What Is the Impact of Antimicrobial Photodynamic Therapy on Oral Candidiasis? An In Vitro Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Biofilm Biomass Assay
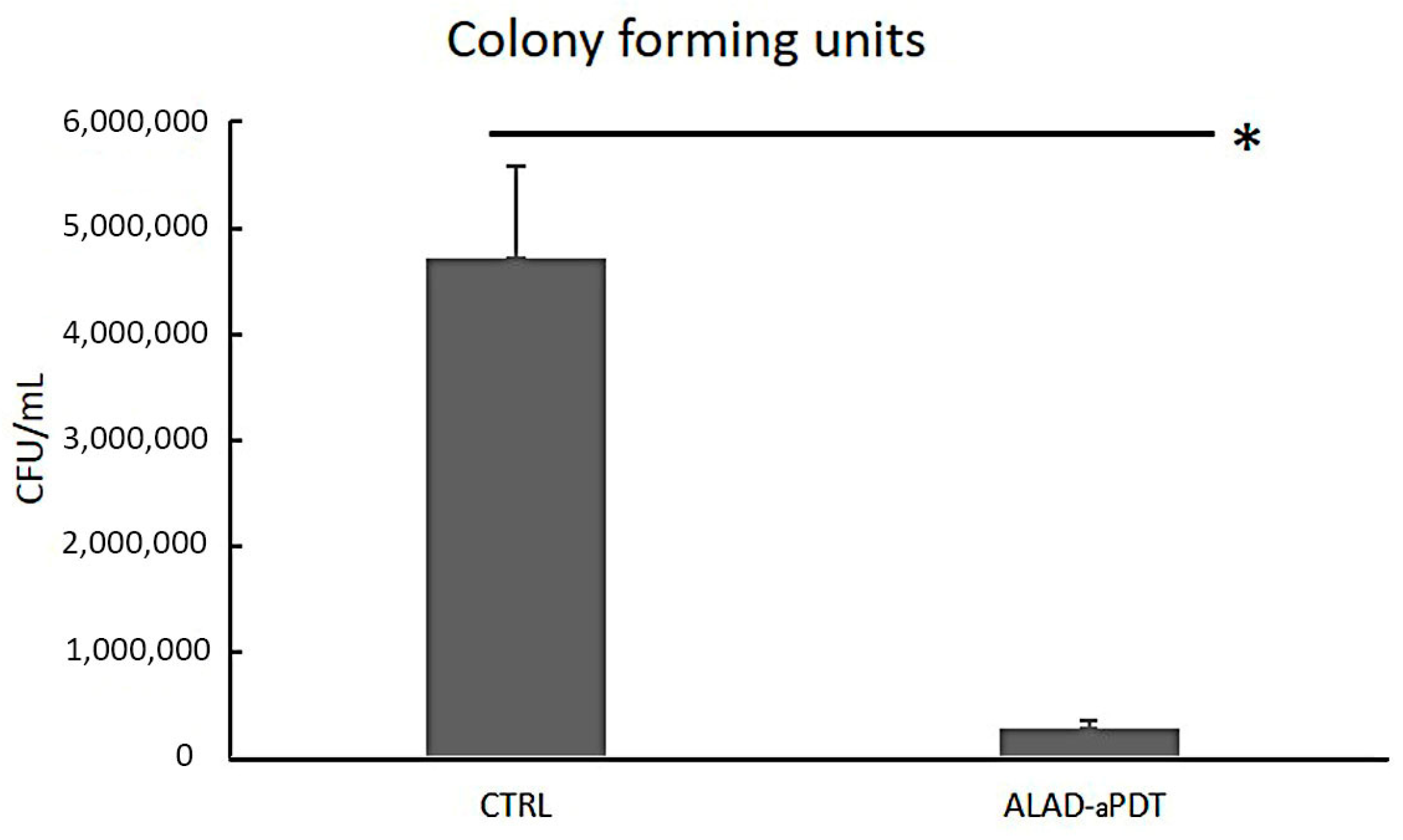
2.2. Colony Forming Units Count
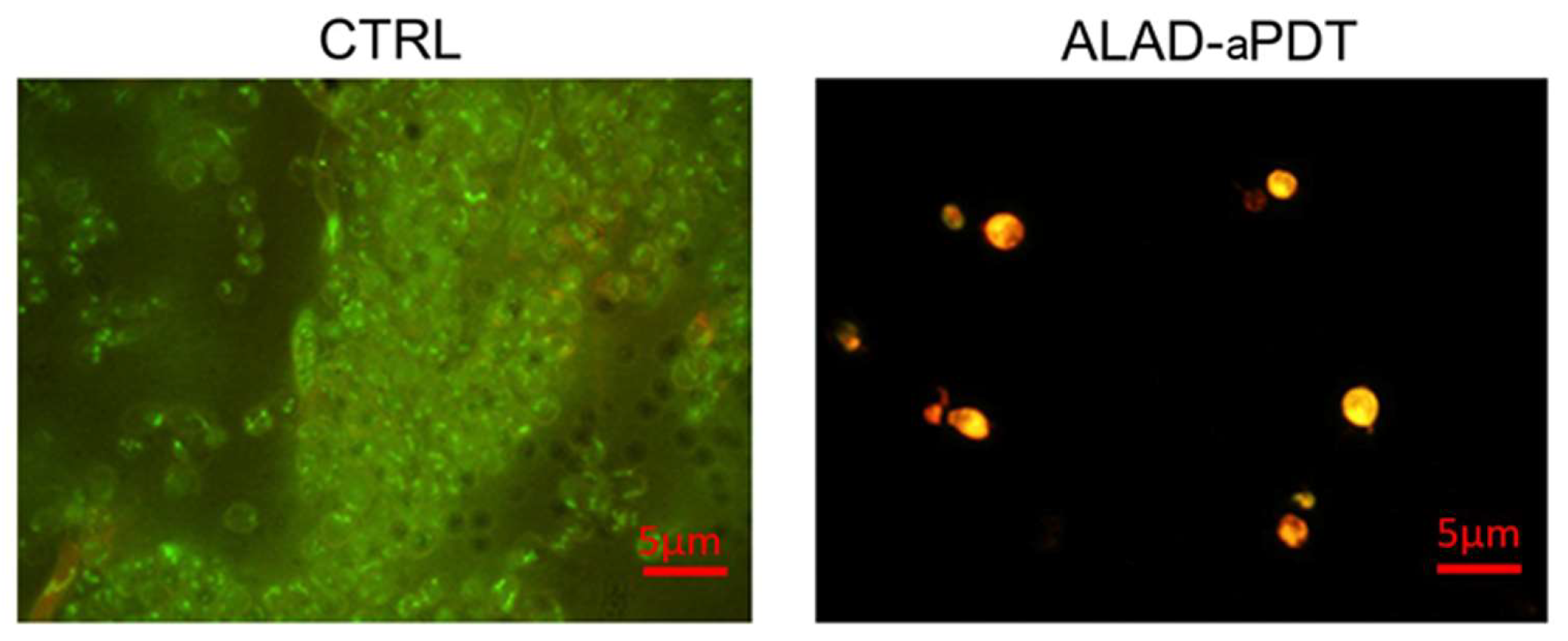
2.3. Live/Dead
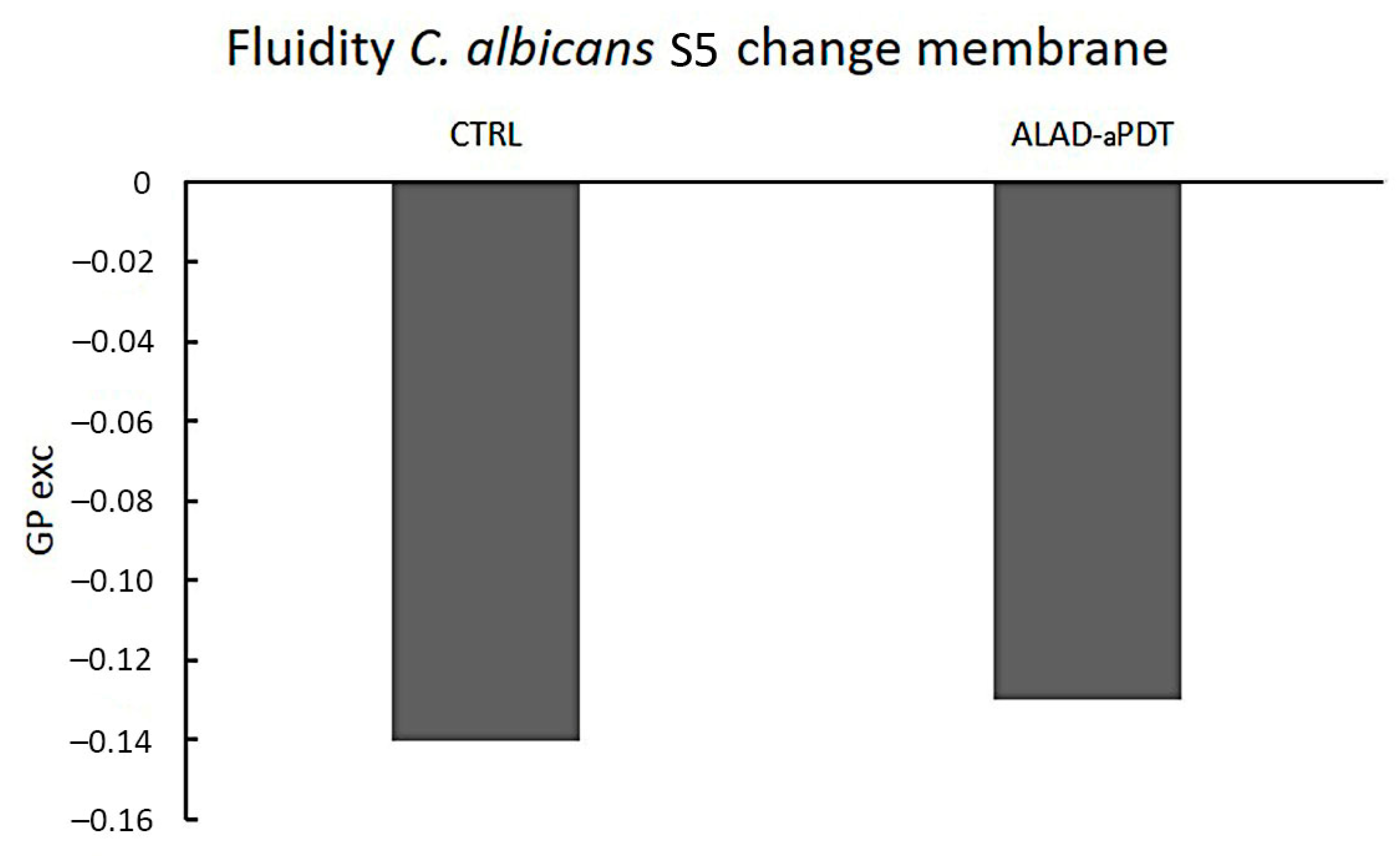
2.4. Membrane Fluidity
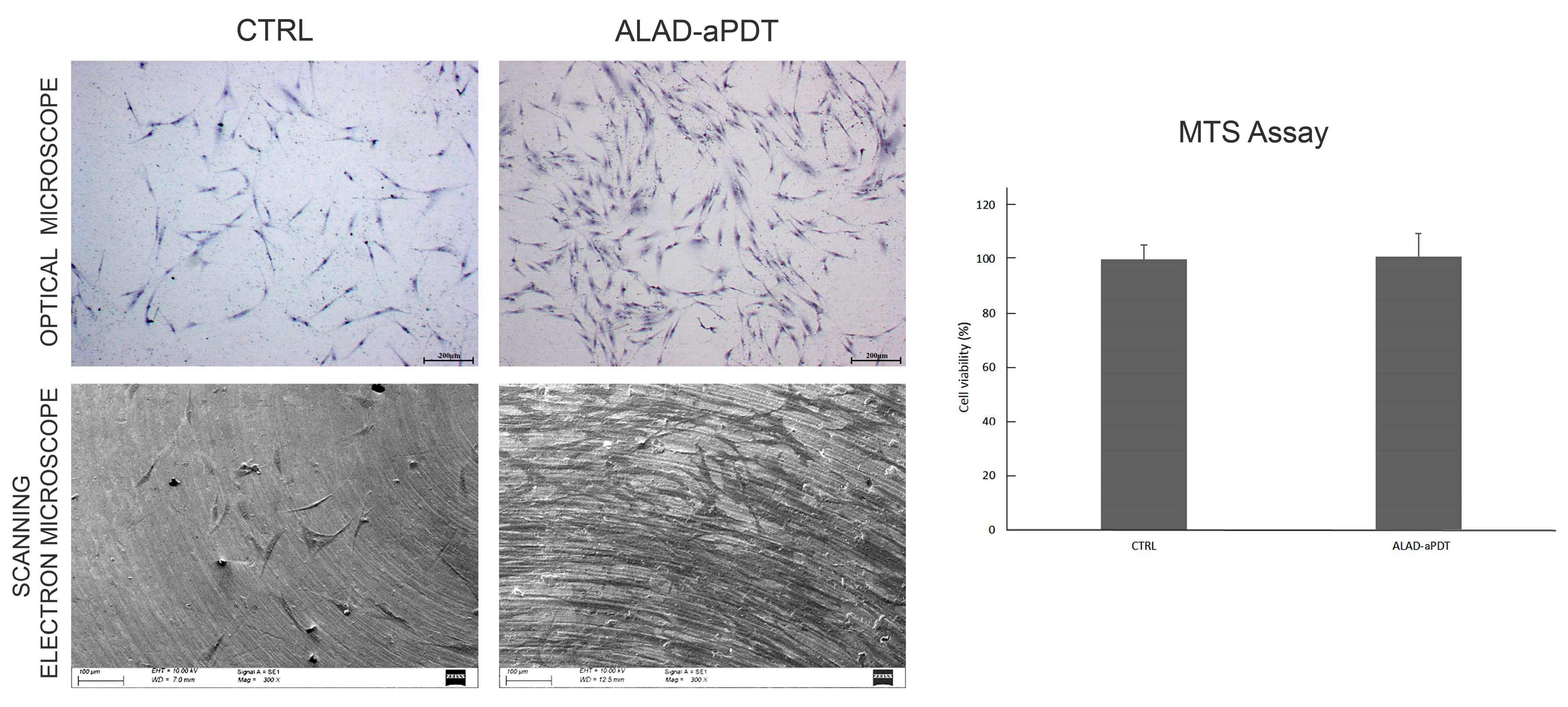
2.5. Cell Viability and Morphological Analysis of Gingival Fibroblasts
