The Recent Progress of the Cellulose-Based Antibacterial Hydrogel
Abstract
1. Introduction
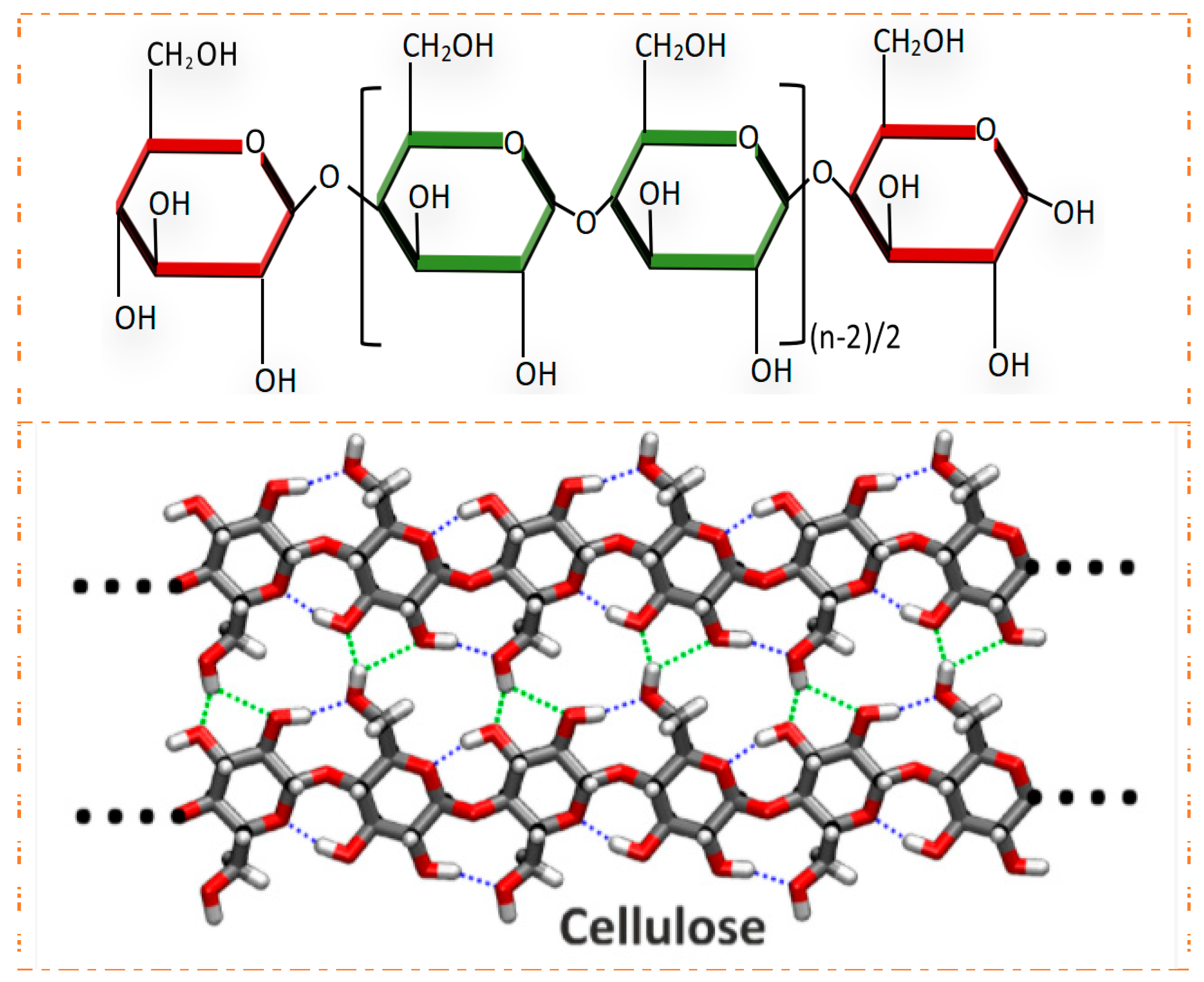
2. Structure and Properties of Cellulose
3. Cellulose-Based Antibacterial Hydrogel
4. Cross-Linking Preparation Method of Cellulose-Based Hydrogel
4.1. Preparation Methods of Physically and Chemically Cross-Linked Hydrogel
4.2. Preparation of Physical Cross-Linking Hydrogels
4.2.1. Hydrogen Bonding
4.2.2. Hydrophobic Interaction
4.2.3. Ionic Bonding
4.3. Chemical Cross-Linking
4.3.1. Free Radical Polymerization
4.3.2. Esterification Reaction
4.3.3. Addition Effect
4.4. Double Cross-Linked Hydrogels
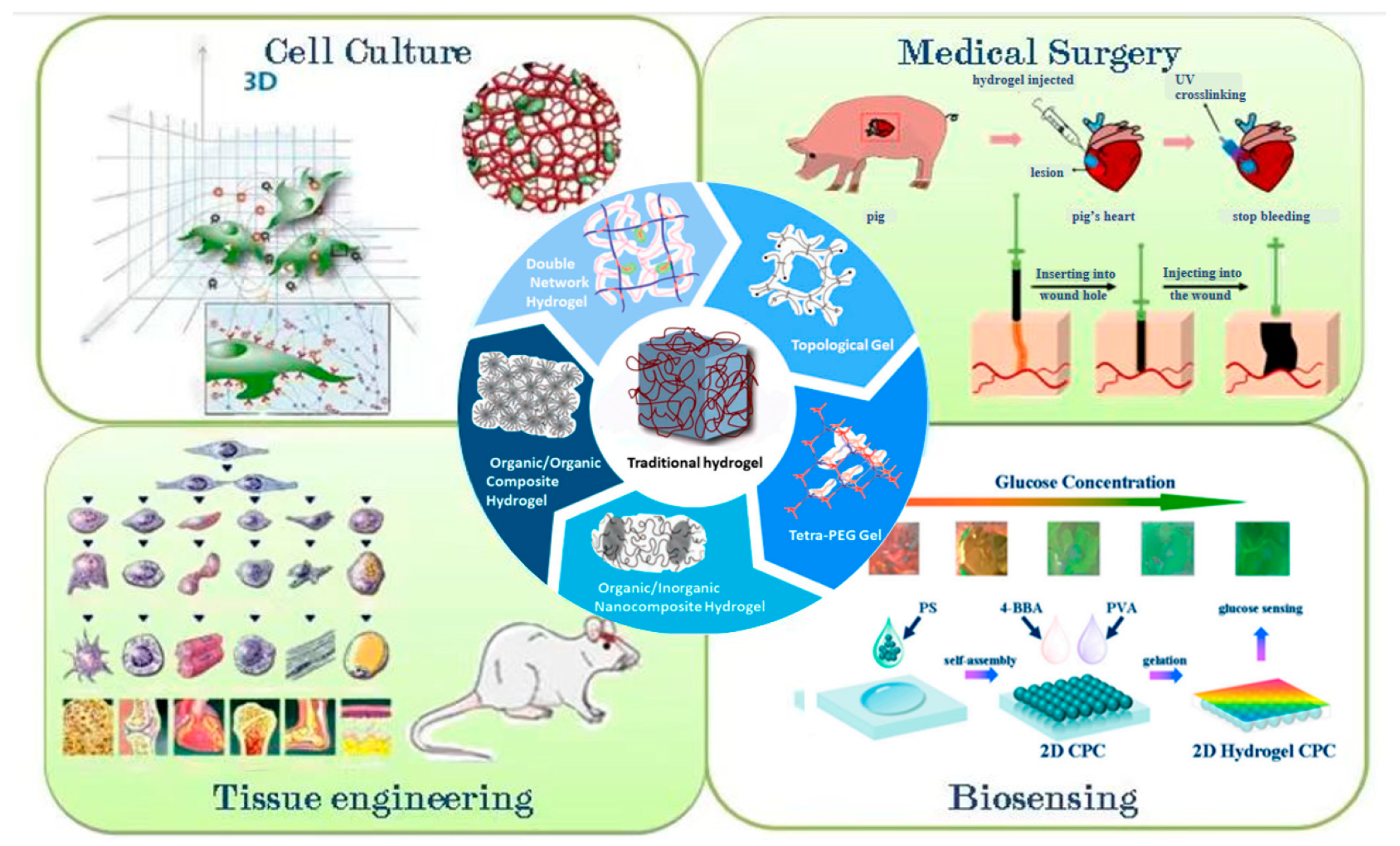
5. Application of Cellulose-Based Hydrogels in the Field of Antibacterial
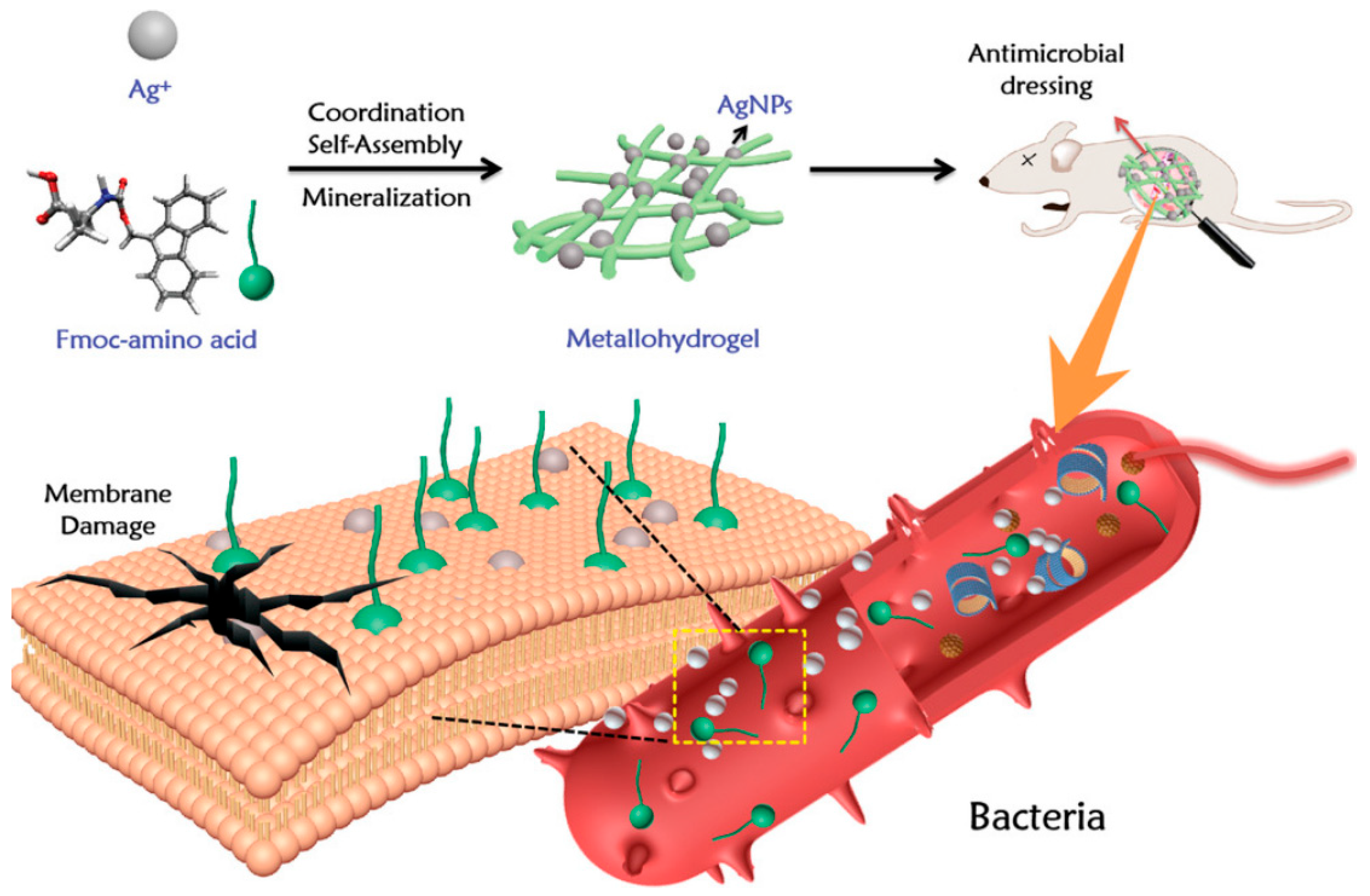
5.1. Cellulose-Based Antibacterial Hydrogels Containing Metal Ion Materials
5.2. Cellulose-Based Antibacterial Hydrogels Containing Metal Oxide Materials
5.3. Cellulose-Based Antibacterial Hydrogels Containing Antibiotics
5.4. Cellulose-Based Antibacterial Hydrogels Containing Biological Extracts
5.5. Cellulose-Based Antibacterial Hydrogels with Synergistic Effects
6. Application of Cellulose-Based Antibacterial Hydrogel
6.1. Wound Dressings
6.2. Tissue Engineering
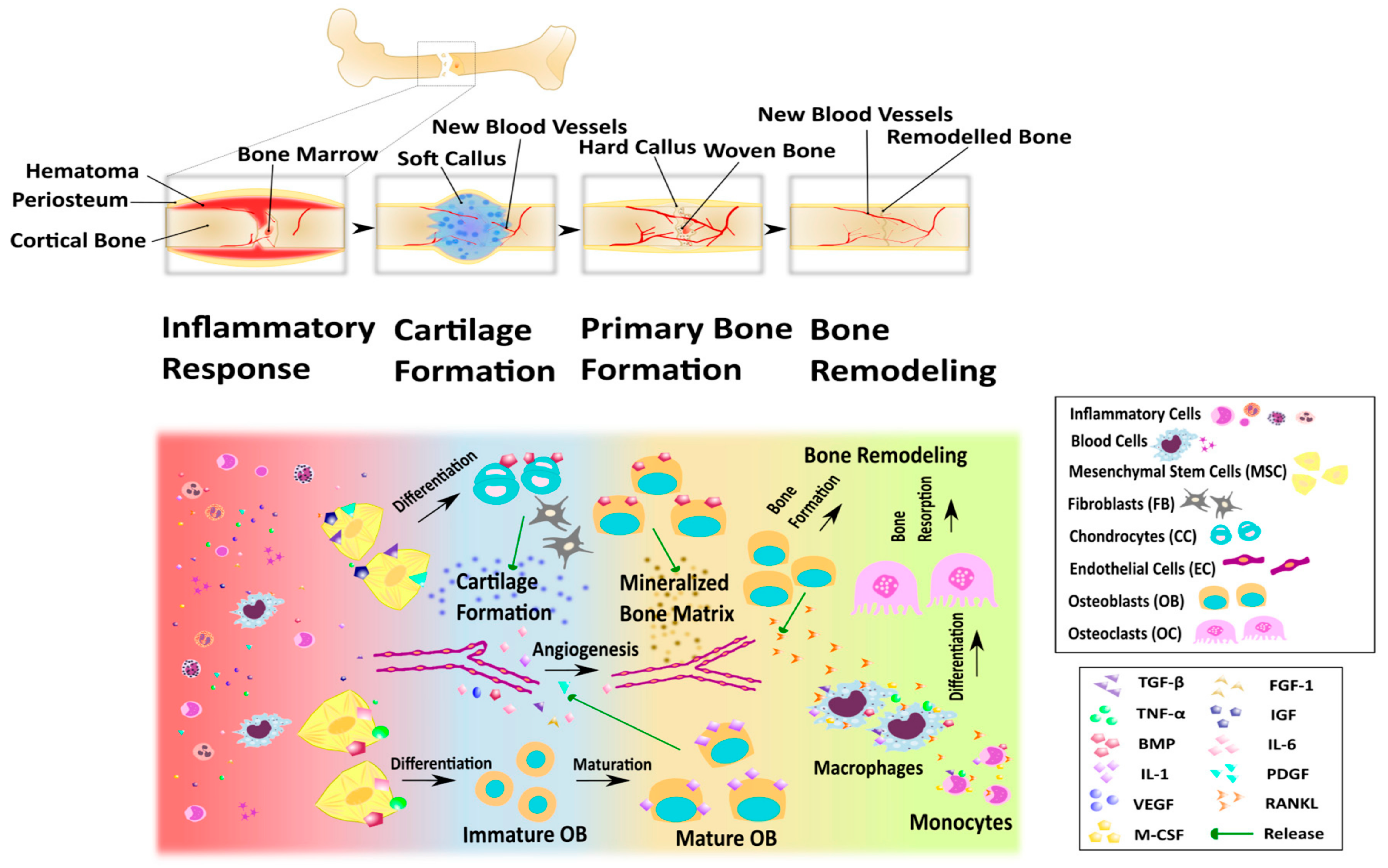
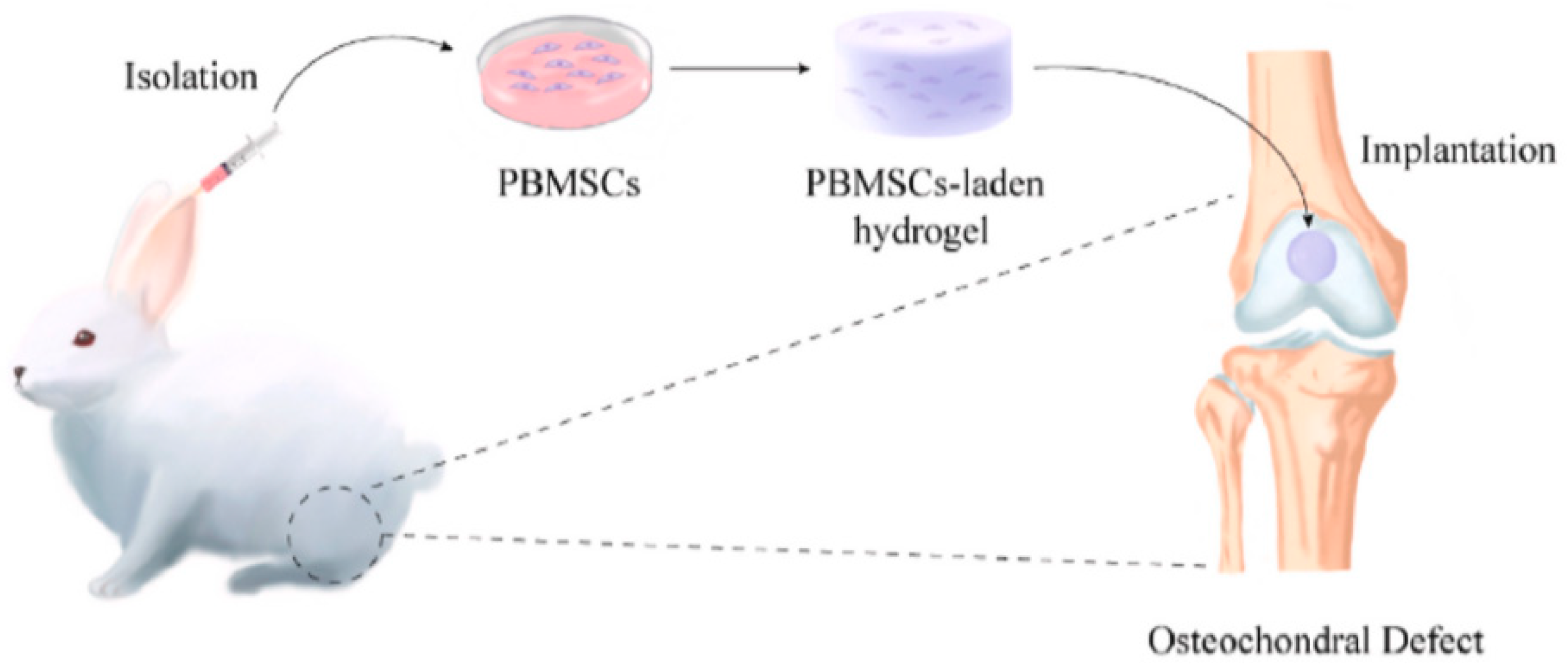
6.3. Bone Tissue
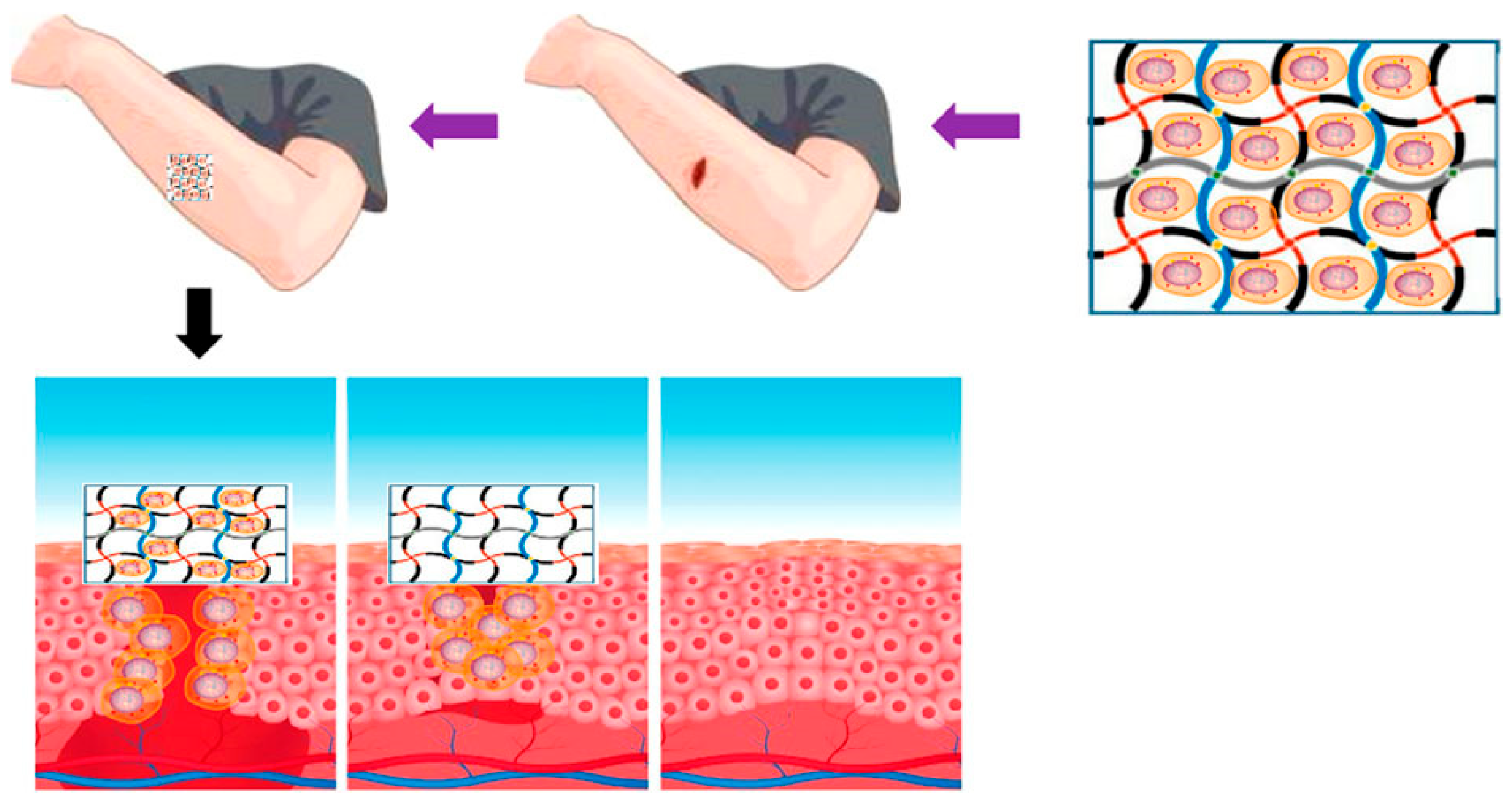
6.4. Self-Healing
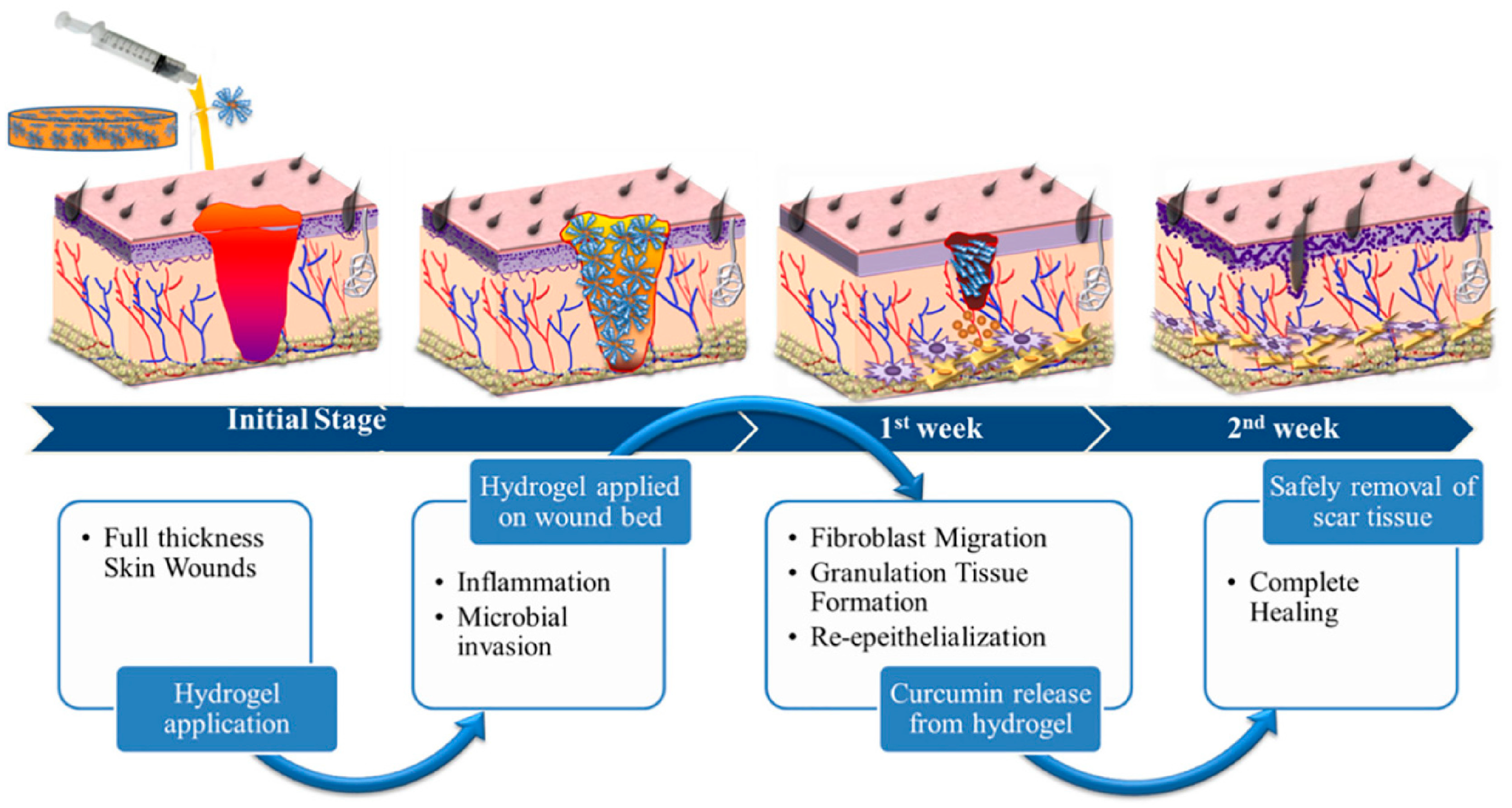
6.5. Skin
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, P.; Kim, K.-S. Membrane Vesicles Derived from Gut Microbiota and Probiotics: Cutting-Edge Therapeutic Approaches for Multidrug-Resistant Superbugs Linked to Neurological Anomalies. Pharmaceutics 2022, 14, 2370. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Martin, R.J. Asthma and Atypical Bacterial Infection. Chest 2007, 132, 1962–1966. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Salick, D.A.; Kretsinger, J.K.; Pochan, D.J.; Scheider, J.P. Inherent Antibacterial Activity of a Peptide-Based β-Hairpin Hydrogel. J. Am. Chem. Soc. 2007, 129, 14793–14799. [Google Scholar] [CrossRef]
- Wang, K.; Hao, Y.T.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.I.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional Hydrogels and Their Application in Drug Delivery, Biosensors, and Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 3160732. [Google Scholar] [CrossRef]
- Hook, A.L.; Chang, C.-Y.; Yang, J.; Atkinson, S.; Langer, R.; Anderson, D.G.; Davies, M.C.; Williams, P.; Alexander, M.R. Discovery of Novel Materials with Broad Resistance to Bacterial Attachment Using Combinatorial Polymer Microarrays. Adv. Mater. 2013, 25, 2542–2547. [Google Scholar] [CrossRef]
- Ahamed, M.; Alsalhi, M.S.; Siddiqui, M.K.J. Silver Nanoparticle Applications and Human Health. Clini. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, Q.; Tai, Y.A.; Wang, Y. Applications of Cellulose Nanomaterials in Stimuli-Responsive Optics. Agric. Food. Chem. 2020, 68, 12940–12955. [Google Scholar] [CrossRef]
