Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering
Abstract
:1. Introduction
2. Hydrogel: Basic Architecture

3. Polymers Used in Hybrid Hydrogels
3.1. Natural Polymers
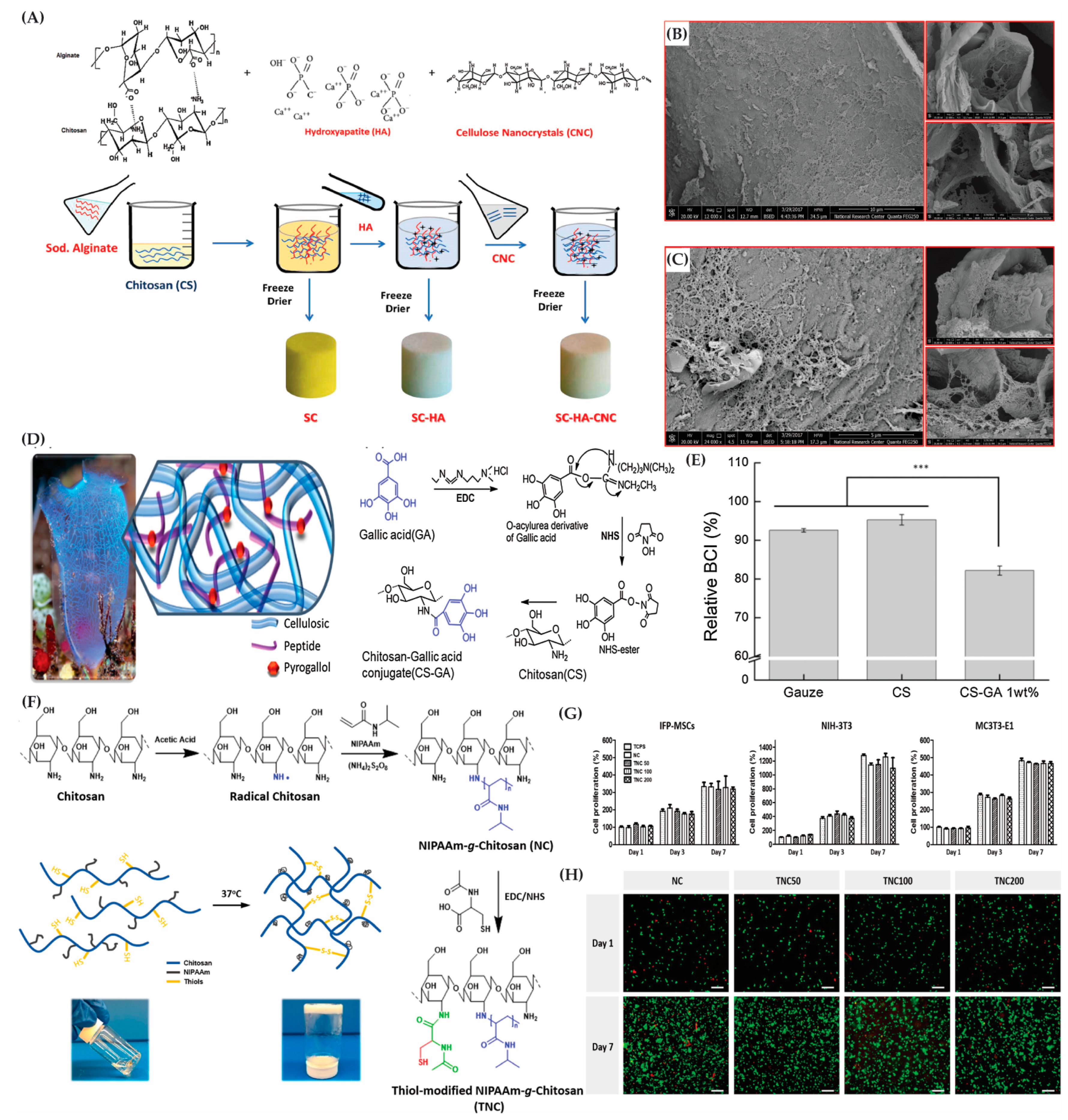
3.1.1. Polysaccharide-Based Polymers
3.1.2. Protein-Based Polymers
3.2. Synthetic Polymers
3.3. Modification of Polymers in Hybrid Hydrogel Fabrication
4. Types of Hybrid Hydrogels
4.1. Reversible Physical Hydrogels
4.2. Multifunctional Hybrid Nanogels
4.3. Self-Assembling Hybrid Hydrogels
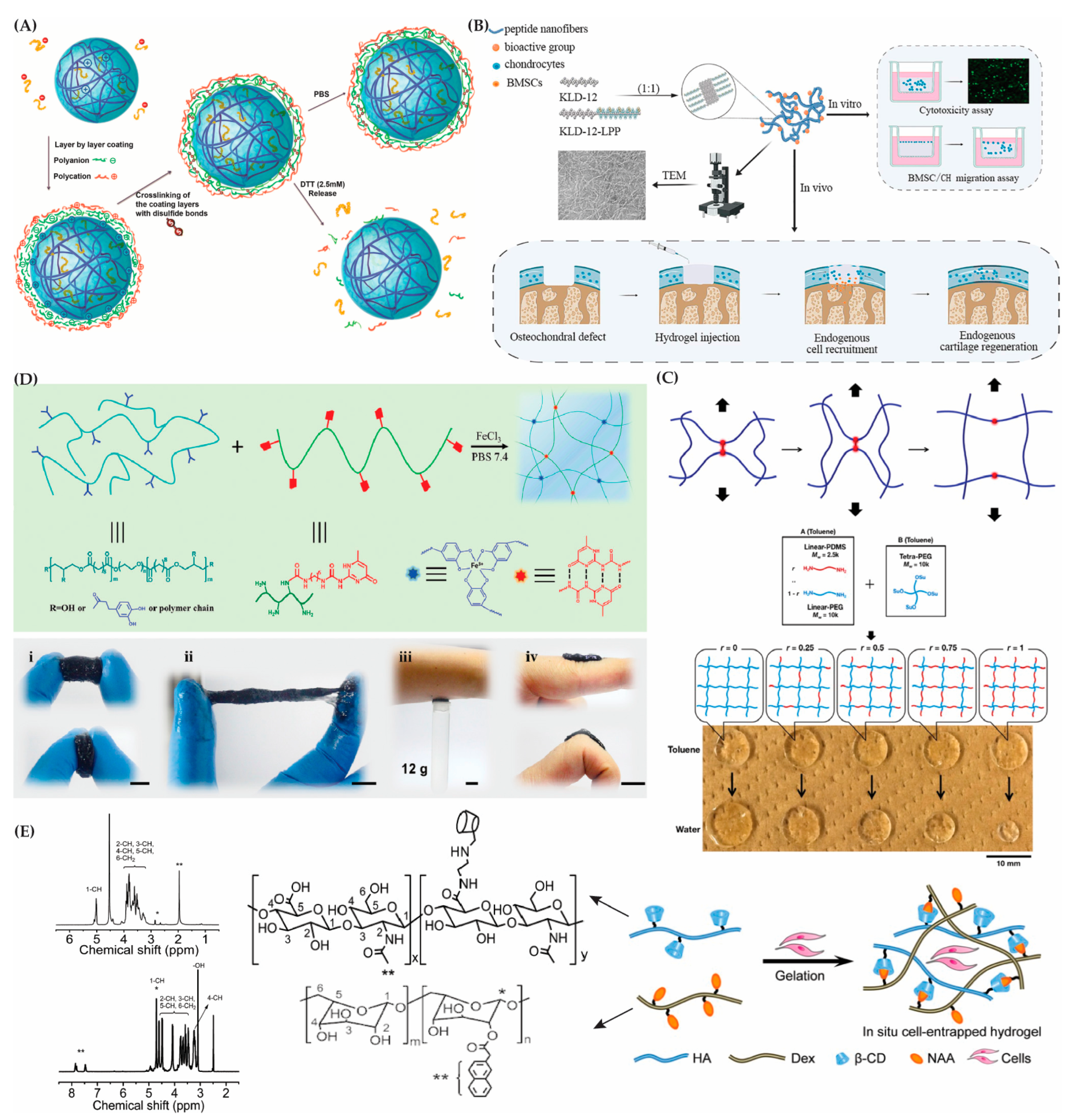
4.4. Chemically (Covalently) Crosslinked Hydrogels
4.5. Core–Shell Hybrid Polymeric Networks
4.6. Interpenetrating Polymer Network (IPN)/Semi-IPN Hydrogels
4.7. Supramolecular Hybrid Hydrogels
5. Hybrid Hydrogel Modification Strategies
5.1. Chemical Modification
5.2. Functionalization
5.3. Stealth Functionalization
5.4. PEGylation
6. Nano/Microstructure Incorporation into Hybrid Hydrogels
6.1. Hybrid Hydrogels with Integrated Nanostructures
6.2. Incorporation of Metallic Nanoparticles in Hybrid Hydrogels
6.3. Hybrid Hydrogels with Integrated Microstructures
7. Mechanisms for Bioactive Molecule Release from Hybrid Hydrogels
8. Encapsulation of Cells and Biomolecules within Hybrid Hydrogels
8.1. Encapsulation of Biomolecules
8.2. Encapsulation of Cells
9. Biomedical Applications of Hybrid Hydrogels
9.1. Hybrid Hydrogels as Drug Delivery Systems (DDSs)
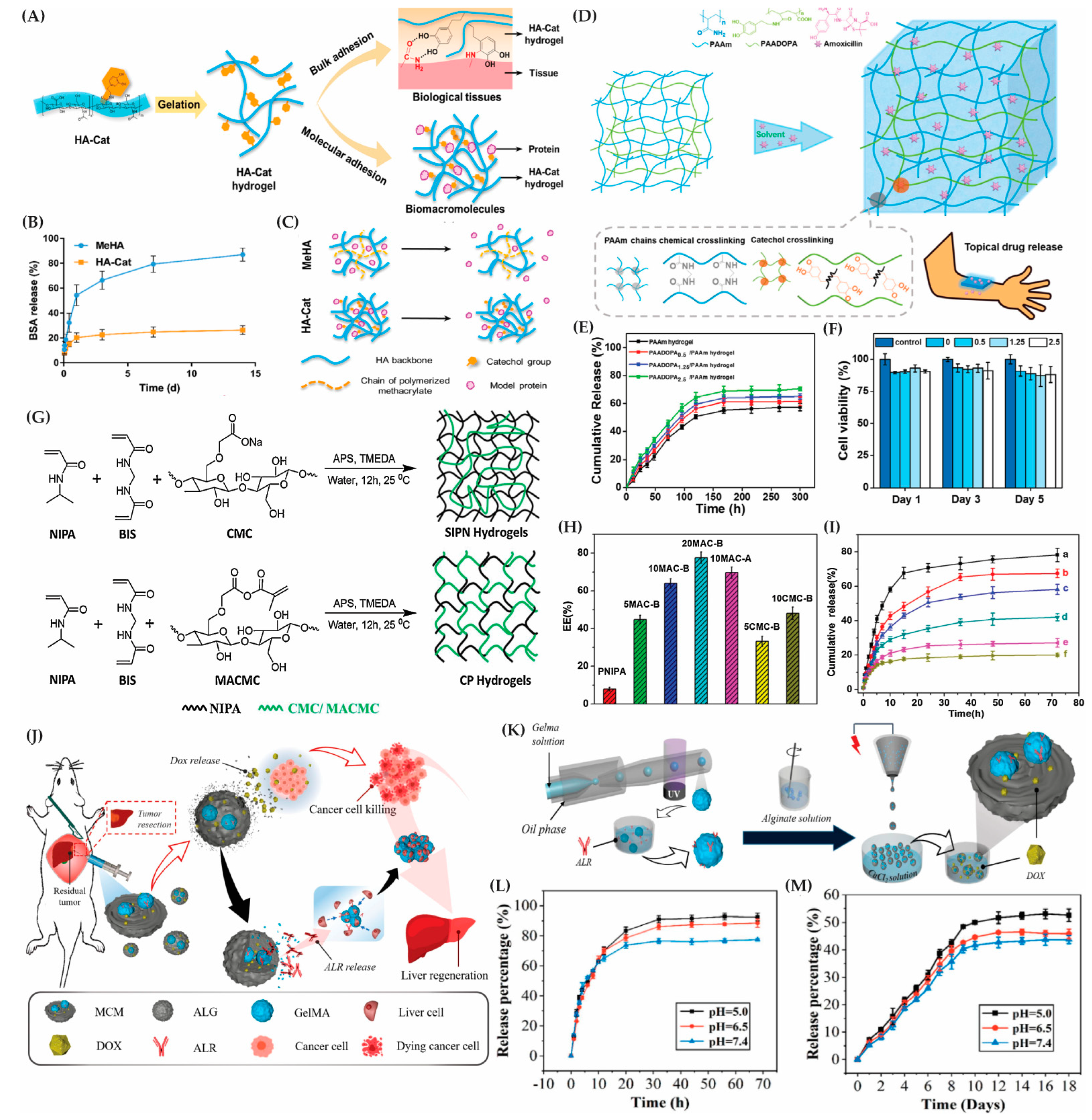
9.2. Hybrid Hydrogels for Tissue Engineering
9.3. Commercialization and Intellectual Property Protection of Hybrid Hydrogels
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid Hydrogels for Biomedical Applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Yesildag, C.; Bartsch, C.; Zhang, X.; Liu, W.; Loebus, A.; Su, Z.; Lensen, M.C. Influence of Network Structure on the Crystallization Behavior in Chemically Crosslinked Hydrogels. Polymers 2018, 10, 970. [Google Scholar] [CrossRef]
- Han, Z.; Wang, P.; Lu, Y.; Jia, Z.; Qu, S.; Yang, W. A Versatile Hydrogel Network–Repairing Strategy Achieved by the Covalent-like Hydrogen Bond Interaction. Sci. Adv. 2022, 8, eabl5066. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Jia, X.; Kiick, K.L. Hybrid Multicomponent Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Tuning the Properties of PNIPAm-Based Hydrogel Scaffolds for Cartilage Tissue Engineering. Polymers 2021, 13, 3154. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, K.; Chen, M.; Leng, X.; Wang, Q.; Lin, M.; Ivanov, A.; Zhang, P.; Andreeva, D.V. Chapter 12—Bioinspired, Biomimetic Hydrogels. In Sustainable Hydrogels; Thomas, S., Sharma, B., Jain, P., Shekhar, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 325–354. ISBN 978-0-323-91753-7. [Google Scholar]
- Mills, D.K.; Luo, Y.; Elumalai, A.; Esteve, S.; Karnik, S.; Yao, S. Creating Structured Hydrogel Microenvironments for Regulating Stem Cell Differentiation. Gels 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a Bioactive Material to Regulate Stem Cell Fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Krishna, O.D.; Kiick, K.L. Protein- and Peptide-Modified Synthetic Polymeric Biomaterials. Pept. Sci. 2010, 94, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Nun, N.; Joy, A. Fabrication and Bioactivity of Peptide-Conjugated Biomaterial Tissue Engineering Constructs. Macromol. Rapid Commun. 2023, 44, 2200342. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Click Chemistry-Based Injectable Hydrogels and Bioprinting Inks for Tissue Engineering Applications. Tissue Eng. Regen. Med. 2018, 15, 531–546. [Google Scholar] [CrossRef]
- Chu, S.; Maples, M.M.; Bryant, S.J. Cell Encapsulation Spatially Alters Crosslink Density of Poly(Ethylene Glycol) Hydrogels Formed from Free-Radical Polymerizations. Acta Biomater. 2020, 109, 37–50. [Google Scholar] [CrossRef]
- Sedighi, M.; Shrestha, N.; Mahmoudi, Z.; Khademi, Z.; Ghasempour, A.; Dehghan, H.; Talebi, S.F.; Toolabi, M.; Préat, V.; Chen, B.; et al. Multifunctional Self-Assembled Peptide Hydrogels for Biomedical Applications. Polymers 2023, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.G.; Massironi, A.; Sugni, M.; Ensign, M.A.; Marzorati, S.; Forouharshad, M. Recent Advances in the Development of Biomimetic Materials. Gels 2023, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Lehn, J.-M.; Meijer, E.W.; Matyjaszewski, K. From Precision Polymers to Complex Materials and Systems. Nat. Rev. Mater. 2016, 1, 16024. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering Hydrogels as Extracellular Matrix Mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Naghib, S.M.; Garshasbi, H.R.; Ghorbanzadeh, S.; Zhang, W. Smart Stimuli-Responsive Injectable Gels and Hydrogels for Drug Delivery and Tissue Engineering Applications: A Review. Front. Bioeng. Biotechnol. 2023, 11, 1104126. [Google Scholar] [CrossRef] [PubMed]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Lau, H.K.; Kiick, K.L. Opportunities for Multicomponent Hybrid Hydrogels in Biomedical Applications. Biomacromolecules 2015, 16, 28–42. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Babu, P.J. Design of 3D Smart Scaffolds Using Natural, Synthetic and Hybrid Derived Polymers for Skin Regenerative Applications. Smart Mater. Med. 2023, 4, 243–256. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide Hydrogels: Functionalization, Construction and Served as Scaffold for Tissue Engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Salave, S.; Rana, D.; Sharma, A.; Bharathi, K.; Gupta, R.; Khode, S.; Benival, D.; Kommineni, N. Polysaccharide Based Implantable Drug Delivery: Development Strategies, Regulatory Requirements, and Future Perspectives. Polysaccharides 2022, 3, 625–654. [Google Scholar] [CrossRef]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides—Naturally Occurring Immune Modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of Polysaccharide Particle-Based Functional Drug Delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Seidi, F.; Youssefi Azarfam, M.; Khodadadi Yazdi, M.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J.; et al. Biopolymer-Based Composites for Tissue Engineering Applications: A Basis for Future Opportunities. Compos. Part B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Shahab, S.; Kasra, M.; Dolatshahi-Pirouz, A. Design and Construction of a Novel Measurement Device for Mechanical Characterization of Hydrogels: A Case Study. PLoS ONE 2021, 16, e0247727. [Google Scholar] [CrossRef]
