Dual-Responsive Hydrogels for Mercury Ion Detection and Removal from Wastewater
Abstract
1. Introduction
2. Results and Discussion
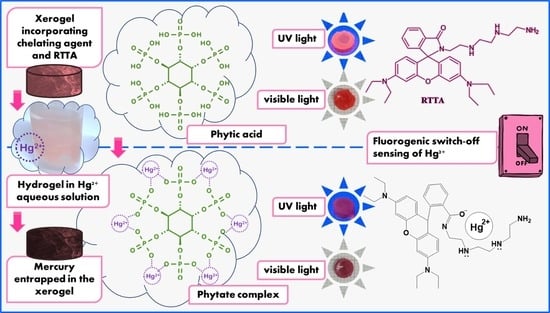
2.1. Method Principles
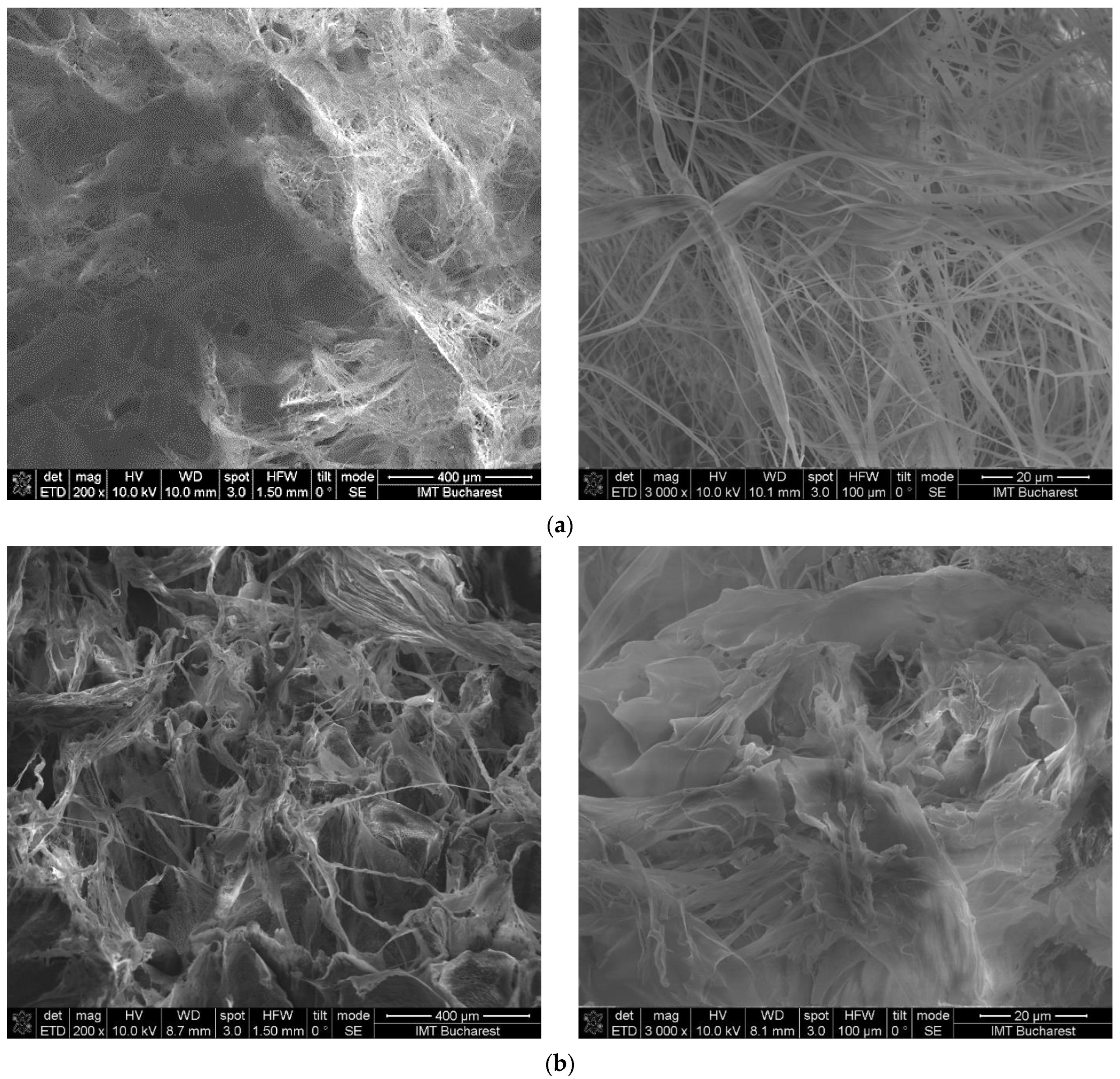
2.2. Morphological Characterization of the Hydrogels
2.3. Gel Fraction and Swelling Degree
2.4. FT-IR Analysis
2.5. Mechanical Properties of the Hydrogels
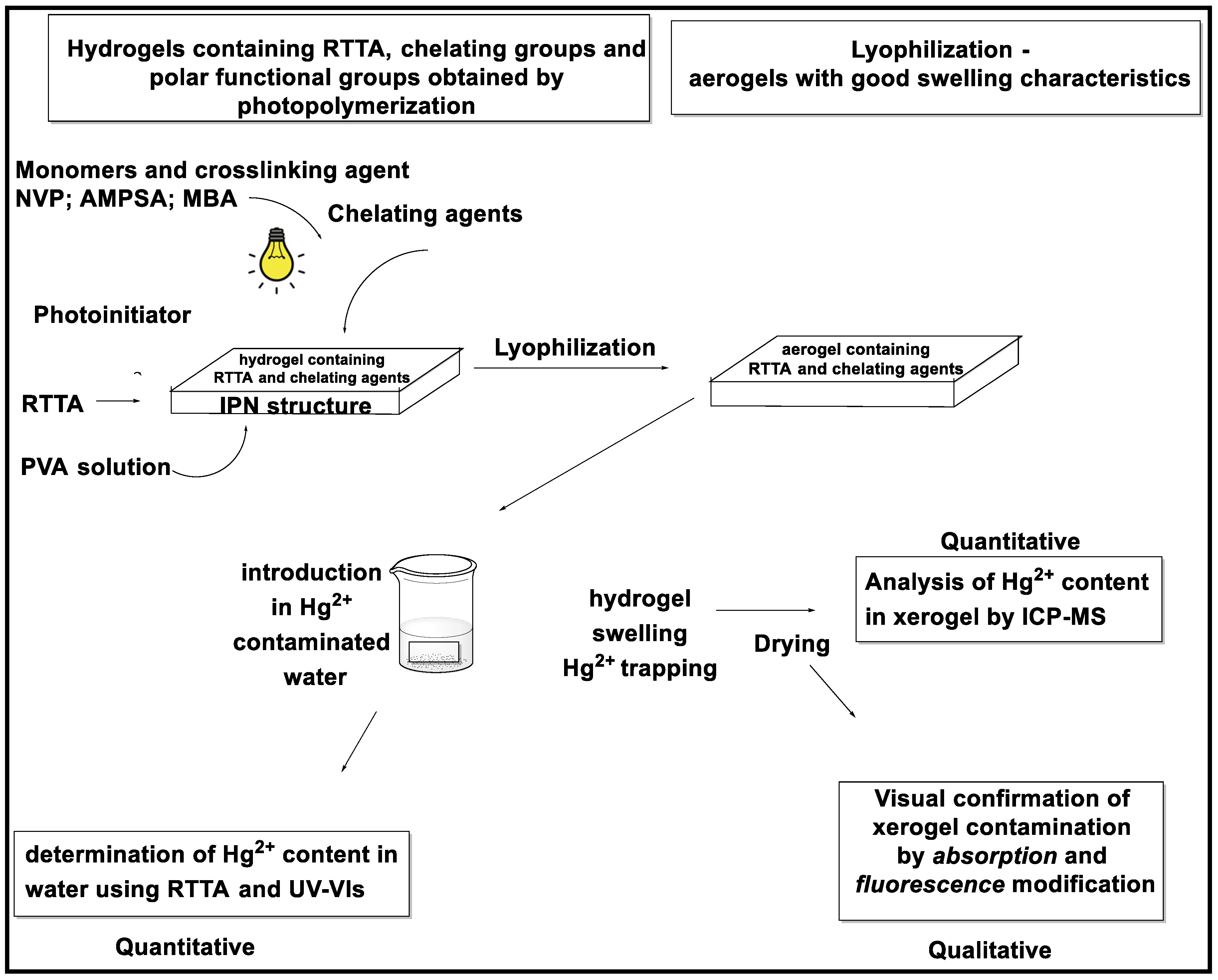
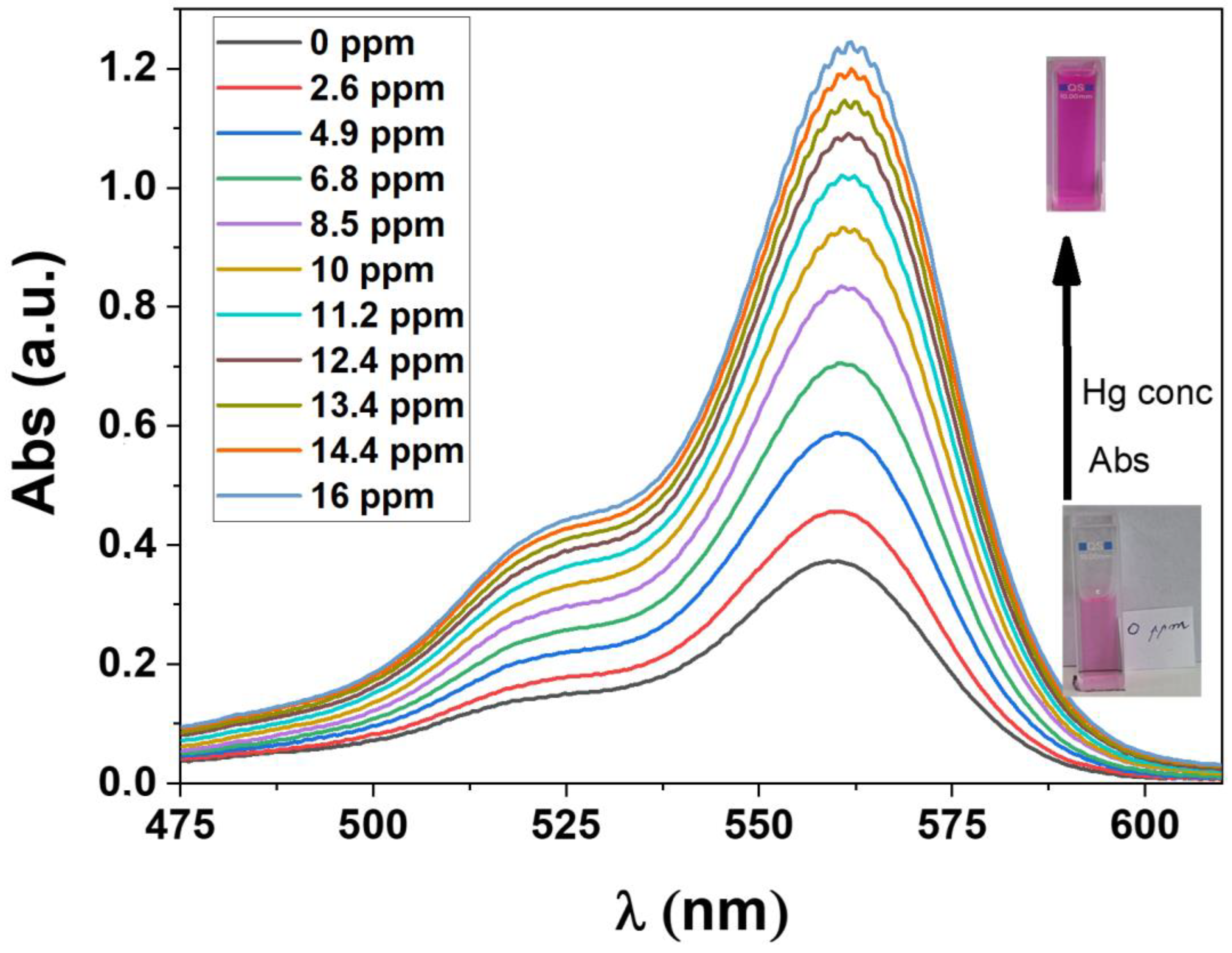
2.6. Hg2+Decontamination Survey
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of Rhodamine-TETA Derivative (RTTA)
4.2.2. Synthesis of RTTA-Based Hydrogels Incorporating Distinct Chelating Agents
4.2.3. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, M.-M.; Liu, A.-F.; Xu, Y.; Xu, D.-M. Synthesis and properties of three novel rhodamine-based fluorescent sensors for Hg2+. Chin. Chem. Lett. 2016, 27, 989–992. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Zhao, Y.; Chen, M.; Liu, J.; Wang, P.; Guo, W. A naphthalimide–rhodamine ratiometric fluorescent probe for Hg2+ based on fluorescence resonance energy transfer. Dye. Pigment. 2012, 92, 909–915. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Wei, J. Enhanced adsorption properties of interpenetrating polymer network hydrogels for heavy metal ion removal. Polym. Bull. 2011, 67, 1709–1720. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, A. Superadsorbent with three-dimensional networks: From bulk hydrogel to granular hydrogel. Eur. Polym. J. 2015, 72, 661–686. [Google Scholar] [CrossRef]
- Sekizkardes, B.; Su, E.; Okay, O. Mechanically Strong Superabsorbent Terpolymer Hydrogels Based on AMPS via Hydrogen-Bonding Interactions. ACS Appl. Polym. Mater. 2023, 5, 2043–2050. [Google Scholar] [CrossRef]
- Su, E.; Yurtsever, M.; Okay, O. A Self-Healing and Highly Stretchable Polyelectrolyte Hydrogel via Cooperative Hydrogen Bonding as a Superabsorbent Polymer. Macromolecules 2019, 52, 3257–3267. [Google Scholar] [CrossRef]
- Porkaew, J.; Somsunan, R.; Nalampang, K.; Molloy, R. Synthesis and Characterization of Sodium AMPS-Based Interpenetrating Network Hydrogels for Use as Temporary Wound Dressing. Adv. Mater. Res. 2014, 894, 300–304. [Google Scholar] [CrossRef]