2.6. ROS Levels Evaluation
2.7. Evaluation of Collagen Production
3. Conclusions
4. Materials and Methods
4.1. Study Design
- −
- biofilm biomass evaluation by Hucker’s crystal violet staining method;
- −
- the colony forming units (CFU/mL) count for the quantification of cultivable cells;
- −
- cell viability via live/dead analysis;
- −
- membrane fluidity changes.
- −
- Cell viability at 24 h;
- −
- Morphological analysis via SEM and optical microscopy at 24 h;
- −
- Laser scanning microscope analysis at 24 h;
- −
- ROS levels at 0, 3, and 24 h;
- −
- Collagen production using Picro-Sirius red staining.
4.2. Microbiological Analysis
4.2.1. Fungal Culture and Biofilm Preparation
4.2.2. Biofilm Biomass Assay
4.2.3. Colony Forming Units Count
4.2.4. Viability Test
4.2.5. Membrane Fluidity
4.3. Cellular Analysis on Mammalian Cells
4.3.1. Cell Culture
4.3.2. Cell Viability
4.3.3. Optical Microscope Analysis
4.3.4. Scanning Electron Microscope
4.3.5. Confocal Laser Scanning Microscope (CLSM)
4.3.6. ROS Levels
4.3.7. Picro-Sirius Red Staining and Spectrophotometric Analysis
4.4. Statistical Analysis
Author Contributions
Funding
 MUR-Fondo Promozione e Sviluppo-DM737/2021, SCIAMI, “Eco-friendly antimicrobial Strategies to fight Chronic-wound Infections Associated with Multidrug-resistant pathogens for the development of Innovative medical systems”.