- Loelovich, M. Adjustment of Hydrophobic Properties of Cellulose Materials. Polymers 2021, 13, 1241. [Google Scholar] [CrossRef]
- Srivastava, D.; Kuklin, M.S.; Ahopelto, J.; Karttunen, A.J. Electronic band structures of pristine and chemically modified cellulose allomorphs. Carbohyd. Polym. 2020, 243, 116440. [Google Scholar] [CrossRef]
- Mehran, A. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. E-Polymers 2019, 19, 103–119. [Google Scholar]
- Jarvis, M.C. Structure of native cellulose microfibrils, the starting point for nanocellulose manufacture. Phil. Trans. R. Soc. 2018, 376, 20170045. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Dogaru, B.-I.; Popescu, C.-M. Effect of Cellulose Nanocrystals Nanofiller on the Structure and Sorption Properties of Carboxymethyl Cellulose–Glycerol–Cellulose Nanocrystals Nanocomposite Systems. Materials 2020, 13, 2900. [Google Scholar] [CrossRef]
- Ioelovich, M.; Leykin, A. Study of sorption properties of cellulose and its derivatives. Bioresources 2011, 6, 178–195. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yu, T.T.; Peng, L.Y.; Sun, O.N.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 00622. [Google Scholar] [CrossRef]
- Yang, K.R.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J.C. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef]
- Li, Y.S.; Han, Y.; Qin, J.T.; Song, Z.Y.; Cai, H.H.; Du, J.F.; Sun, S.F.; Liu, Y. Photosensitive antibacterial and cytotoxicity performances of a TiO2/carboxymethyl chitosan/poly(vinyl alcohol) nanocomplex hydrogel byin situradiation construction. J. Appl. Polym. Sci. 2016, 133, 44150. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Hu, Y.; Chen, A.; Zhou, L.; Gao, H.; Liu, Y.; Liu, S. Study on photocatalytic antibacterial and sustained-release properties of cellulose/TiO2/β-CD complex hydrogel. J. Nanomater. 2019, 2019, 2326042. [Google Scholar] [CrossRef]
- Qu, J.; Li, J.; Zhu, W.; Xu, Y.; Zhou, S.; Yang, Y.; Qian, X. Hybrid nanocomposite multinetwork hydrogel containing magnesium hydroxide nanoparticles with enhanced antibacterial activity for wound dressing applications. Polymer 2022, 251, 124902. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, C.P.; Chen, Y.; Li, W.; Wang, L.; Yuan, Z.G. A novel mechanical-based injective hydrogel for treatment with aromatase inhibitors caused joint inflammation via the NF-kappaB pathway. ACS Omega 2021, 6, 10242–10249. [Google Scholar] [CrossRef] [PubMed]
- Suljovrujic, E.; Miladinovic, Z.R.; Micic, M.; Suljovrujic, D.; Milicevic, D. The influence of monomer/solvent feed ratio on POEGDMA thermoresponsive hydrogels: Radiation-induced synthesis, swelling properties and VPTT. Radiat. Phys. Chem. 2019, 158, 37–45. [Google Scholar] [CrossRef]
- Patwa, R.; Zandraa, O.; Capáková, Z.; Saha, N.; Sáha, P. Effect of Iron-Oxide Nanoparticles Impregnated Bacterial Cellulose on Overall Properties of Alginate/Casein Hydrogels: Potential Injectable Biomaterial for Wound Healing Applications. Polymers 2020, 12, 2690. [Google Scholar] [CrossRef] [PubMed]
