google Scholar] [CrossRef]
- Persano, F.; Malitesta, C.; Mazzotta, E. Cellulose-Based Hydrogels for Wastewater Treatment: A Focus on Metal Ions Removal. Polymers 2024, 16, 1292. [Google Scholar] [CrossRef]


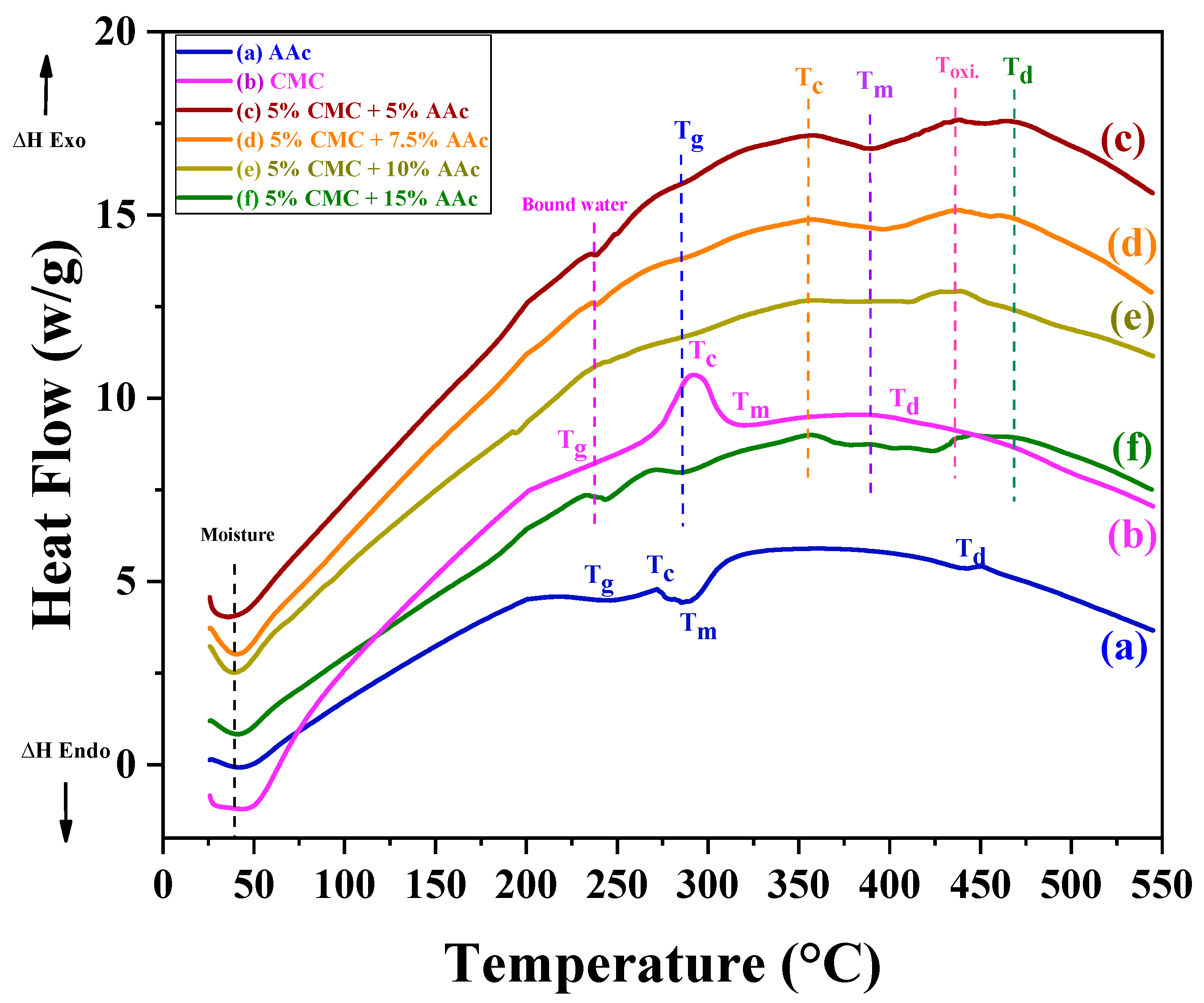

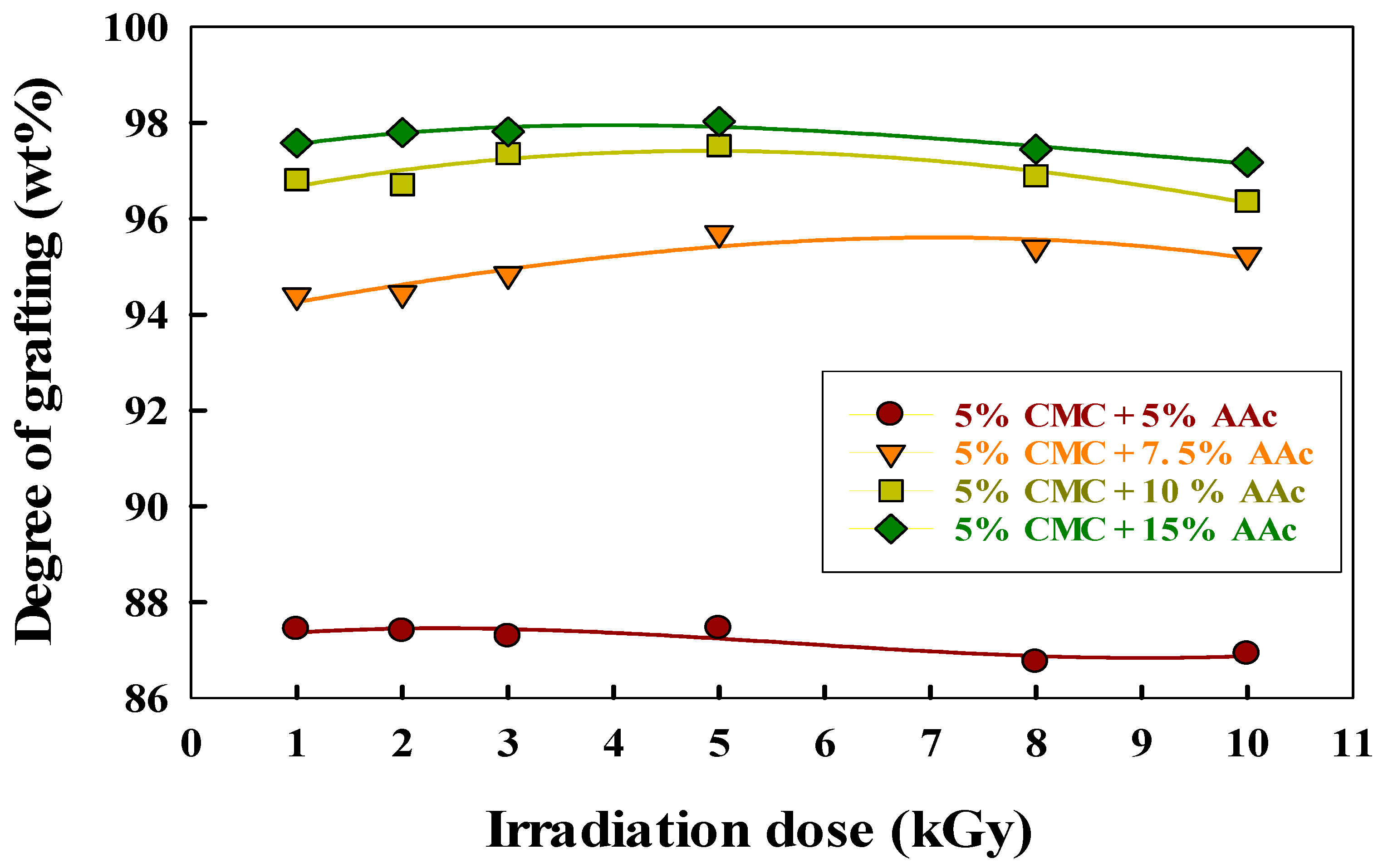

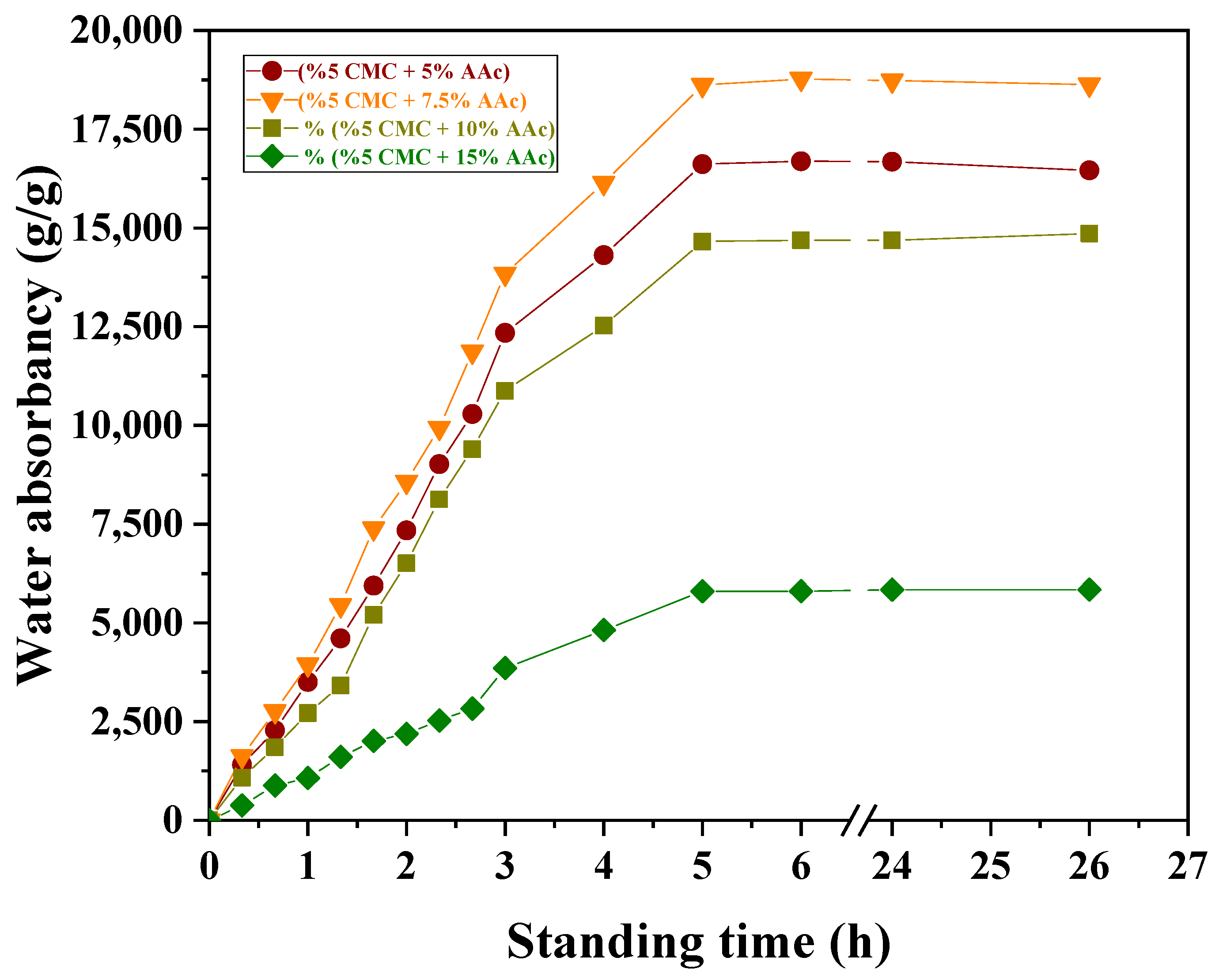
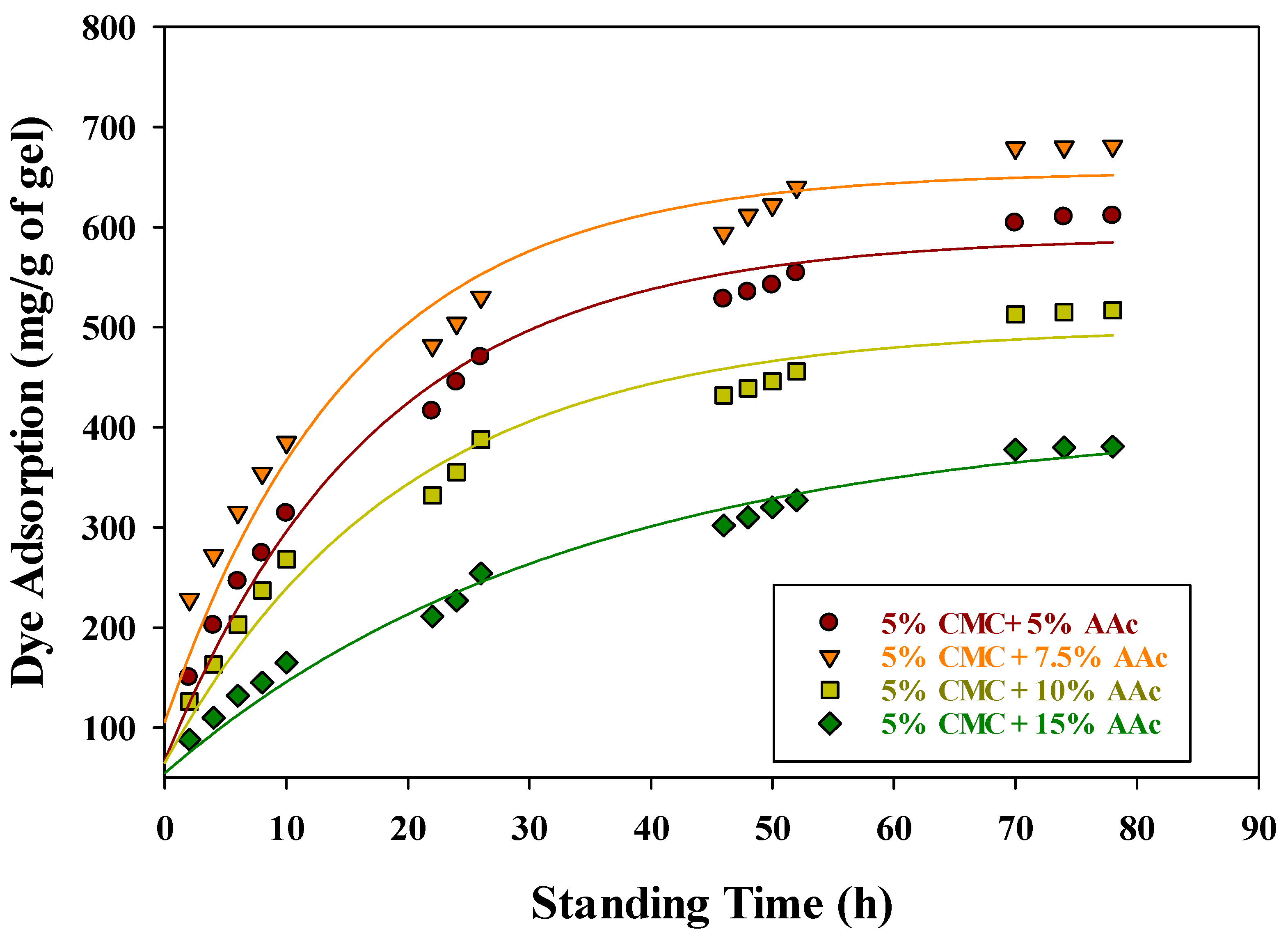




| Samples | T1 a | Tg | Tc | Tm | Toxi | Td |
|---|---|---|---|---|---|---|
| CMC | 231.49 | 228.73 | 293.32 | 322.64 | n. d | 403.58 |
| AAc | 242.01 | 242.01 | 272.05 | 287.84 | n. d | 451.38 |
| 5% CMC + 5% AAc | 237.84 | 285.34 | 356.16 | 389.2 | 436.71 | 467.9 |
| 5% CMC + 7.5% AAc | 238.76 | 290.41 | 357.99 | 396.01 | 438.7 | 468.06 |
| 5% CMC + 10% AAc | 241.76 | 291.58 | 359.65 | 412.55 | 439.53 | 469.88 |
| 5% CMC + 15% AAc | 243.67 | 292.82 | 360.06 | 425.15 | 446.99 | 473.62 |