- Nayak, K.K.; Gupta, P. In Vitro Biocompatibility Study of Keratin/Agar Scaffold for Tissue Engineering. Int. J. Biol. Macromol. 2015, 81, 1–10. [Google Scholar] [CrossRef]
- Khodadadi Yazdi, M.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-Based Biomaterials for Advanced Drug Delivery. J. Control. Release 2020, 326, 523–543. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Madry, H.; Cucchiarini, M. Application of Alginate Hydrogels for Next-Generation Articular Cartilage Regeneration. Int. J. Mol. Sci. 2022, 23, 1147. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of Alginate Microspheres in Therapeutics Delivery and Cell Culture: Past, Present and Future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wang, B.; Huang, Y.; Qu, Y.; Peng, J.; Qian, Z. Injectable Alginate Hydrogel Cross-Linked by Calcium Gluconate-Loaded Porous Microspheres for Cartilage Tissue Engineering. ACS Omega 2017, 2, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent Progress of Collagen, Chitosan, Alginate and Other Hydrogels in Skin Repair and Wound Dressing Applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.-U.; Jaber, F.; Dera, A.A.; Awwad, N.S.; Ibrahium, H.A. Recent Developments in Natural Biopolymer Based Drug Delivery Systems. RSC Adv. 2023, 13, 23087–23121. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, S.-H.; Kim, G.H. Three-Dimensional Collagen/Alginate Hybrid Scaffolds Functionalized with a Drug Delivery System (DDS) for Bone Tissue Regeneration. Chem. Mater. 2012, 24, 881–891. [Google Scholar] [CrossRef]
- Mobaraki, M.; Bizari, D.; Soltani, M.; Khshmohabat, H.; Raahemifar, K.; Akbarzade Amirdehi, M. The Effects of Curcumin Nanoparticles Incorporated into Collagen-Alginate Scaffold on Wound Healing of Skin Tissue in Trauma Patients. Polymers 2021, 13, 4291. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the Role of Hydrogen Bonds: Not in Charge of Everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Rana, M.d.M.; De la Hoz Siegler, H. Influence of Ionic Liquid (IL) Treatment Conditions in the Regeneration of Cellulose with Different Crystallinity. J. Mater. Res. 2022, 38, 328–336. [Google Scholar] [CrossRef]
- Melnikova, N.; Knyazev, A.; Nikolskiy, V.; Peretyagin, P.; Belyaeva, K.; Nazarova, N.; Liyaskina, E.; Malygina, D.; Revin, V. Wound Healing Composite Materials of Bacterial Cellulose and Zinc Oxide Nanoparticles with Immobilized Betulin Diphosphate. Nanomaterials 2021, 11, 713. [Google Scholar] [CrossRef]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key Advances of Carboxymethyl Cellulose in Tissue Engineering & 3D Bioprinting Applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef] [PubMed]
- Madub, K.; Goonoo, N.; Gimié, F.; Ait Arsa, I.; Schönherr, H.; Bhaw-Luximon, A. Green Seaweeds Ulvan-Cellulose Scaffolds Enhance in Vitro Cell Growth and in Vivo Angiogenesis for Skin Tissue Engineering. Carbohydr. Polym. 2021, 251, 117025. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Buzzacchera, I.; Vorobii, M.; Kostina, N.Y.; de los Santos Pereira, A.; Riedel, T.; Bruns, M.; Ogieglo, W.; Möller, M.; Wilson, C.J.; Rodriguez-Emmenegger, C. Polymer Brush-Functionalized Chitosan Hydrogels as Antifouling Implant Coatings. Biomacromolecules 2017, 18, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.; Abdelkhalek, A.A.; Mahmoud, A.A.; Salah, S.; Ammar, M.M.; Ghorab, M.M. Mesenchymal Stem Cells Associated with Chitosan Scaffolds Loaded with Rosuvastatin to Improve Wound Healing. Eur. J. Pharm. Sci. 2019, 127, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.A.; Grenha, A. Chapter Seven—Polysaccharide Nanoparticles for Protein and Peptide Delivery: Exploring Less-Known Materials. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Protein and Peptide Nanoparticles for Drug Delivery; Academic Press: Cambridge, MA, USA, 2015; Volume 98, pp. 223–261. [Google Scholar]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; da Jeremias, T.S.; de Carvalho, R.G.; Iucif Vieira, D.G.; Oliveira, L.F.C.; Fernandes Maia, L.; Trentin, A.G.; et al. Carrageenan Hydrogel as a Scaffold for Skin-Derived Multipotent Stromal Cells Delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef]
- Lipinska, A.P.; Collén, J.; Krueger-Hadfield, S.A.; Mora, T.; Ficko-Blean, E. To Gel or Not to Gel: Differential Expression of Carrageenan-Related Genes between the Gametophyte and Tetasporophyte Life Cycle Stages of the Red Alga Chondrus Crispus. Sci. Rep. 2020, 10, 11498. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent Advance in Delivery System and Tissue Engineering Applications of Chondroitin Sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- Li, Q.; Niu, Y.; Xing, P.; Wang, C. Bioactive Polysaccharides from Natural Resources Including Chinese Medicinal Herbs on Tissue Repair. Chin. Med. 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Dhaneshwar, S.; Bhilare, N.; Roy, S. Dextran Pharmaceutical Applications. In Polysaccharides of Microbial Origin: Biomedical Applications; Oliveira, J., Radhouani, H., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–28. ISBN 978-3-030-35734-4. [Google Scholar]