- Luthfianti, H.R.; Waresindo, W.X.; Edikresnha, D.; Chahyadi, A.; Suciati, T.; Noor, F.A.; Khairurrijal, K. Physicochemical characteristics and antibacterial activities of freeze-thawed polyvinyl alcohol/andrographolide hydrogels. ACS Omega 2023, 8, 2915–2930. [Google Scholar] [CrossRef]
- Huo, J.; Jia, Q.; Huang, H.; Zhang, J.; Li, P.; Dong, X.; Huang, W. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chem. Soc. Rev. 2021, 50, 8762–8789. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, L.; Ling, Q.; Liu, J.; Gu, H. Mussel-induced nano-silver antibacterial, self-healing, self-adhesive, anti-freezing, and moisturizing dual-network organohydrogel based on SA-PBA/PVA/CNTs as flexible wearable strain sensors. Polymer 2022, 256, 125270. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, Y.; Xu, S.; Linna, C.; Zhao, X.; Mei, X.; Gao, X. NIR-triggered tea polyphenol-modified gold nanoparticles-loaded hydrogel treats periodontitis by inhibiting bacteria and inducing bone regeneration. Mater. Des. 2023, 225, 111487. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, H.; Zu, Y.; Yin, W. Biodegradable MoOx@MB incorporated hydrogel as light-activated dressing for rapid and safe bacteria eradication and wound healing. RSC Adv. 2022, 12, 8862–8877. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Hu, D.; Dong, A.; Tian, J.; Zhang, W. Highly antibacterial and adhesive hyaluronic acid hydrogel for wound repair. Biomacromolecules 2022, 23, 4766–4777. [Google Scholar] [CrossRef] [PubMed]
- Farnaz, A.; Ali Reza, K. Injectable photosensitizing supramolecular hydrogels: A robust physically cross-linked system based on polyvinyl alcohol/chitosan/tannic acid with self-healing and antioxidant properties. React. Funct. Polym. 2022, 173, 105212. [Google Scholar]
- Laurano, R.; Boffito, M. Thermosensitive micellar hydrogels as vehicles to deliver drugs with different wettability. Front. Bioeng. Biotechnol. 2020, 8, 708. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Supramolecular nanofiber-reinforced Puerarin hydrogels as drug carriers with synergistic controlled release and antibacterial properties. J. Mater. Sci. 2020, 55, 6669–6677. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, T.; Gan, J.; Sun, L.; Guan, C.; Zhang, Q.; Pan, S.; Chen, H. Synthesis and Rheological Characterization of a Novel Salecan Hydrogel. Pharmaceutics 2022, 14, 1492. [Google Scholar] [CrossRef] [PubMed]
- Fatema, N.; Ceballos, R.M.; Fan, C. Modifications of cellulose-based biomaterials for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 993711. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, O.; Budtova, T. Gelation of cellulose-NaOH solutions in the presence of cellulose fibers. Carbohydr. Polym. 2019, 224, 115152. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.M.; El Awad, S.M.; Forti, E.S.; Schueneman, G.T.; Moon, R.J.; Youngblood, J.P. Recent Developments in Cellulose Nanomaterial Composites. Adv. Mater. 2021, 33, 2000718. [Google Scholar] [CrossRef]