| Hydrogel Composition | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | Elovich Kinetic Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe, exp. (mg/g) | Qe, cal (mg/g) | k1 (h−1) | R2 | Qe, cal (mg/g) | k1 × 10−4 (h−1) | R2 | α (mg. g−1 h−1) | β (mg. g−1) | R2 | |
| 5% CMC + 5% AAc | 611 | 448.85 | 0.0387 | 0.9865 | 666.67 | 1.38 | 0.9950 | 156.35 | 0.0074 | 0.9911 |
| 5% CMC + 7.5% AAc | 681 | 533.84 | 0.0585 | 0.9895 | 769.23 | 1.42 | 0.9988 | 193.43 | 0.0064 | 0.9783 |
| 5% CMC + 10% AAc | 517 | 373.34 | 0.0341 | 0.9831 | 588.24 | 1.4 | 0.9854 | 128.30 | 0.0089 | 0.9879 |
| 5% CMC + 15% AAc | 381 | 303.46 | 0.0316 | 0.9934 | 454.55 | 1.31 | 0.9977 | 101.69 | 0.0116 | 0.9612 |
| Hydrogel Composition | Liquid Film Diffusion Model | Intraparticle Diffusion (mg.g−1 h−1/2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kfd | Intercept | R2 | R2 | R2 | R2 | κi1 | κi2 | κi3 | |
| 5% CMC + 5% AAc | −0.08598 | 0.99553 | 0.9950 | 0.9970 | 0.9860 | 0.8601 | 92.2542 | 41.6503 | 15.0960 |
| 5% CMC + 7.5% AAc | −0.01859 | 0.99507 | 0.9988 | 0.9962 | 0.9841 | 0.9999 | 121.473 | 37.6668 | 4.2993 |
| 5% CMC + 10% AAc | −0.08519 | 0.99464 | 0.9854 | 0.9935 | 0.9731 | 0.9999 | 82.0731 | 36.4603 | 8.5986 |
| 5% CMC + 15% AAc | −0.03512 | 0.99362 | 0.9977 | 0.9940 | 0.9883 | 0.9671 | 43.4481 | 35.7869 | 6.4586 |
| Adsorbent | Methods | Morphology | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| Alkali-modified corn straw biochar | Pyrolysis | Rough surface and porous structure | 290.71 | [66] |
| CMC-g-Poly (MAA-co-AAm)/cloisite 30B | Free radicle polymerization | Porous structure | 77.51 | [54] |