- Podaru, A.-I.; Moldovan, A.E.; Ionita, M.; Rusen, E.; Diacon, A.; Toader, G.; Ginghina, E.R.; Gavrila, A.-M. N-Vinylpyrrolidone-based polymeric networks incorporating functionalized carbon nanofibers. UPB Sci. Bull. Ser. B 2023, 85, 31–42. [Google Scholar]
- Jiang, D.; Zheng, M.; Ma, X.; Zhang, Y.; Jiang, S.; Li, J.; Zhang, C.; Liu, K.; Li, L. Rhodamine-Anchored Polyacrylamide Hydrogel for Fluorescent Naked-Eye Sensing of Fe3+. Molecules 2023, 28, 6572. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, J.; Li, C.; Dai, L.; Li, H.; Zhong, Y.; Si, C. pH-Responsive Lignin Hydrogel for Lignin Fractionation. ACS Sustain. Chem. Eng. 2021, 9, 13972–13978. [Google Scholar] [CrossRef]
- Roy, A.; Manna, K.; Pal, S. Recent advances in various stimuli-responsive hydrogels: From synthetic designs to emerging healthcare applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Lin, S.-B.; Yuan, C.-H.; Ke, A.-R.; Quan, Z.-L. Electrical response characterization of PVA–P(AA/AMPS) IPN hydrogels in aqueous Na2SO4 solution. Sens. Actuators B Chem. 2008, 134, 281–286. [Google Scholar] [CrossRef]
- Ali, I.; Ali Shah, L.; Rehman, T.U.; Faizan, S. Investigation of the viscoelastic behavior of PVA-P(AAm/AMPS) IPN hydrogel with enhanced mechanical strength and excellent recoverability. J. Polym. Res. 2021, 29, 7. [Google Scholar] [CrossRef]
- Dragan, E.S. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Qu, Z.; Meng, X.; Duan, H.; Qin, D.; Wang, L. Rhodamine-immobilized optical hydrogels with shape deformation and Hg2+-sensitive fluorescence behaviors. Sci. Rep. 2020, 10, 7723. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seo, H.; Lee, T.G. Removal of Hg(II) ions from aqueous solution by poly(allylamine-co-methacrylamide-co-dimethylthiourea). J. Ind. Eng. Chem. 2020, 84, 82–86. [Google Scholar] [CrossRef]
- Rani, L.; Srivastav, A.L.; Kaushal, J.; Nguyen, X.C. Recent advances in nanomaterial developments for efficient removal of Hg(II) from water. Environ. Sci. Pollut. Res. 2022, 29, 62851–62869. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, S.; Xi, C.; Jiang, B.; Zhang, F. Adsorption and Removal of Mercury(II) by a Crosslinked Hyperbranched Polymer Modified via Sulfhydryl. ACS Omega 2022, 7, 12231–12241. [Google Scholar] [CrossRef]