- Shen, X.P.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green. Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Xu, X.; Hu, Y.; Wang, S.C.; Chen, X.; Jiang, Y.Y.; Su, J.C. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bio. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Wang, Q.H.; Zhang, Y.; Ma, Y.; Pan, G.Q. Nano-crosslinked dynamic hydrogels for biomedical applications. Mater. Today Bio. 2023, 20, 100640. [Google Scholar] [CrossRef]
- Liu, X.; Pang, J.; Zhang, X.; Wu, Y.; Sun, R. Regenerated cellulose film with enhanced tensile strength prepared with ionic liquid 1-ethyl-3-methylimidazolium acetate (EMIMAc). Cellulose 2013, 20, 1391–1399. [Google Scholar] [CrossRef]
- Shao, C.; Yang, J. Dynamics in cellulose-based hydrogels with reversible cross-links. Self-Heal. Self-Recover. Hydrogels 2020, 285, 319–354. [Google Scholar]
- Jung, H.S.; Kim, H.C.; Prak, W.H. Robust methylcellulose hydrogels reinforced with chitin nanocrystals. Carbohyd. Polym. 2019, 213, 311–319. [Google Scholar] [CrossRef]
- Kaiser, P.; Werner, M.; Jérôme, V.; Hübner, H.; Buchholz, R.; Freitag, R. Cell retention by encapsulation for the cultivation of Jurkat cells in fixed and fluidized bed reactors. Biotechnol. Bioeng. 2014, 111, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Keßler, B. Release of pamidronate from poly(ethyleneimine)/cellulose sulphate complex nanoparticle films: An in situ ATR-FTIR study. Pharm. Biomed. Anal. 2012, 66, 183. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Wang, Y.; Boluk, Y. Investigation of the scaling law on gelation of oppositely charged nanocrystalline cellulose and polyelectrolyte. Carbohydr. Polym. 2014, 105, 214. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Sayed, S.M.; Liu, S.; Oderinde, O.; Kang, M.; Yao, F.; Fu, G. Enhancing the mechanical properties and self-healing efficiency of hydroxyethyl cellulose-based conductive hydrogels via supramolecular interactions. Eur. Polym. J. 2018, 105, 85–94. [Google Scholar] [CrossRef]
- Hennink, W.E.; Van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Duquette, D.; Dumont, M.J. Comparative studies of chemical crosslinking reactions and applications of bio-based hydrogels. Polym. Bull. 2019, 76, 2683–2710. [Google Scholar] [CrossRef]
- Maity, J.; Ray, S.K. Removal of Cu (II) ion from water using sugar cane bagasse cellulose and gelatin based composite hydrogels. Int. J. Biol. Macromol. 2017, 97, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kumar, A.; Han, S.S. Poly(acrylamidoglycolic acid)nanocomposite hydrogels reinforced with cellulose nanocrystals for pH-sensitive controlled release of diclofenac sodium. Polym. Test. 2017, 64, 175–182. [Google Scholar] [CrossRef]
- Kono, H.; Fujita, S. Biodegradable superabsorbent hydrogels derived from cellulose by esterification crosslinking with 1,2,3,4-butanetetracarboxylic dianhydride. Carbohydr. Polym. 2012, 87, 2582–2588. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Senna, A.M.; Novack, K.M.; Botaro, V.R. Synthesis and characterization of hydrogels from cellulose acetate by esterification crosslinking with EDTA dianhydride. Carbohydr. Polym. 2014, 114, 260–268. [Google Scholar] [CrossRef]