| Starch/poly (acrylic acid)-based hydrogels | Free radicle polymerization | Amorphous porous structure | 66.7 | [67] |
| MIL-100(Fe)/cellulose | Nanocomposite | Porous hexagonal structure | 384.62 | [68] |
| Lignin nanoparticles | Hydrotropic | Microporous structure | 127.91 | [69] |
| Acrylic acid-chitosan hydrogel | γ- radiation polymerization | Porous structure | 322 | [70] |
| Acrylic acid-grafted-starch | γ- radiation polymerization | Porous structure | 576 | [71] |
| Carboxymethylcellulose-Acrylic acid | γ- radiation polymerization | Porous structure | 681 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, S.C.; Banik, N.; Islam, M.; Rahman Khan, M.M.; Jeong, J.-H. Gamma Radiation-Induced Synthesis of Carboxymethyl Cellulose-Acrylic Acid Hydrogels for Methylene Blue Dye Removal. Gels 2024, 10, 785. https://doi.org/10.3390/gels10120785
Sutradhar SC, Banik N, Islam M, Rahman Khan MM, Jeong J-H. Gamma Radiation-Induced Synthesis of Carboxymethyl Cellulose-Acrylic Acid Hydrogels for Methylene Blue Dye Removal. Gels. 2024; 10(12):785. https://doi.org/10.3390/gels10120785
Chicago/Turabian StyleSutradhar, Sabuj Chandra, Nipa Banik, Mobinul Islam, Mohammad Mizanur Rahman Khan, and Jae-Ho Jeong. 2024. "Gamma Radiation-Induced Synthesis of Carboxymethyl Cellulose-Acrylic Acid Hydrogels for Methylene Blue Dye Removal" Gels 10, no. 12: 785. https://doi.org/10.3390/gels10120785
APA StyleSutradhar, S. C., Banik, N., Islam, M., Rahman Khan, M. M., & Jeong, J.-H. (2024). Gamma Radiation-Induced Synthesis of Carboxymethyl Cellulose-Acrylic Acid Hydrogels for Methylene Blue Dye Removal. Gels, 10(12), 785. https://doi.org/10.3390/gels10120785