- Ryu, J.; Lee, M.Y.; Song, M.G.; Baeck, S.-H.; Shim, S.E.; Qian, Y. Highly selective removal of Hg(II) ions from aqueous solution using thiol-modified porous polyaminal-networked polymer. Sep. Purif. Technol. 2020, 250, 117120. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Roy, P.; Bonilla-Petriciolet, A.; Badawi, M.; Ganachari, S.V.; Shetti, N.P.; Aminabhavi, T.M. Polymeric hydrogels-based materials for wastewater treatment. Chemosphere 2023, 331, 138743. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Gao, R.; Kan, C.; Xu, J. Portable quantitative detection of Fe3+ by integrating a smartphone with colorimetric responses of a rhodamine-functionalized polyacrylamide hydrogel chemosensor. Sens. Actuators B Chem. 2021, 340, 129958. [Google Scholar] [CrossRef]
- Ali, I.; Shah, L.A. Rheological investigation of the viscoelastic thixotropic behavior of synthesized polyethylene glycol-modified polyacrylamide hydrogels using different accelerators. Polym. Bull. 2021, 78, 1275–1291. [Google Scholar] [CrossRef]
- Choudhury, N.; Ruidas, B.; Mukhopadhyay, C.D.; De, P. Rhodamine-Appended Polymeric Probe: An Efficient Colorimetric and Fluorometric Sensing Platform for Hg2+ in Aqueous Medium and Living Cells. ACS Appl. Polym. Mater. 2020, 2, 5077–5085. [Google Scholar] [CrossRef]
- Nshnsh, K.M.; Cavoura, O.; Davidson, C.M.; Gibson, L.T. Low-cost colorimetric mercury sensor based on immobilisation of rhodamine B thiolactone in a sustainable agar-agar gel substrate. Microchem. J. 2023, 195, 109481. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, S. Silica nanospheres functionalized by a rhodamine-based Hg(II)-sensing probe having two sensing channels: Preparation, characterization and sensing performance. J. Lumin. 2014, 145, 760–766. [Google Scholar] [CrossRef]
- Huang, W.; Wu, D.; Wu, G.; Wang, Z. Dual functional rhodamine-immobilized silica toward sensing and extracting mercury ions in natural water samples. Dalton Trans. 2012, 41, 2620–2625. [Google Scholar] [CrossRef] [PubMed]
- Chockala, B.; Krishnan, S.; Aruliah, R.; Kadarkarai, M.; Benelli, G.; Kannaiyan, D. Organic-inorganic hybrid fluorescent sensor thin films of rhodamine B embedded Ag-SBA15 for selective recognition of Hg (II) ions in water. Chin. Chem. Lett. 2017, 28, 1399–1405. [Google Scholar] [CrossRef]