- Esposito, A.; Sannino, A.; Cozzolino, A.; Quintiliano, S.N.; Lamberti, M.; Ambrosio, L.; Nicolais, L. Response of intestinal cells and macrophages to an orally administered cellulose-PEG based polymer as a potential treatment for intractable edemas. Biomaterials 2005, 26, 4101–4110. [Google Scholar] [CrossRef]
- Butun, S.; Ince, F.G.; Erdugan, H.; Sahiner, N. One-step fabrication of biocompatible carboxymethyl cellulose polymeric particles for drug delivery systems. Carbohydr. Polym. 2011, 86, 636–643. [Google Scholar] [CrossRef]
- Tavakolian, M.; Munguia-Lopez, J.G.; Valiei, A.; Islam, M.S.; Kinsella, J.M.; Tufenkji, N.; Van de Ven, T.G.M. Highly absorbent antibacterial and biofilm-disrupting hydrogels from cellulose for wound dressing applications. ACS Appl. Mater. Interfaces 2020, 12, 39991–40001. [Google Scholar] [CrossRef]
- Oyarzun-Ampuero, F.; Vidal, A.; Concha, M.; Morales, J.; Orellana, S.; Moreno-Villoslada, I. Nanoparticles for the treatment of wounds. Curr. Pharm. Des. 2015, 21, 4329–4341. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel asymmetric wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Mokhena, T.C.; Luyt, A.S. Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydr. Polym. 2017, 165, 304–312. [Google Scholar] [CrossRef]
- Shao, W.; Wu, J.; Wang, S.; Huang, M.; Liu, X.; Zhang, R. Construction of silver sulfadiazine loaded chitosan composite sponges as potential wound dressings. Carbohydr. Polym. 2017, 157, 1963–1970. [Google Scholar] [CrossRef]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-extract-mediated synthesis of metal nanoparticles. J. Chem. 2021, 2021, 6562687. [Google Scholar] [CrossRef]
- Celebioglu, A.; Aytac, Z.; Umu, O.C.O.; Dana, A.; Tekinay, T.; Uyar, T. One-step synthesis of size-tunable Ag nanoparticles incorporated in electrospun PVA/cyclodextrin nanofibers. Carbohydr. Polym. 2014, 99, 808–816. [Google Scholar] [CrossRef]
- Csoka, L.; Bozanic, D.K.; Nagy, V.; Dimitrijevic-Brankovic, S.; Luyt, A.S.; Grozdits, G.; Djokovic, V. Viscoelastic properties and antimicrobial activity of cellulose fiber sheets impregnated with Ag nanoparticles. Carbohydr. Polym. 2012, 90, 1139–1146. [Google Scholar] [CrossRef]
- Emam, H.E.; Mowafi, S.; Mashaly, H.M.; Rehan, M. Production of antibacterial colored viscose fibers using in situ prepared spherical Ag nanoparticles. Carbohydr. Polym. 2014, 110, 148–155. [Google Scholar] [CrossRef]
- Lavorgna, M.; Attianese, I.; Buonocore, G.G.; Conte, A.; Del Nobile, M.A.; Tescione, F.; Amendola, E. MMT-supported Ag nanoparticles for chitosan nanocomposites: Structural properties and antibacterial activity. Carbohydr. Polym. 2014, 102, 385–392. [Google Scholar] [CrossRef]
- Qu, F.; Ding, Y.; Lv, X.; Xia, L.; You, J.; Han, W. Emissions of terbium metal-organic frameworks modulated by dispersive/agglomerated gold nanoparticles for the construction of prostate-specific antigen biosensor. Anal. Bioanal. Chem. 2019, 411, 3979–3988. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ahamad, T.; Al-Hajji, A.B.; Ahmed, J.; Chaudhary, A.A.; Alshehri, S.M. Cellulose gum and copper nanoparticles based hydrogel as antimicrobial agents against urinary tract infection (UTI) pathogens. Int. Biol. Macromol. 2018, 109, 803–809. [Google Scholar] [CrossRef]