- Sun, G.; Zhang, X.; Shen, Y.-I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran Hydrogel Scaffolds Enhance Angiogenic Responses and Promote Complete Skin Regeneration during Burn Wound Healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kiick, K.L. Heparin-Functionalized Polymeric Biomaterials in Tissue Engineering and Drug Delivery Applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef]
- Zang, L.; Zhu, H.; Wang, K.; Liu, Y.; Yu, F.; Zhao, W. Not Just Anticoagulation—New and Old Applications of Heparin. Molecules 2022, 27, 6968. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.S.; Svechkarev, D.; Souchek, J.J.; Hill, T.K.; Taylor, M.A.; Natarajan, A.; Mohs, A.M. Impact of Structurally Modifying Hyaluronic Acid on CD44 Interaction. J. Mater. Chem. B 2017, 5, 8183–8192. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Alam, K.; Roy, N.S.; Kaur, K.; Kaity, S.; Ravichandiran, V.; Roy, S. Exploring the Interaction between Extracellular Matrix Components in a 3D Organoid Disease Model to Replicate the Pathophysiology of Breast Cancer. J. Exp. Clin. Cancer Res. 2023, 42, 343. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.O.; Marinho, E.; Silva, C.; Antunes, J.C.; Felgueiras, H.P. Pullulan Hydrogels as Drug Release Platforms in Biomedicine. J. Drug Deliv. Sci. Technol. 2023, 89, 105066. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M.V. Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. Int. J. Mol. Sci. 2023, 24, 4962. [Google Scholar] [CrossRef]
- Lee, C.-S.; Hwang, H.S. Starch-Based Hydrogels as a Drug Delivery System in Biomedical Applications. Gels 2023, 9, 951. [Google Scholar] [CrossRef]
- Moghadam, M.; Seyed Dorraji, M.S.; Dodangeh, F.; Ashjari, H.R.; Mousavi, S.N.; Rasoulifard, M.H. Design of a New Light Curable Starch-Based Hydrogel Drug Delivery System to Improve the Release Rate of Quercetin as a Poorly Water-Soluble Drug. Eur. J. Pharm. Sci. 2022, 174, 106191. [Google Scholar] [CrossRef]
- Moayedzadeh, S.; Madadlou, A.; Khosrowshahiasl, A. Formation Mechanisms, Handling and Digestibility of Food Protein Nanofibrils. Trends Food Sci. Technol. 2015, 45, 50–59. [Google Scholar] [CrossRef]
- Noro, J.; Vilaça-Faria, H.; Reis, R.L.; Pirraco, R.P. Extracellular Matrix-Derived Materials for Tissue Engineering and Regenerative Medicine: A Journey from Isolation to Characterization and Application. Bioact. Mater. 2024, 34, 494–519. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Sousa, D.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Polymeric Biomaterials for Wound Healing. Front. Bioeng. Biotechnol. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Boekema, B.K.H.L.; Vlig, M.; Olde Damink, L.; Middelkoop, E.; Eummelen, L.; Bühren, A.V.; Ulrich, M.M.W. Effect of Pore Size and Cross-Linking of a Novel Collagen-Elastin Dermal Substitute on Wound Healing. J. Mater. Sci. Mater. Med. 2014, 25, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Wang, Y.; Fathi, A.; Parungao, R.; Maitz, P.K.; Li, Z. Skin Wound Repair: Results of a Pre-Clinical Study to Evaluate Electropsun Collagen–Elastin–PCL Scaffolds as Dermal Substitutes. Burns 2019, 45, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Griswold, E.; Cappello, J.; Ghandehari, H. Silk-Elastinlike Protein-Based Hydrogels for Drug Delivery and Embolization. Adv. Drug Deliv. Rev. 2022, 191, 114579. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D. Fibrin Sealant: Past, Present, and Future: A Brief Review. World J. Surg. 2010, 34, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Horta, R.; Matesanz, A.; Gallardo, A.; Reinecke, H.; Jorcano, J.L.; Acedo, P.; Velasco, D.; Elvira, C. Technological Advances in Fibrin for Tissue Engineering. J. Tissue Eng. 2023, 14, 20417314231190288. [Google Scholar] [CrossRef] [PubMed]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-Based Hydrogels for Biomedical Applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Pierce, B.F.; Pittermann, E.; Ma, N.; Gebauer, T.; Neffe, A.T.; Hölscher, M.; Jung, F.; Lendlein, A. Viability of Human Mesenchymal Stem Cells Seeded on Crosslinked Entropy-Elastic Gelatin-Based Hydrogels. Macromol. Biosci. 2012, 12, 312–321. [Google Scholar] [CrossRef]
- Hölzl, K.; Fürsatz, M.; Göcerler, H.; Schädl, B.; Žigon-Branc, S.; Markovic, M.; Gahleitner, C.; Hoorick, J.V.; Van Vlierberghe, S.; Kleiner, A.; et al. Gelatin Methacryloyl as Environment for Chondrocytes and Cell Delivery to Superficial Cartilage Defects. J. Tissue Eng. Regen. Med. 2022, 16, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin—Based Materials for Biomedical Applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Tomblyn, S.; Pettit Kneller, E.L.; Walker, S.J.; Ellenburg, M.D.; Kowalczewski, C.J.; Van Dyke, M.; Burnett, L.; Saul, J.M. Keratin Hydrogel Carrier System for Simultaneous Delivery of Exogenous Growth Factors and Muscle Progenitor Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, R.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Surface Modifications of Scaffolds for Bone Regeneration. J. Mater. Res. Technol. 2023, 24, 7938–7973. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug Delivery Systems Based on Polyethylene Glycol Hydrogels for Enhanced Bone Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Rubio-Elizalde, I.; Bernáldez-Sarabia, J.; Moreno-Ulloa, A.; Vilanova, C.; Juárez, P.; Licea-Navarro, A.; Castro-Ceseña, A.B. Scaffolds Based on Alginate-PEG Methyl Ether Methacrylate-Moringa Oleifera-Aloe Vera for Wound Healing Applications. Carbohydr. Polym. 2019, 206, 455–467. [Google Scholar] [CrossRef]
- Zahedi, E.; Ansari, S.; Wu, B.M.; Bencharit, S.; Moshaverinia, A. 4—Hydrogels in Craniofacial Tissue Engineering. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Thorston, UK, 2017; pp. 47–64. ISBN 978-0-08-100961-1. [Google Scholar]