MUR-Fondo Promozione e Sviluppo-DM737/2021, SCIAMI, “Eco-friendly antimicrobial Strategies to fight Chronic-wound Infections Associated with Multidrug-resistant pathogens for the development of Innovative medical systems”.Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Denk-Lobnig, M.; Wood, K.B. Antibiotic Resistance in Bacterial Communities. Curr. Opin. Microbiol. 2023, 74, 102306. [Google Scholar] [CrossRef] [PubMed]
- Akpan, A.; Morgan, R. Oral Candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Ayuningtyas, N.F.; Mahdani, F.Y.; Pasaribu, T.A.S.; Chalim, M.; Ayna, V.K.P.; Santosh, A.B.R.; Santacroce, L.; Surboyo, M.D.C. Role of Candida Albicans in Oral Carcinogenesis. Pathophysiology 2022, 29, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.; Miranda, I.M.; Rodrigues, A.G. Candida Parapsilosis Virulence and Antifungal Resistance Mechanisms: A Comprehensive Review of Key Determinants. J. Fungi 2023, 9, 80. [Google Scholar] [CrossRef]
- Stasiewicz, M.; Karpiński, T.M. The Oral Microbiota and Its Role in Carcinogenesis. Semin. Cancer Biol. 2022, 86, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Fazel, N. Oral Candidiasis. Clin. Derm. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharm. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Carvalho, G.G.; Felipe, M.P.; Costa, M.S. The Photodynamic Effect of Methylene Blue and Toluidine Blue on Candida Albicans Is Dependent on Medium Conditions. J. Microbiol. 2009, 47, 619–623. [Google Scholar] [CrossRef]
- Petrini, M.; Pierfelice, T.V.; D’amico, E.; Carlesi, T.; Iezzi, G.; D’arcangelo, C.; Di Lodovico, S.; Piattelli, A.; D’ercole, S. Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus Faecalis and Human Gingival Fibroblasts. Int. J. Environ. Res. Public Health 2022, 19, 3048. [Google Scholar] [CrossRef]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a Novel Gel Containing 5-Aminolevulinic Acid and Red LED against Bacteria Involved in Peri-Implantitis and Other Oral Infections. J. Photochem. Photobiol. B 2020, 205, 111826. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Di Lodovico, S.; Iezzi, G.; Cellini, L.; Tripodi, D.; Piattelli, A.; D’ercole, S. Photodynamic Antibiofilm and Antibacterial Activity of a New Gel with 5-Aminolevulinic Acid on Infected Titanium Surfaces. Biomedicines 2022, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Lepore, S.; Tripodi, D.; Piattelli, A.; D’Ercole, S.; Petrini, M. Efficacy of 5% Aminolaevulinic Acid and Red Light on Enterococcus Faecalis in Infected Root Canals. Gels 2023, 9, 125. [Google Scholar] [CrossRef]
- Bohm, G.C.; Gándara, L.; Di Venosa, G.; Mamone, L.; Buzzola, F.; Casas, A. Photodynamic Inactivation Mediated by 5-Aminolevulinic Acid of Bacteria in Planktonic and Biofilm Forms. Biochem. Pharm. 2020, 177, 114016. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Di Piazza, S.; Chan, J.; Zotti, M.; Hanna, R.; Gheno, E.; Zekiy, A.O.; Pasquale, C.; De Angelis, N.; Amaroli, A. Newly Formulated 5% 5-Aminolevulinic Acid Photodynamic Therapy on Candida Albicans. Photodiagnosis Photodyn. Ther. 2020, 29, 101575. [Google Scholar] [CrossRef] [PubMed]