- Bundjaja, V.; Santoso, S.P.; Angkawijaya, A.E.; Yuliana, M.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Gunarto, C.; Ju, Y.-H.; Ho, M.-H. Fabrication of cellulose carbamate hydrogel-dressing with rarasaponin surfactant for enhancing adsorption of silver nanoparticles and antibacterial activity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111542–111583. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Functionalization and Antibacterial Applications of Cellulose-Based Composite Hydrogels. Polymers 2022, 14, 769. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Pugh, T.; Johnson, A.L.; Jenkins, A.T.A. An antimicrobial zinc based molecule for cross linking polyacrylic acid. Eur. Polym. J. 2011, 47, 1338–1345. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Acrylamide-based hydrogel drug delivery systems: Release of acyclovir from MgO nanocomposite hydrogel. J. Taiwan Inst. Chem. Eng. 2017, 72, 182–193. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Hrenovic, J.; Milenkovic, J.; Daneu, N.; Kepcija, R.M.; Rajic, N. Antimicrobial activity of metal oxide nanoparticles supported onto natural clinoptilolite. Chemosphere 2012, 88, 1103–1107. [Google Scholar] [CrossRef]
- Song, J.; Yuan, C.; Jiao, T.; Xing, R.; Yang, M.; Adams, D.; Yan, X. Multifunctional Antimicrobial Biometallohydrogels Based on Amino Acid coordinated self-Aassembly. Small 2020, 16, 1907309. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethyl cellulose/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 74, 136–141. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Sheriffa Begum, K.M.M.S.; Arthanareeswaran, G. Biomass-derived dialdehyde cellulose cross-linked chitosan-based nanocomposite hydrogel with phytosynthesized zinc oxide nanoparticles for enhanced curcumin delivery and bioactivity. J. Agric. Food Chem. 2019, 67, 10880–10890. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Hoque, J.; Bhattacharjee, B.; Prakash, R.G.; Paramanandham, K.; Haldar, J. Dual function injectable hydrogel for controlled release of antibiotic and local antibacterial therapy. Biomacromolecules 2018, 19, 267–278. [Google Scholar] [CrossRef]
- Taccone, F.S.; Bond, O.; Cavicchi, F.Z.; Hites, M. Individualized antibiotic strategies. Curr. Opin. Anesth. 2016, 29, 166–171. [Google Scholar] [CrossRef]
- Sattari, S.; Tehrani, A.D.; Adeli, M. PH-responsive hybrid hydrogels as antibacterial and drug delivery systems. Polymers 2018, 10, 660. [Google Scholar] [CrossRef]
- Xu, W.; Dong, S.; Han, Y.; Li, S.; Liu, Y. Hydrogels as antibacterial biomaterials. Curr. Pharm. Des. 2018, 24, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Forero-Doria, O.; Polo, E.; Marican, A.; Guzman, L.; Venegas, B.; Vijayakumar, S.; Wehinger, S.; Guerrero, M.; Gallego, J.; Duran-Lara, E.F. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr. Polym. 2020, 242, 116383–116393. [Google Scholar] [CrossRef] [PubMed]