- Wen, Q.; Chen, Z.; Zhao, Y.; Zhang, H.; Feng, Y. Biodegradation of Polyacrylamide by Bacteria Isolated from Activated Sludge and Oil-Contaminated Soil. J. Hazard. Mater. 2010, 175, 955–959. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A. Recent Advances in the Use of Polyhydroyalkanoates in Biomedicine. Bioengineering 2019, 6, 82. [Google Scholar] [CrossRef]
- Novikova, L.N.; Pettersson, J.; Brohlin, M.; Wiberg, M.; Novikov, L.N. Biodegradable Poly-β-Hydroxybutyrate Scaffold Seeded with Schwann Cells to Promote Spinal Cord Repair. Biomaterials 2008, 29, 1198–1206. [Google Scholar] [CrossRef]
- Asran, A.S.; Razghandi, K.; Aggarwal, N.; Michler, G.H.; Groth, T. Nanofibers from Blends of Polyvinyl Alcohol and Polyhydroxy Butyrate As Potential Scaffold Material for Tissue Engineering of Skin. Biomacromolecules 2010, 11, 3413–3421. [Google Scholar] [CrossRef]
- Harpaz, D.; Axelrod, T.; Yitian, A.L.; Eltzov, E.; Marks, R.S.; Tok, A.I.Y. Dissolvable Polyvinyl-Alcohol Film, a Time-Barrier to Modulate Sample Flow in a 3D-Printed Holder for Capillary Flow Paper Diagnostics. Materials 2019, 12, 343. [Google Scholar] [CrossRef]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of Chitosan-Polyvinyl Alcohol and Silk Electrospun Fiber Seeded with Differentiated Keratinocyte for Skin Tissue Regeneration in Animal Wound Model. J. Biol. Eng. 2020, 14, 27. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-Based Biodegradable Microspheres in Drug Delivery: Recent Advances in Research and Application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, P.; Huang, Y.; Zhang, W.; Chen, F.; Zhang, X.; Mao, J.; Chen, D.; Cai, Q.; Yang, X. Injectable PLGA Microspheres with Tunable Magnesium Ion Release for Promoting Bone Regeneration. Acta Biomater. 2019, 85, 294–309. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(Lactic Acid) Nanofibrous Scaffolds for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt Electrowriting of PLA, PCL, and Composite PLA/PCL Scaffolds for Tissue Engineering Application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef] [PubMed]
- Gharibshahian, M.; Salehi, M.; Beheshtizadeh, N.; Kamalabadi-Farahani, M.; Atashi, A.; Nourbakhsh, M.-S.; Alizadeh, M. Recent Advances on 3D-Printed PCL-Based Composite Scaffolds for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1168504. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.-T.; Woodruff, M.A.; Teoh, S.H. Evaluation of Polycaprolactone Scaffold Degradation for 6 Months in Vitro and in Vivo. J. Biomed. Mater. Res. Part A 2009, 90A, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tong, Z.; Jia, X.; Kiick, K.L. Resilin-like Polypeptide Hydrogels Engineered for Versatile Biological Function. Soft Matter 2012, 9, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.; Gama, N.; Ferreira, A. Different Methods of Synthesizing Poly(Glycerol Sebacate) (PGS): A Review. Front. Bioeng. Biotechnol. 2022, 10, 1033827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Wang, Z.; Zhang, J.; Zhu, J.; Ma, Y.; Yang, Z.; Yuan, Y. Optimized Synthesis of Biodegradable Elastomer PEGylated Poly(Glycerol Sebacate) and Their Biomedical Application. Polymers 2019, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wei, Z.; Xu, X.; Feng, Q.; Xu, J.; Bian, L. Efficient Catechol Functionalization of Biopolymeric Hydrogels for Effective Multiscale Bioadhesion. Mater. Sci. Eng. C 2019, 103, 109835. [Google Scholar] [CrossRef] [PubMed]
- Carrow, J.K.; Gaharwar, A.K. Bioinspired Polymeric Nanocomposites for Regenerative Medicine. Macromol. Chem. Phys. 2015, 216, 248–264. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Asfour, M.H.; Abd El-Alim, S.H.; Awad, G.E.A.; Kassem, A.A. Chitosan/β-Glycerophosphate in Situ Forming Thermo-Sensitive Hydrogel for Improved Ocular Delivery of Moxifloxacin Hydrochloride. Eur. J. Pharm. Sci. 2021, 167, 106041. [Google Scholar] [CrossRef]
- Nasr, F.H.; Khoee, S. Design, Characterization and in Vitro Evaluation of Novel Shell Crosslinked Poly(Butylene Adipate)-Co-N-Succinyl Chitosan Nanogels Containing Loteprednol Etabonate: A New System for Therapeutic Effect Enhancement via Controlled Drug Delivery. Eur. J. Med. Chem. 2015, 102, 132–142. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Mastrobattista, E.; van Nostrum, C.F.; Hennink, W.E.; Vermonden, T. Reduction-Sensitive Polymer-Shell-Coated Nanogels for Intracellular Delivery of Antigens. ACS Biomater. Sci. Eng. 2017, 3, 42–48. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. Smart Core−Shell Hybrid Nanogels with Ag Nanoparticle Core for Cancer Cell Imaging and Gel Shell for pH-Regulated Drug Delivery. Chem. Mater. 2010, 22, 1966–1976. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angew. Chem. Int. Ed. 2012, 51, 7396–7417. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, Q.; Li, H.; Chen, M.; Jin, Y.; Liu, D. Self-Assembled Peptide Hydrogels in Regenerative Medicine. Gels 2023, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Sun, C.; Hu, B.; Chen, S.; Wang, Z.; Wu, Q.; Fu, K.; Xia, Z.; Shao, Z.; Wang, B. Simultaneous Recruitment of Stem Cells and Chondrocytes Induced by a Functionalized Self-Assembling Peptide Hydrogel Improves Endogenous Cartilage Regeneration. Front. Cell Dev. Biol. 2020, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; Qi, Y.; Zhao, Y.; Nie, G.; Wang, X.; Zheng, S. Self-Assembled Peptide Hydrogel Scaffolds with VEGF and BMP-2 Enhanced in Vitro Angiogenesis and Osteogenesis. Oral Dis. 2022, 28, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Zhang, L.; Chae, B.-S.; Palla, C.S.; Furst, E.M.; Kiick, K.L. Growth Factor Mediated Assembly of Cell Receptor-Responsive Hydrogels. J. Am. Chem. Soc. 2007, 129, 3040–3041. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Sun, R.; Xia, K.; Zhang, Q.; Li, W.; Wang, F.; Zhang, X.; Ge, Z.; Wang, L.; Fan, C.; et al. DNA-Based Hybrid Hydrogels Sustain Water-Insoluble Ophthalmic Therapeutic Delivery against Allergic Conjunctivitis. ACS Appl. Mater. Interfaces 2019, 11, 26704–26710. [Google Scholar] [CrossRef]