- Wei, S.; Li, Z.; Lu, W.; Liu, H.; Zhang, J.; Chen, T.; Tang, B.Z. Multicolor Fluorescent Polymeric Hydrogels. Angew. Chem. Int. Ed. 2021, 60, 8608–8624. [Google Scholar] [CrossRef]
- He, J.; Yun, L.; Cheng, X. Organic-soluble chitosan-g-PHMA (PEMA/PBMA)-bodipy fluorescent probes and film by RAFT method for selective detection of Hg2+/Hg+ ions. Int. J. Biol. Macromol. 2023, 237, 124255. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y. Recent advances in fluorescent materials for mercury(ii) ion detection. RSC Adv. 2023, 13, 19429–19446. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Yang, L.; Zhang, Z.; Zhang, Z.; Xu, D. A novel rhodamine-based colorimetric and fluorescent sensor for the dual-channel detection of Cu2+ and Fe3+ in aqueous solutions. Dye. Pigment. 2013, 99, 472–479. [Google Scholar] [CrossRef]
- Hong, M.; Lu, S.; Lv, F.; Xu, D. A novel facilely prepared rhodamine-based Hg2+ fluorescent probe with three thiourea receptors. Dye. Pigment. 2016, 127, 94–99. [Google Scholar] [CrossRef]
- Ozmen, M.M.; Okay, O. Superfast responsive ionic hydrogels with controllable pore size. Polymer 2005, 46, 8119–8127. [Google Scholar] [CrossRef]
- Kim, B.; Hong, D.; Chang, W.V. EDTA and pH-sensitive crosslinking polymerization of acrylic acid, 2-acrylamidoglycolic acid, and 2-acrylamide-2-methyl-1-propanesulfonic acid. J. Appl. Polym. Sci. 2014, 131, 41026. [Google Scholar] [CrossRef]
- Milakin, K.A.; Morávková, Z.; Acharya, U.; Kašparová, M.; Breitenbach, S.; Taboubi, O.; Hodan, J.; Hromádková, J.; Unterweger, C.; Humpolíček, P.; et al. Enhancement of conductivity, mechanical and biological properties of polyaniline-poly(N-vinylpyrrolidone) cryogels by phytic acid. Polymer 2021, 217, 123450. [Google Scholar] [CrossRef]
- Elhady, M.A.; Awadallah, A.M. A Comparative Study of Poly(vinyl alcohol)/Poly(N-vinyl-2-pyrrolidinone) Hydrogels Induced by Ultrasound and Gamma Rays for Ionoprinting Technique. Egypt. J. Radiat. Sci. Appl. 2018, 31, 19–29. [Google Scholar] [CrossRef]
- Sheth, G.N. Studies in interaction between poly(vinyl pyrrolidone) and azo dyes. J. Appl. Polym. Sci. 1985, 30, 4659–4668. [Google Scholar] [CrossRef]
- Barleany, D.R.; Ananta, C.V.; Maulina, F.; Erizal, H.A.; Rochmat, A. Controlled Release of Metformin Hydrogen Chloride from Stimuli-Responsive Hydrogel Based on Poly(N-Isopropylacrylamide)/Chitosan/Polyvinyl Alcohol Composite. Int. J. Technol. 2020, 11, 291–319. [Google Scholar] [CrossRef]