- Iman, M.; Barati, A.; Safari, S. Characterization, in vitro antibacterial activity, and toxicity for rat of tetracycline in a nanocomposite hydrogel based on PEG and cellulose. Cellulose 2019, 27, 347–356. [Google Scholar] [CrossRef]
- Antunes, J.C.; Domingues, J.M.; Miranda, C.S.; Silva, A.F.G.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Bioactivity of chitosan-based particles loaded with plant-derived extracts for biomedical applications: Emphasis on antimicrobial fiber-based systems. Mar. Drugs 2021, 19, 359. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Cao, S.; Shen, F.; Wang, Y.; Ren, J.; Wang, X. Rapid self-healing, stretchable, moldable, antioxidant and antibacterial tannic acid-cellulose nanofibril composite hydrogels. Carbohydr. Polym. 2019, 224, 115147–115155. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, S.; Mulaba Bafubiandi, A.F.; Rajinikanth, V.; Varaprasad, K.; Reddy, N.N.; Raju, K.M. Development and characterization of curcumin loaded silver nanoparticle hydrogels for antibacterial and drug delivery applications. J. Inorg. Organomet. Polym. Mater. 2012, 22, 1254–1262. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in woundhealing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Nho, Y.C.; Park, J.S.; Lim, Y.M. Preparation of hydrogel by radiation for the healing of diabetic ulcer. Radiat. Phys. Chem. 2014, 94, 176–180. [Google Scholar] [CrossRef]
- Omali, N.B.; Subbaraman, L.N.; Coles Brennan, C.; Fadli, Z.; Jones, L.W. Biological and clinical implications of lysozyme deposition on soft contact lenses. Optom. Vision Sci. 2015, 92, 750–757. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, L.; Wang, C.; Gao, Z.H.; Zhou, S.W.; Cui, P.F.; Jiang, P.J.; Hu, H.Z.; Ni, X.Y.; Du, X.C.; et al. Nanodot-doped peptide hydrogels for antibacterial phototherapy and wound healing. Biomater. Sci. 2022, 10, 654–664. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xu, F.J. Rational design and latest advances of polysaccharide-based hydrogels forwound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H. Sprayed in-situ synthesis of polyvinyl alcohol/chitosan loaded silver nanocomposite hydrogel for improved antibacterial effects. Int. J. Biol. Macromol. 2020, 145, 950–964. [Google Scholar] [CrossRef]
- Sun, X.; Ma, C.; Gong, W.; Ma, Y.; Ding, Y.; Liu, L. Biological properties of sulfanilamide-loaded alginate hydrogel fibers based on ionic and chemical crosslinking for wound dressings. Int. J. Biol. Macromol. 2020, 157, 522–529. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Graft and crosslinked polymerization of polysaccharide gum to form hydrogel wound dressings for drug delivery applications. Carbohyd. Res. 2020, 489, 107949–107954. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Saleh, B.; Soltantabar, P.; Webster, T.J. Chitosan/pva hydrogels incorporated with green synthesized cerium oxide nanoparticles for wound healing applications. Eur. Polym. J. 2020, 134, 108853–108860. [Google Scholar] [CrossRef]