- Kondo, S.; Hiroi, T.; Han, Y.-S.; Kim, T.-H.; Shibayama, M.; Chung, U.; Sakai, T. Reliable Hydrogel with Mechanical “Fuse Link” in an Aqueous Environment. Adv. Mater. 2015, 27, 7407–7411. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Chen, J.-X.; Cao, L.-J.; Shi, Y.; Wang, P.; Chen, J.-H. In Situ Supramolecular Hydrogel Based on Hyaluronic Acid and Dextran Derivatives as Cell Scaffold. Journal of Biomedical Materials Research Part A 2016, 104, 2263–2270. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem. Rev. 2020, 120, 10695–10743. [Google Scholar] [CrossRef] [PubMed]
- Maji, K.; Dasgupta, S.; Bhaskar, R.; Gupta, M.K. Photo-Crosslinked Alginate Nano-Hydroxyapatite Paste for Bone Tissue Engineering. Biomed. Mater. 2020, 15, 055019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Pan, J.; He, H.; Wang, Z.; Deng, M.; Liu, X.; Fu, F. Silk Fibroin Enhanced Double-Network Hydrogels with Extreme Stretchability, Self-Adhesive and Biocompatibility for Ultrasensitive Strain Sensors. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133035. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked Alginate Hydrogels with Tunable Biodegradation Rates and Mechanical Properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, H.-W. Core–Shell Designed Scaffolds for Drug Delivery and Tissue Engineering. Acta Biomater. 2015, 21, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Kareem, M.M.; Hodgkinson, T.; Sanchez, M.S.; Dalby, M.J.; Tanner, K.E. Hybrid Core–Shell Scaffolds for Bone Tissue Engineering. Biomed. Mater. 2019, 14, 025008. [Google Scholar] [CrossRef] [PubMed]
- Srouji, S.; Ben-David, D.; Lotan, R.; Livne, E.; Avrahami, R.; Zussman, E. Slow-Release Human Recombinant Bone Morphogenetic Protein-2 Embedded Within Electrospun Scaffolds for Regeneration of Bone Defect: In Vitro and In Vivo Evaluation. Tissue Eng. Part A 2011, 17, 269–277. [Google Scholar] [CrossRef]
- Choi, D.H.; Park, C.H.; Kim, I.H.; Chun, H.J.; Park, K.; Han, D.K. Fabrication of Core–Shell Microcapsules Using PLGA and Alginate for Dual Growth Factor Delivery System. J. Control. Release 2010, 147, 193–201. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Zhang, X.; Xu, M.; Liu, F.; Ma, Q.; Cai, Q.; Deng, X. PLGA/PDLLA Core–Shell Submicron Spheres Sequential Release System: Preparation, Characterization and Promotion of Bone Regeneration in Vitro and in Vivo. Chem. Eng. J. 2015, 273, 490–501. [Google Scholar] [CrossRef]
- Dragan, E.S. Advances in Interpenetrating Polymer Network Hydrogels and Their Applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A Sodium Alginate-Based Sustained-Release IPN Hydrogel and Its Applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef]
- Li, C.; Rowland, M.J.; Shao, Y.; Cao, T.; Chen, C.; Jia, H.; Zhou, X.; Yang, Z.; Scherman, O.A.; Liu, D. Responsive Double Network Hydrogels of Interpenetrating DNA and CB[8] Host–Guest Supramolecular Systems. Adv. Mater. 2015, 27, 3298–3304. [Google Scholar] [CrossRef]
- Daniele, M.A.; Adams, A.A.; Naciri, J.; North, S.H.; Ligler, F.S. Interpenetrating Networks Based on Gelatin Methacrylamide and PEG Formed Using Concurrent Thiol Click Chemistries for Hydrogel Tissue Engineering Scaffolds. Biomaterials 2014, 35, 1845–1856. [Google Scholar] [CrossRef]
- Molina, M.; Wedepohl, S.; Miceli, E.; Calderón, M. Overcoming Drug Resistance with On-Demand Charged Thermoresponsive Dendritic Nanogels. Nanomedicine 2017, 12, 117–129. [Google Scholar] [CrossRef]
- Lyu, Y.; Azevedo, H.S. Supramolecular Hydrogels for Protein Delivery in Tissue Engineering. Molecules 2021, 26, 873. [Google Scholar] [CrossRef]
- Bernhard, S.; Tibbitt, M.W. Supramolecular Engineering of Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2021, 171, 240–256. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Dang-i, A.Y.; Kousar, A.; Liu, J.; Mukwaya, V.; Zhao, C.; Wang, F.; Hou, L.; Feng, C.-L. Mechanically Stable C2-Phenylalanine Hybrid Hydrogels for Manipulating Cell Adhesion. ACS Appl. Mater. Interfaces 2019, 11, 28657–28664. [Google Scholar] [CrossRef] [PubMed]
- van de Manakker, F.; van der Pot, M.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Self-Assembling Hydrogels Based on β-Cyclodextrin/Cholesterol Inclusion Complexes. Macromolecules 2008, 41, 1766–1773. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Wischke, C. Opportunities and Challenges of Switchable Materials for Pharmaceutical Use. Pharmaceutics 2022, 14, 2331. [Google Scholar] [CrossRef] [PubMed]
- Gwardys, P.; Marcisz, K.; Jagleniec, D.; Romanski, J.; Karbarz, M. Electrochemically Controlled Release from a Thin Hydrogel Layer. ACS Appl. Mater. Interfaces 2023, 15, 49865–49873. [Google Scholar] [CrossRef]
- Zou, H.; Guo, W.; Yuan, W. Supramolecular Hydrogels from Inclusion Complexation of α-Cyclodextrin with Densely Grafted Chains in Micelles for Controlled Drug and Protein Release. J. Mater. Chem. B 2013, 1, 6235–6244. [Google Scholar] [CrossRef]
- Shahrokhinia, A.; Biswas, P.; Reuther, J.F. Orthogonal Synthesis and Modification of Polymer Materials. J. Polym. Sci. 2021, 59, 1748–1786. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Rehmann, M.S.; Skeens, K.M.; Maverakis, E.; Kloxin, A.M. Thiol–Ene Click Hydrogels for Therapeutic Delivery. ACS Biomater. Sci. Eng. 2016, 2, 165–179. [Google Scholar] [CrossRef]
- Ooi, H.W.; Mota, C.; ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol–Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive Hydrogel Loaded with Chitosan-Carbon Nanotubes for near Infrared Light Triggered Drug Delivery. Colloids Surf. B Biointerfaces 2017, 154, 253–262. [Google Scholar] [CrossRef]