- Alcântara, M.T.S.; Brant, A.J.C.; Giannini, D.R.; Pessoa, J.O.C.P.; Andrade, A.B.; Riella, H.G.; Lugão, A.B. Influence of dissolution processing of PVA blends on the characteristics of their hydrogels synthesized by radiation—Part I: Gel fraction, swelling, and mechanical properties. Radiat. Phys. Chem. 2012, 81, 1465–1470. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Mun, G.A.; Khutoryanskiy, V.V.; Bitekenova, A.B.; Dubolazov, A.V.; Esirkegenova, S.Z. pH effects in the formation of interpolymer complexes between poly(N-vinylpyrrolidone) and poly(acrylic acid) in aqueous solutions. Eur. Phys. J. E 2003, 10, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Pakuro, N.; Yakimansky, A.; Chibirova, F.; Arest-Yakubovich, A. Thermo- and pH-sensitivity of aqueous poly(N-vinylpyrrolidone) solutions in the presence of organic acids. Polymer 2009, 50, 148–153. [Google Scholar] [CrossRef]
- Rivero, R.E.; Alustiza, F.; Rodríguez, N.; Bosch, P.; Miras, M.C.; Rivarola, C.R.; Barbero, C.A. Effect of functional groups on physicochemical and mechanical behavior of biocompatible macroporous hydrogels. React. Funct. Polym. 2015, 97, 77–85. [Google Scholar] [CrossRef]
- Ghilan, A.; Nita, L.E.; Pamfil, D.; Simionescu, N.; Tudorachi, N.; Rusu, D.; Rusu, A.G.; Bercea, M.; Rosca, I.; Ciolacu, D.E.; et al. One-Step Preparation of Carboxymethyl Cellulose—Phytic Acid Hydrogels with Potential for Biomedical Applications. Gels 2022, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Manish, V.; Arockiarajan, A.; Tamadapu, G. Influence of water content on the mechanical behavior of gelatin based hydrogels: Synthesis, characterization, and modeling. Int. J. Solids Struct. 2021, 233, 111219. [Google Scholar] [CrossRef]
- Dave, P.N.; Macwan, P.M.; Kamaliya, B. Synthesis and rheological investigations of gum-ghatti-cl-poly(NIPA-co-AA)-graphene oxide based hydrogels. Mater. Adv. 2023, 4, 2971–2980. [Google Scholar] [CrossRef]