- Pierfelice, T.V.; D’Amico, E.; Petrini, M.; Pandolfi, A.; D’Arcangelo, C.; Di Pietro, N.; Piattelli, A.; Iezzi, G. The Effects of 5% 5-Aminolevulinic Acid Gel and Red Light (ALAD-PDT) on Human Fibroblasts and Osteoblasts. Gels 2022, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Cabral, F.V.; Silva, C.R.; Silva, D.F.T.; Freitas, A.Z.; Fontes, A.; Ribeiro, M.S. New Insights in Phenothiazinium-Mediated Photodynamic Inactivation of Candida Auris. J. Fungi 2023, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Leonel, L.; Carvalho, M.L.; da Silva, B.M.; Zamuner, S.; Alberto-Silva, C.; Silva Costa, M. Photodynamic Antimicrobial Chemotherapy (PACT) Using Methylene Blue Inhibits the Viability of the Biofilm Produced by Candida Albicans. Photodiagnosis Photodyn. Ther. 2019, 26, 316–323. [Google Scholar] [CrossRef]
- de Souza, S.C.; Junqueira, J.C.; Balducci, I.; Koga-Ito, C.Y.; Munin, E.; Jorge, A.O.C. Photosensitization of Different Candida Species by Low Power Laser Light. J. Photochem. Photobiol. B 2006, 83, 34–38. [Google Scholar] [CrossRef]
- Pupo, Y.M.; Gomes, G.M.; Santos, E.B.; Chaves, L.; Michel, M.D.; Kozlowski, V.A.; Gomes, O.M.M.; Gomes, J.C. Susceptibility of Candida Albicans to Photodynamic Therapy Using Methylene Blue and Toluidine Blue as Photosensitizing Dyes. Acta Odontol. Lat. 2011, 24, 188–192. [Google Scholar]
- Serini, S.M.; Cannizzaro, M.V.; Dattola, A.; Garofalo, V.; Del Duca, E.; Ventura, A.; Milani, M.; Campione, E.; Bianchi, L. The Efficacy and Tolerability of 5-aminolevulinic Acid 5% Thermosetting Gel Photodynamic Therapy (PDT) in the Treatment of Mild-to-moderate Acne Vulgaris. A Two-center, Prospective Assessor-blinded, Proof-of-concept Study. J. Cosmet Derm. 2019, 18, 156–162. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Capezzera, R.; Sala, R.; Zane, C. Photodynamic Therapy of Interdigital Mycoses of the Feet with Topical Application of 5-aminolevulinic Acid. Photodermatol. Photoimmunol. Photomed. 2004, 20, 144–147. [Google Scholar] [CrossRef]
- Oriel, S.; Nitzan, Y. Photoinactivation of Candida Albicans by Its Own Endogenous Porphyrins. Curr. Microbiol. 2010, 60, 117–123. [Google Scholar] [CrossRef]
- Krishnamurthy, S.S.; Prasad, R. Membrane Fluidity Affects Functions of Cdr1p, a Multidrug ABC Transporter of Candida Albicans. FEMS Microbiol. Lett. 1999, 173, 475–481. [Google Scholar] [CrossRef]
- Bessa, L.J.; Ferreira, M.; Gameiro, P. Evaluation of Membrane Fluidity of Multidrug-Resistant Isolates of Escherichia Coli and Staphylococcus Aureus in Presence and Absence of Antibiotics. J. Photochem. Photobiol. B 2018, 181, 150–156. [Google Scholar] [CrossRef]
- Sharom, F.J. The P-Glycoprotein Multidrug Transporter: Interactions with Membrane Lipids, and Their Modulation of Activity. Biochem. Soc. Trans. 1997, 25, 1088–1096. [Google Scholar] [CrossRef]
- Moore, C.M.; Hoh, I.M.; Bown, S.G.; Emberton, M. Does Photodynamic Therapy Have the Necessary Attributes to Become a Future Treatment for Organ-confined Prostate Cancer? BJU Int. 2005, 96, 754–758. [Google Scholar] [CrossRef]
- Kashtan, H.; Konikoff, F.; Haddad, R.; Umansky, M.; Skornick, Y.; Halpern, Z. Photodynamic Therapy for Dysphagia Due to Esophageal Carcinoma. Harefuah 1999, 137, 441–444. [Google Scholar]