- Oroojalian, F.; Babaei, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Encapsulation of Thermo-Responsive Gel in pH-Sensitive Polymersomes as Dual-Responsive Smart Carriers for Controlled Release of Doxorubicin. J. Control. Release 2018, 288, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.D.; Kiick, K.L. Tunable Degradation of Maleimide–Thiol Adducts in Reducing Environments. Bioconjugate Chem. 2011, 22, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click Hydrogels, Microgels and Nanogels: Emerging Platforms for Drug Delivery and Tissue Engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, D.; Lim, D.-K.; Koo, H.; Kim, K. Copper-Free Click Chemistry: Applications in Drug Delivery, Cell Tracking, and Tissue Engineering. Adv. Mater. 2022, 34, 2107192. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Shin, J.J.; Xi, Y.; Hawker, C.J. Click Chemistry Strategies for the Accelerated Synthesis of Functional Macromolecules. J. Polym. Sci. 2021, 59, 963–1042. [Google Scholar] [CrossRef]
- Yousefi, F.; Kandel, S.; Pleshko, N. Infrared Spectroscopic Quantification of Methacrylation of Hyaluronic Acid: A Scaffold for Tissue Engineering Applications. Appl. Spectrosc. 2018, 72, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent Advances in Photo-Crosslinkable Hydrogels for Biomedical Applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Xu, Z.; Bratlie, K.M. Click Chemistry and Material Selection for in Situ Fabrication of Hydrogels in Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2018, 4, 2276–2291. [Google Scholar] [CrossRef]
- Nolan, M.D.; Scanlan, E.M. Applications of Thiol-Ene Chemistry for Peptide Science. Front. Chem. 2020, 8, 583272. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Lueckgen, A.; Garske, D.S.; Ellinghaus, A.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Enzymatically-Degradable Alginate Hydrogels Promote Cell Spreading and in Vivo Tissue Infiltration. Biomaterials 2019, 217, 119294. [Google Scholar] [CrossRef]
- Ji, S.; Abaci, A.; Morrison, T.; Gramlich, W.M.; Guvendiren, M. Novel Bioinks from UV-Responsive Norbornene-Functionalized Carboxymethyl Cellulose Macromers. Bioprinting 2020, 18, e00083. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, H.H.; Park, Y.H.; Lin, C.-C.; Um, I.C.; Ki, C.S. Dual Mode Gelation Behavior of Silk Fibroin Microgel Embedded Poly(Ethylene Glycol) Hydrogels. J. Mater. Chem. B 2016, 4, 4574–4584. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, A.M.; Ford, E.M.; Guo, C.; Sloppy, J.D.; Kloxin, A.M. Hierarchically Structured Hydrogels Utilizing Multifunctional Assembling Peptides for 3D Cell Culture. Biomater. Sci. 2020, 8, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Ouyang, L.; Highley, C.B.; Burdick, J.A. Norbornene-Modified Poly(Glycerol Sebacate) as a Photocurable and Biodegradable Elastomer. Polym. Chem. 2017, 8, 5091–5099. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Chang, C.-W.; Yeh, Y.-C. Formation of Highly Elastomeric and Property-Tailorable Poly(Glycerol Sebacate)-Co-Poly(Ethylene Glycol) Hydrogels through Thiol–Norbornene Photochemistry. Biomater. Sci. 2020, 8, 4728–4738. [Google Scholar] [CrossRef]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc. Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Hahn, S.K.; Oh, E.J.; Miyamoto, H.; Shimobouji, T. Sustained Release Formulation of Erythropoietin Using Hyaluronic Acid Hydrogels Crosslinked by Michael Addition. Int. J. Pharm. 2006, 322, 44–51. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Jones, C.N.; Revzin, A.; Tae, G. Heparin-Based Hydrogel as a Matrix for Encapsulation and Cultivation of Primary Hepatocytes. Biomaterials 2010, 31, 3596–3603. [Google Scholar] [CrossRef]
- Xu, K.; Cantu, D.A.; Fu, Y.; Kim, J.; Zheng, X.; Hematti, P.; Kao, W.J. Thiol-Ene Michael-Type Formation of Gelatin/Poly(Ethylene Glycol) Biomatrices for Three-Dimensional Mesenchymal Stromal/Stem Cell Administration to Cutaneous Wounds. Acta Biomater. 2013, 9, 8802–8814. [Google Scholar] [CrossRef] [PubMed]
- McGann, C.L.; Levenson, E.A.; Kiick, K.L. Resilin-Based Hybrid Hydrogels for Cardiovascular Tissue Engineering. Macromol. Chem. Phys. 2013, 214, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Nwe, K.; Brechbiel, M.W. Growing Applications of “Click Chemistry” for Bioconjugation in Contemporary Biomedical Research. Cancer Biother. Radiopharm. 2009, 24, 289–302. [Google Scholar] [CrossRef]
- Li, M.; De, P.; Gondi, S.R.; Sumerlin, B.S. Responsive Polymer-Protein Bioconjugates Prepared by RAFT Polymerization and Copper-Catalyzed Azide-Alkyne Click Chemistry. Macromol. Rapid Commun. 2008, 29, 1172–1176. [Google Scholar] [CrossRef]
- Piluso, S.; Vukićević, R.; Nöchel, U.; Braune, S.; Lendlein, A.; Neffe, A.T. Sequential Alkyne-Azide Cycloadditions for Functionalized Gelatin Hydrogel Formation. Eur. Polym. J. 2018, 100, 77–85. [Google Scholar] [CrossRef]
- Truong, V.X.; Ablett, M.P.; Gilbert, H.T.J.; Bowen, J.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P. In Situ-Forming Robust Chitosan-Poly(Ethylene Glycol) Hydrogels Prepared by Copper-Free Azide–Alkyne Click Reaction for Tissue Engineering. Biomater. Sci. 2013, 2, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; Heilshorn, S.C. Tyrosine-Selective Functionalization for Bio-Orthogonal Cross-Linking of Engineered Protein Hydrogels. Bioconjugate Chem. 2017, 28, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gong, Y.; Guo, T.; Deng, J.; Ji, J.; Wang, B.; Hao, S. Thermo-Sensitive Keratin Hydrogel against Iron-Induced Brain Injury after Experimental Intracerebral Hemorrhage. Int. J. Pharm. 2019, 566, 342–351. [Google Scholar] [CrossRef]
- Tan, H.; Ramirez, C.M.; Miljkovic, N.; Li, H.; Rubin, J.P.; Marra, K.G. Thermosensitive Injectable Hyaluronic Acid Hydrogel for Adipose Tissue Engineering. Biomaterials 2009, 30, 6844–6853. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Luo, L.-J. Chitosan-g-Poly(N-Isopropylacrylamide) Copolymers as Delivery Carriers for Intracameral Pilocarpine Administration. Eur. J. Pharm. Biopharm. 2017, 113, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-R.; Ou, L.-H.; Lin, Y.-J.; Ling, M.-H. Hollow, pH-Sensitive Calcium–Alginate/Poly(Acrylic Acid) Hydrogel Beads as Drug Carriers for Vancomycin Release. J. Appl. Polym. Sci. 2010, 118, 1878–1886. [Google Scholar] [CrossRef]