- Böhning, M.; Frasca, D.; Schulze, D.; Schartel, B. Chapter Six—Multilayer Graphene/Elastomer Nanocomposites. In Carbon-Based Nanofillers and Their Rubber Nanocomposites; Yaragalla, S., Mishra, R.K., Thomas, S., Kalarikkal, N., Maria, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–200. [Google Scholar]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Akhtar, R.; Draper, E.R.; Adams, D.J.; Hay, J. Oscillatory nanoindentation of highly compliant hydrogels: A critical comparative analysis with rheometry. J. Mater. Res. 2018, 33, 873–883. [Google Scholar] [CrossRef]
- Vildanova, R.R.; Sigaeva, N.N.; Kukovinets, O.S.; Kolesov, S.V. Preparation and rheological properties of hydrogels based on N-succinyl chitosan and hyaluronic acid dialdehyde. Polym. Test. 2021, 96, 107120. [Google Scholar] [CrossRef]
- Udhayakumari, D. Review on fluorescent sensors-based environmentally related toxic mercury ion detection. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 451–476. [Google Scholar] [CrossRef]
- Radiul, S.M.; Chowdhury, J.; Hazarika, S. Fluorescent H-aggregates of pure rhodamine B (RhB) in glycerol, ethylene glycol, methanol and butanol under ambient condition. J. Mol. Struct. 2023, 1275, 12. [Google Scholar] [CrossRef]
- Thomas, S.A.; Gaillard, J.-F. The Molecular Structure of Aqueous Hg(II)-EDTA As Determined by X-ray Absorption Spectroscopy. J. Phys. Chem. A 2015, 119, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, S.; De Stefano, C.; Gianguzza, A.; Pettignano, A. Sequestration of (CH3)Hg+ by amino-polycarboxylic chelating agents. J. Mol. Liq. 2012, 172, 46–52. [Google Scholar] [CrossRef]
- Bretti, C.; Cigala, R.M.; De Stefano, C.; Lando, G.; Sammartano, S. Interaction of Phytate with Ag+, CH3Hg+, Mn2+, Fe2+, Co2+, and VO2+: Stability Constants and Sequestering Ability. J. Chem. Eng. Data 2012, 57, 2838–2847. [Google Scholar] [CrossRef]
- Genc, H.N.; Guctekin Yasar, O.; Karuk Elmas, S.N.; Arslan, F.N.; Yilmaz, I.; Sirit, A. Naked-eye colorimetric and switch-on fluorescence chemosensor based on a rhodamine derivative for Hg2+: Smartphone device, test-kit and food sample applications. J. Photochem. Photobiol. A Chem. 2023, 438, 114558. [Google Scholar] [CrossRef]
- Liang, B.; Wang, B.; Ma, Q.; Xie, C.; Li, X.; Wang, S. A lysosome-targetable turn-on fluorescent probe for the detection of thiols in living cells based on a 1,8-naphthalimide derivative. Spectrochim. Acta Mol. Biomol. Spectros. 2018, 192, 67–74. [Google Scholar] [CrossRef]