- Jang, Y.H.; Koo, G.-B.; Kim, J.-Y.; Kim, Y.-S.; Kim, Y.C. Prolonged Activation of ERK Contributes to the Photorejuvenation Effect in Photodynamic Therapy in Human Dermal Fibroblasts. J. Investig. Dermatol. 2013, 133, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Taninaka, A.; Ugajin, S.; Kurokawa, H.; Nagoshi, Y.; Kamiyanagi, M.; Takeuchi, O.; Matsui, H.; Shigekawa, H. Direct Analysis of the Actin-Filament Formation Effect in Photodynamic Therapy. RSC Adv. 2022, 12, 5878–5889. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Han, J.; Wei, M.; Xu, Y.; Zhang, G.; Zhang, H.; Shi, L.; Liu, X.; Hamblin, M.R.; Wang, X. Remodeling of Dermal Collagen in Photoaged Skin Using Low-dose 5-aminolevulinic Acid Photodynamic Therapy Occurs via the Transforming Growth Factor-β Pathway. J. Biophotonics 2018, 11, e201700357. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M. d. C.; Nader, H.B. Effect of Photodynamic Therapy on the Extracellular Matrix and Associated Components. Braz. J. Med. Biol. Res. 2007, 40, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Orringer, J.S.; Hammerberg, C.; Hamilton, T.; Johnson, T.M.; Kang, S.; Sachs, D.L.; Fisher, G.; Voorhees, J.J. Molecular Effects of Photodynamic Therapy for Photoaging. Arch. Derm. 2008, 144, 1296–1302. [Google Scholar] [CrossRef]
- Pierfelice, T.V.; Lazarevic, M.; Mitic, D.; Nikolic, N.; Radunovic, M.; Iezzi, G.; Piattelli, A.; Milasin, J. Red Light and 5% Aminolaevulinic Acid (5%) Inhibit Proliferation and Migration of Dysplastic Oral Keratinocytes via ROS Production: An In Vitro Study. Gels 2023, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, J.; Zhang, H.; Zhang, J.; Sun, H. Effect of 5-Aminolevulinic Acid Photodynamic Therapy on Candida Albicans Biofilms: An in Vitro Study. Photodiagnosis Photodyn. Ther. 2016, 15, 40–45. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Palmieri, A.; Vella, F. Della; Petruzzi, M.; Botticelli, D.; Carinci, F. Photodynamic Therapy Using 5-Aminolevulinic Acid (Ala) for the Treatment of Chronic Periodontitis: A Prospective Case Series. Appl. Sci. 2022, 12, 3102. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, E.; Di Lodovico, S.; Pierfelice, T.V.; Tripodi, D.; Piattelli, A.; Iezzi, G.; Petrini, M.; D’Ercole, S. What Is the Impact of Antimicrobial Photodynamic Therapy on Oral Candidiasis? An In Vitro Study. Gels 2024, 10, 110. https://doi.org/10.3390/gels10020110
D’Amico E, Di Lodovico S, Pierfelice TV, Tripodi D, Piattelli A, Iezzi G, Petrini M, D’Ercole S. What Is the Impact of Antimicrobial Photodynamic Therapy on Oral Candidiasis? An In Vitro Study. Gels. 2024; 10(2):110. https://doi.org/10.3390/gels10020110
Chicago/Turabian StyleD’Amico, Emira, Silvia Di Lodovico, Tania Vanessa Pierfelice, Domenico Tripodi, Adriano Piattelli, Giovanna Iezzi, Morena Petrini, and Simonetta D’Ercole. 2024. "What Is the Impact of Antimicrobial Photodynamic Therapy on Oral Candidiasis? An In Vitro Study" Gels 10, no. 2: 110. https://doi.org/10.3390/gels10020110
APA StyleD’Amico, E., Di Lodovico, S., Pierfelice, T. V., Tripodi, D., Piattelli, A., Iezzi, G., Petrini, M., & D’Ercole, S. (2024). What Is the Impact of Antimicrobial Photodynamic Therapy on Oral Candidiasis? An In Vitro Study. Gels, 10(2), 110. https://doi.org/10.3390/gels10020110