- De Cicco, F.; Reverchon, E.; Adami, R.; Auriemma, G.; Russo, P.; Calabrese, E.C.; Porta, A.; Aquino, R.P.; Del Gaudio, P. In situ forming antibacterial dextran blend hydrogel for wound dressing: SAA technology vs. spray drying. Carbohyd. Polym. 2014, 101, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, N.; Li, X.; Li, Y.; Xie, Z.; Ma, Z.; Zhao, J.; Hou, X.; Yuan, X. Bioinspired double-dynamic-bond crosslinked bioadhesive enables post-wound closure care. Adv. Funct. Mater. 2020, 30, 2000130. [Google Scholar] [CrossRef]
- Sajjad, W.; He, F.; Ullah, M.W.; Ikram, M.; Shah, S.M.; Khan, R.; Khan, T.; Khalid, A.; Yang, G.; Wahid, F. Fabrication of Bacterial Cellulose-Curcumin Nanocomposite as a Novel Dressing for Partial Thickness Skin Burn. Front. Bioeng. Biotechnol. 2020, 8, 553037. [Google Scholar] [CrossRef] [PubMed]
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.; Farrell, M.; Kostov, Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 2004, 25, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rendon, J.G.; Femmer, T.; De Laporte, L.; Tigges, T.; Rahimi, K.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M. Bioactive Gyroid Scaffolds Formed by Sacrificial Templating of Nanocellulose and Nanochitin Hydrogels as Instructive Platforms for Biomimetic Tissue Engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef]
- Witzler, M.; Büchner, D.; Shoushrah, S.H.; Babczyk, P.; Baranova, J.; Witzleben, S.; Tobiasch, E.; Schulze, M. Polysaccharide-Based Systems for Targeted Stem Cell Differentiation and Bone Regeneration. Biomolecules 2019, 9, 840. [Google Scholar] [CrossRef]
- Stevens, M.; George, J. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J.C. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.C.; Yuan, F.Z.; Deng, R.H.; Yan, X.; Mao, F.B.; Chen, Y.R.; Lu, H.; Yu, J.K. An immunomodulatory polypeptide hydrogel for osteochondral defect repair. Bioact. Mater. 2022, 19, 678–689. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care. 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Mohd Amin, M.C.I.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313–108326. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosanand Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Cheng, X.; Zhang, A.; Liu, W.; Zhang, S.; Jian, X. Tunicate inspired gelatin-based tough hydrogel wound dressing containing twisted phthalazinone with adhesive, self-healing and antibacterial properties. Biol. Macromol. 2022, 218, 639–653. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, J.; Li, D.; Cheng, F. The Recent Progress of the Cellulose-Based Antibacterial Hydrogel. Gels 2024, 10, 109. https://doi.org/10.3390/gels10020109
Sun Y, Wang J, Li D, Cheng F. The Recent Progress of the Cellulose-Based Antibacterial Hydrogel. Gels. 2024; 10(2):109. https://doi.org/10.3390/gels10020109
Chicago/Turabian StyleSun, Ying, Jiayi Wang, Duanxin Li, and Feng Cheng. 2024. "The Recent Progress of the Cellulose-Based Antibacterial Hydrogel" Gels 10, no. 2: 109. https://doi.org/10.3390/gels10020109
APA StyleSun, Y., Wang, J., Li, D., & Cheng, F. (2024). The Recent Progress of the Cellulose-Based Antibacterial Hydrogel. Gels, 10(2), 109. https://doi.org/10.3390/gels10020109