- Guo, B.; Pan, X.; Liu, Y.; Nie, L.; Zhao, H.; Liu, Y.; Jing, J.; Zhang, X. A reversible water-soluble naphthalimide-based chemosensor for imaging of cellular copper(II) ion and cysteine. Sens. Actuators B Chem. 2018, 256, 632–638. [Google Scholar] [CrossRef]
- Singh, S.; Coulomb, B.; Boudenne, J.-L.; Bonne, D.; Dumur, F.; Simon, B.; Robert-Peillard, F. Sub-ppb mercury detection in real environmental samples with an improved rhodamine-based detection system. Talanta 2021, 224, 121909. [Google Scholar] [CrossRef]
- Bhalla, V.; Tejpal, R.; Kumar, M. Rhodamine appended terphenyl: A reversible “off–on” fluorescent chemosensor for mercury ions. Sens. Actuators B Chem. 2010, 151, 180–185. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, H.; Zhu, Z.; Fan, C.; Tu, Y.; Liu, G.; Pu, S. Selective rhodamine–based probe for detecting Hg2+ and its application as test strips and cell staining. J. Photochem. Photobiol. A Chem. 2020, 390, 112302. [Google Scholar] [CrossRef]
- Mao, J.; Wang, L.; Dou, W.; Tang, X.; Yan, Y.; Liu, W. Tuning the Selectivity of Two Chemosensors to Fe(III) and Cr(III). Org. Lett. 2007, 9, 4567–4570. [Google Scholar] [CrossRef]
- Bag, B.; Pal, A. Rhodamine-based probes for metal ion-induced chromo-/fluorogenic dual signaling and their selectivity towards Hg(ii) ion. Org. Biomol. Chem. 2011, 9, 4467–4480. [Google Scholar] [CrossRef]
- Macron, J.; Bresson, B.; Tran, Y.; Hourdet, D.; Creton, C. Equilibrium and Out-of-Equilibrium Adherence of Hydrogels against Polymer Brushes. Macromolecules 2018, 51, 7556–7566. [Google Scholar] [CrossRef]
- Ninciuleanu, C.M.; Ianchiş, R.; Alexandrescu, E.; Mihăescu, C.I.; Scomoroşcenco, C.; Nistor, C.L.; Preda, S.; Petcu, C.; Teodorescu, M. The effects of monomer, crosslinking agent, and filler concentrations on the viscoelastic and swelling properties of poly (methacrylic acid) hydrogels: A comparison. Materials 2021, 14, 2305. [Google Scholar] [CrossRef]
- Toader, G.; Ginghina, R.E.; Diacon, A.; Rusen, E.; Bratu, A.E.; Podaru, A.; Rotariu, T. Design and Application of Photocrosslinkable Hydrogel Films for Fast and Efficient Decontamination of Chemical Warfare Agents. ACS Appl. Polym. Mater. 2023, 5, 877–891. [Google Scholar] [CrossRef]





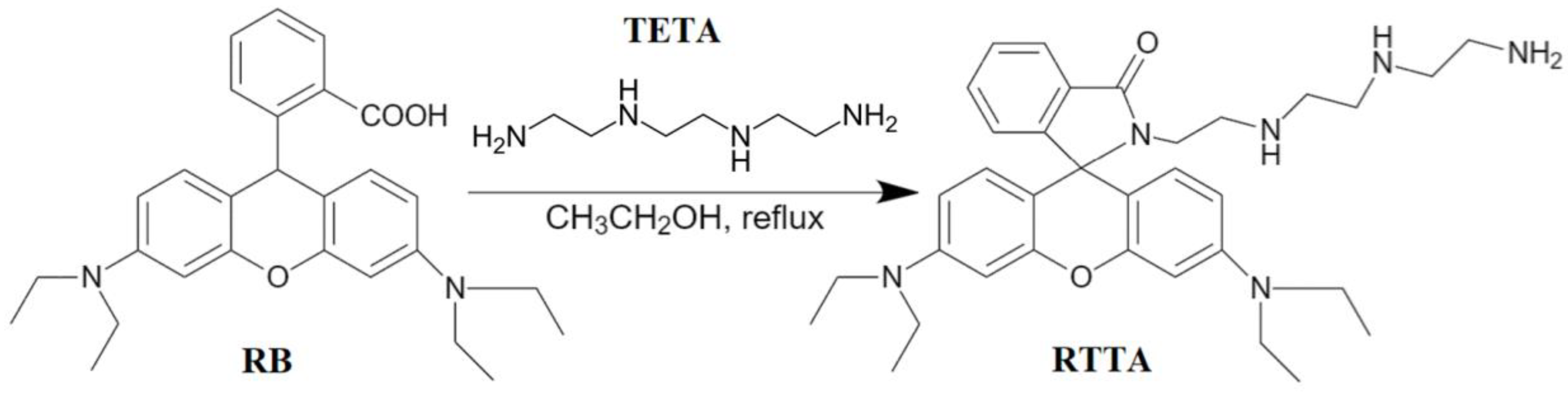
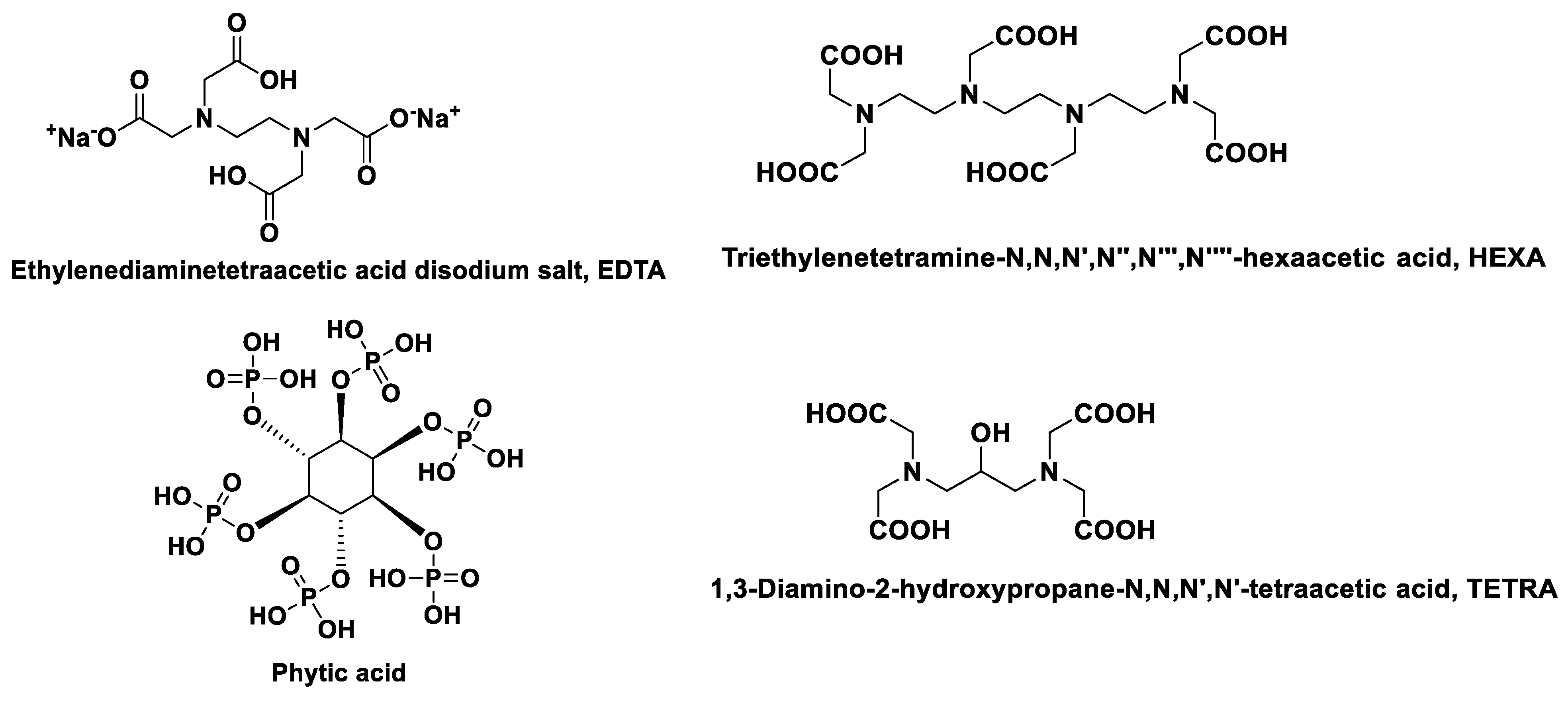
| Molecule | Solvent System | Type | Target | LOD | Ref. |
|---|---|---|---|---|---|
 | HEPES: MeOH 7:3 | Fluorimetric | Hg2+ | 0.11 μM | [56] |
 | DMF | Fluorimetric | Hg2+ | 0.87 μM | [57] |
 | CH3CN: HEPES 1:99 | Fluorimetric | Hg2+ | 0.14 μM | [58] |
 | Acetic acid 50 mM pH 5.25 | Fluorimetric | Hg2+ | 0.001 nM (0.27μg L−1) | [59] |
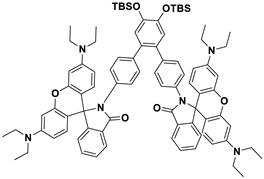 | THF | Colorimetric | Hg2+ | 0.1 μM | [60] |
 | EtOH: HEPES 1:1 | Colorimetric | Hg2 | 0.13 μM | |
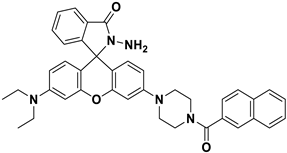 | CH3CN: H2O 7:3 | Fluorimetric | Hg2+ | 0.38 μM | [61] |
 | EtOH: H2O 1:1 pH 5 | Colorimetric | Hg2+ | 2.75 nM (0.55 ppm) | This work |
| Sample Code | Chelating Agent (g) | RTTA (g) | PVA Solution, 5 wt.%, (g) | AMPSA (g) | NVP (g) | MBA (g) | Photoinitiator, (g) |
|---|---|---|---|---|---|---|---|
| Bk | - | 0.005 | 10 | 1.725 | 3.33 | 0.1 | 0.028 g |
| Phytic acid | 0.30 | 0.005 | 10 | 1.725 | 3.33 | 0.1 | 0.028 g |
| HEXA | 0.20 | 0.005 | 10 | 1.725 | 3.33 | 0.1 | 0.028 g |
| TETRA | 0.13 | 0.005 | 10 | 1.725 | 3.33 | 0.1 | 0.028 g |
| EDTA | 0.15 | 0.005 | 10 | 1.725 | 3.33 | 0.1 | 0.028 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diacon, A.; Albota, F.; Mocanu, A.; Brincoveanu, O.; Podaru, A.I.; Rotariu, T.; Ahmad, A.A.; Rusen, E.; Toader, G. Dual-Responsive Hydrogels for Mercury Ion Detection and Removal from Wastewater. Gels 2024, 10, 113. https://doi.org/10.3390/gels10020113
Diacon A, Albota F, Mocanu A, Brincoveanu O, Podaru AI, Rotariu T, Ahmad AA, Rusen E, Toader G. Dual-Responsive Hydrogels for Mercury Ion Detection and Removal from Wastewater. Gels. 2024; 10(2):113. https://doi.org/10.3390/gels10020113
Chicago/Turabian StyleDiacon, Aurel, Florin Albota, Alexandra Mocanu, Oana Brincoveanu, Alice Ionela Podaru, Traian Rotariu, Ahmad A. Ahmad, Edina Rusen, and Gabriela Toader. 2024. "Dual-Responsive Hydrogels for Mercury Ion Detection and Removal from Wastewater" Gels 10, no. 2: 113. https://doi.org/10.3390/gels10020113
APA StyleDiacon, A., Albota, F., Mocanu, A., Brincoveanu, O., Podaru, A. I., Rotariu, T., Ahmad, A. A., Rusen, E., & Toader, G. (2024). Dual-Responsive Hydrogels for Mercury Ion Detection and Removal from Wastewater. Gels, 10(2), 113. https://doi.org/10.3390/gels10020113