- Wischerhoff, E.; Uhlig, K.; Lankenau, A.; Börner, H.G.; Laschewsky, A.; Duschl, C.; Lutz, J.-F. Controlled Cell Adhesion on PEG-Based Switchable Surfaces. Angew. Chem. Int. Ed. 2008, 47, 5666–5668. [Google Scholar] [CrossRef]
- Ren, K.; Hu, M.; Zhang, H.; Li, B.; Lei, W.; Chen, J.; Chang, H.; Wang, L.; Ji, J. Layer-by-Layer Assembly as a Robust Method to Construct Extracellular Matrix Mimic Surfaces to Modulate Cell Behavior. Prog. Polym. Sci. 2019, 92, 1–34. [Google Scholar] [CrossRef]
- Gentile, P.; Ghione, C.; Ferreira, A.M.; Crawford, A.; Hatton, P.V. Alginate-Based Hydrogels Functionalised at the Nanoscale Using Layer-by-Layer Assembly for Potential Cartilage Repair. Biomater. Sci. 2017, 5, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.B.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. A Bioinspired Omniphobic Surface Coating on Medical Devices Prevents Thrombosis and Biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Elisseeff, J.H. Mimicking Biological Functionality with Polymers for Biomedical Applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Eslami, P.; Rossi, F.; Fedeli, S. Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications. Pharmaceutics 2019, 11, 71. [Google Scholar] [CrossRef]
- Karg, M. Functional Materials Design through Hydrogel Encapsulation of Inorganic Nanoparticles: Recent Developments and Challenges. Macromol. Chem. Phys. 2016, 217, 242–255. [Google Scholar] [CrossRef]
- Foster, L.J.R. PEGylation and BioPEGylation of Polyhydroxyalkanoates: Synthesis, Characterisation and Applications. In Biopolymers; IntechOpen: London, UK, 2010; ISBN 978-953-307-109-1. [Google Scholar]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef]
- Lv, F.; Mao, L.; Liu, T. Thermosensitive Porphyrin-Incorporated Hydrogel with Four-Arm PEG–PCL Copolymer: Preparation, Characterization and Fluorescence Imaging in Vivo. Mater. Sci. Eng. C 2014, 43, 221–230. [Google Scholar] [CrossRef]
- Wei, C.; Dong, X.; Zhang, Y.; Liang, J.; Yang, A.; Zhu, D.; Liu, T.; Kong, D.; Lv, F. Simultaneous Fluorescence Imaging Monitoring of the Programmed Release of Dual Drugs from a Hydrogel-Carbon Nanotube Delivery System. Sens. Actuators B Chem. 2018, 273, 264–275. [Google Scholar] [CrossRef]
- Barkat, K.; Ahmad, M.; Usman Minhas, M.; Khalid, I.; Nasir, B. Development and Characterization of pH-Responsive Polyethylene Glycol-Co-Poly(Methacrylic Acid) Polymeric Network System for Colon Target Delivery of Oxaliplatin: Its Acute Oral Toxicity Study. Adv. Polym. Technol. 2018, 37, 1806–1822. [Google Scholar] [CrossRef]
- Carles-Carner, M.; Saleh, L.S.; Bryant, S.J. The Effects of Hydroxyapatite Nanoparticles Embedded in a MMP-Sensitive Photoclickable PEG Hydrogel on Encapsulated MC3T3-E1 Pre-Osteoblasts. Biomed. Mater. 2018, 13, 045009. [Google Scholar] [CrossRef]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.C.; Duan, B.; Malone, E.; Wu, J.; Girardi, L.N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D Printing of Anatomically Accurate and Mechanically Heterogeneous Aortic Valve Hydrogel Scaffolds. Biofabrication 2012, 4, 035005. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.C.; Wang, S.; Padera, R.F.; Grodzinsky, A.J.; Hammond, P.T. Cartilage Penetrating Nanocarriers Improve Delivery and Efficacy of Growth Factor Treatment of Osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Xavier, J.R.; Carrow, J.K.; Desai, P.; Alge, D.; Gaharwar, A.K. Mechanically Stiff Nanocomposite Hydrogels at Ultralow Nanoparticle Content. ACS Nano 2016, 10, 246–256. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-Nanotube-Embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef]
- Marcelo, G.; López-González, M.; Mendicuti, F.; Tarazona, M.P.; Valiente, M. Poly(N-Isopropylacrylamide)/Gold Hybrid Hydrogels Prepared by Catechol Redox Chemistry. Characterization and Smart Tunable Catalytic Activity. Macromolecules 2014, 47, 6028–6036. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Lu, C.; Yoganathan, R.B.; Kociolek, M.; Allen, C. Hydrogel Containing Silica Shell Cross-Linked Micelles for Ocular Drug Delivery. J. Pharm. Sci. 2013, 102, 627–637. [Google Scholar] [CrossRef]
- Bini, R.A.; Silva, M.F.; Varanda, L.C.; da Silva, M.A.; Dreiss, C.A. Soft Nanocomposites of Gelatin and Poly(3-Hydroxybutyrate) Nanoparticles for Dual Drug Release. Colloids Surf. B Biointerfaces 2017, 157, 191–198. [Google Scholar] [CrossRef]
- Liu, T.; Wu, T.; Liu, H.; Ke, B.; Huang, H.; Jiang, Z.; Xie, M. Ultraviolet-Crosslinked Hydrogel Sustained-Release Hydrophobic Antibiotics with Long-Term Antibacterial Activity and Limited Cytotoxicity. J. Appl. Polym. Sci. 2014, 131, 40438. [Google Scholar] [CrossRef]
- Liang, Y.; Kiick, K.L. Liposome-Cross-Linked Hybrid Hydrogels for Glutathione-Triggered Delivery of Multiple Cargo Molecules. Biomacromolecules 2016, 17, 601–614. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, H.; Xiao, P.; Yang, A.; Situ, X.; Wang, Y.; Zhang, X.; Li, W.; Pan, W.; Wang, Y. Sustained Release of Magnesium Ions Mediated by a Dynamic Mechanical Hydrogel to Enhance BMSC Proliferation and Differentiation. ACS Omega 2020, 5, 24477–24486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jia, Z.; Yang, B.; Feng, Q.; Xu, X.; Yuan, W.; Li, X.; Chen, X.; Duan, L.; Wang, D.; et al. Adaptable Hydrogels Mediate Cofactor-Assisted Activation of Biomarker-Responsive Drug Delivery via Positive Feedback for Enhanced Tissue Regeneration. Adv. Sci. 2018, 5, 1800875. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.J.; Lee, A.L.Z.; Voo, Z.X.; Venkataraman, S.; Koh, B.W.; Yang, Y.Y.; Hedrick, J.L. Biodegradable Strain-Promoted Click Hydrogels for Encapsulation of Drug-Loaded Nanoparticles and Sustained Release of Therapeutics. Biomacromolecules 2017, 18, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.K.; Li, L.; Jurusik, A.K.; Sabanayagam, C.R.; Kiick, K.L. Aqueous Liquid–Liquid Phase Separation of Resilin-Like Polypeptide/Polyethylene Glycol Solutions for the Formation of Microstructured Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Stocco, T.D.; Zhang, T.; Dimitrov, E.; Ghosh, A.; da Silva, A.M.H.; Melo, W.C.M.A.; Tsumura, W.G.; Silva, A.D.R.; Sousa, G.F.; Viana, B.C.; et al. Carbon Nanomaterial-Based Hydrogels as Scaffolds in Tissue Engineering: A Comprehensive Review. Int. J. Nanomed. 2023, 18, 6153–6183. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.W.; El-Hotaby, W.; Hemdan, B.; Abdel-Fattah, W.I. Thermosensitive Chitosan/Phosphate Hydrogel-Composites Fortified with Ag versus Ag@Pd for Biomedical Applications. Life Sci. 2018, 194, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, F.H.; Hussain, F.S.J.; Zeyohannes, S.S.; Rasad, M.S.B.A.; Yusuff, M.M. A Facile Synthesis Method of Hydroxyethyl Cellulose-Silver Nanoparticle Scaffolds for Skin Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 79, 151–160. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, M.I.; Perni, S.; Tommasi, G.; Morris, N.G.; Hawkins, K.; López-Cabarcos, E.; Prokopovich, P. Silver Nanoparticle Based Antibacterial Methacrylate Hydrogels Potential for Bone Graft Applications. Mater. Sci. Eng. C 2015, 50, 332–340. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, X.; Lin, X.; Yi, W.; Jiang, J. An Injectable Metal Nanoparticle Containing Cellulose Derivative-Based Hydrogels: Evaluation of Antibacterial and in Vitro-Vivo Wound Healing Activity in Children with Burn Injuries. Int. Wound J. 2022, 19, 666–678. [Google Scholar] [CrossRef]
- You, J.-O.; Rafat, M.; Ye, G.J.C.; Auguste, D.T. Nanoengineering the Heart: Conductive Scaffolds Enhance Connexin 43 Expression. Nano Lett. 2011, 11, 3643–3648. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic Natural Biomaterials for Tissue Engineering and Regenerative Medicine: New Biosynthesis Methods, Recent Advances, and Emerging Applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Iliut, M.; Zhou, M.; Moffat, J.; Elsawy, M.A.; Pinheiro, W.A.; Hoyland, J.A.; Miller, A.F.; Vijayaraghavan, A.; Saiani, A. Designing Peptide/Graphene Hybrid Hydrogels through Fine-Tuning of Molecular Interactions. Biomacromolecules 2018, 19, 2731–2741. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Ganji, F. Vancomycin-Loaded HPMC Microparticles Embedded within Injectable Thermosensitive Chitosan Hydrogels. Prog. Biomater. 2017, 6, 49–56. [Google Scholar] [CrossRef]
- Sivakumaran, D.; Maitland, D.; Hoare, T. Injectable Microgel-Hydrogel Composites for Prolonged Small-Molecule Drug Delivery. Biomacromolecules 2011, 12, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, A. Role of Particle Size, Shape, and Stiffness in Design of Intravascular Drug Delivery Systems: Insights from Computations, Experiments, and Nature. WIREs Nanomed. Nanobiotechnology 2016, 8, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Zhang, Y.; Zhang, L. Fabrication and Characterization of a 3D Bioprinted Nanoparticle-Hydrogel Hybrid Device for Biomimetic Detoxification. Nanoscale 2017, 9, 14506–14511. [Google Scholar] [CrossRef]
- Hossain, M.A.; Zhumabekova, A.; Paul, S.C.; Kim, J.R. A Review of 3D Printing in Construction and Its Impact on the Labor Market. Sustainability 2020, 12, 8492. [Google Scholar] [CrossRef]
- Duan, B.; Xu, C.; Das, S.; Chen, J.M.; Butcher, J.T. Spatial Regulation of Valve Interstitial Cell Phenotypes within Three-Dimensional Micropatterned Hydrogels. ACS Biomater. Sci. Eng. 2019, 5, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Nawroth, J.C.; Scudder, L.L.; Halvorson, R.T.; Tresback, J.; Ferrier, J.P.; Sheehy, S.P.; Cho, A.; Kannan, S.; Sunyovszki, I.; Goss, J.A.; et al. Automated Fabrication of Photopatterned Gelatin Hydrogels for Organ-on-Chips Applications. Biofabrication 2018, 10, 025004. [Google Scholar] [CrossRef]
- DeFail, A.J.; Chu, C.R.; Izzo, N.; Marra, K.G. Controlled Release of Bioactive TGF-Β1 from Microspheres Embedded within Biodegradable Hydrogels. Biomaterials 2006, 27, 1579–1585. [Google Scholar] [CrossRef]
- Das, N. Biodegradable Hydrogels for Controlled Drug Delivery. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Polymers and Polymeric Composites: A Reference Series; Springer International Publishing: Cham, Switzerland, 2019; pp. 1433–1472. ISBN 978-3-319-77830-3. [Google Scholar]
- Kim, H.T.; Park, J.S.; Kang, M.J. Nanocomplex System of Bupivacaine with Dextran Sulfate for Parenteral Prolonged Delivery. Bull. Korean Chem. Soc. 2020, 41, 981–988. [Google Scholar] [CrossRef]
- Shin, Y.; Husni, P.; Kang, K.; Lee, D.; Lee, S.; Lee, E.; Youn, Y.; Oh, K. Recent Advances in pH- or/and Photo-Responsive Nanovehicles. Pharmaceutics 2021, 13, 725. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of Drug Release in pH-Sensitive Micelles for Tumour Targeted Drug Delivery System: A Review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-Responsive Nanomaterials for Controlled Drug Delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed]
- Kotova, S.; Kostjuk, S.; Rochev, Y.; Efremov, Y.; Frolova, A.; Timashev, P. Phase Transition and Potential Biomedical Applications of Thermoresponsive Compositions Based on Polysaccharides, Proteins and DNA: A Review. Int. J. Biol. Macromol. 2023, 249, 126054. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nayak, B.R.; Lowe, T.L. Synthesis and Characterization of Novel Thermoresponsive-Co-Biodegradable Hydrogels Composed of N-Isopropylacrylamide, Poly(L-Lactic Acid), and Dextran. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5054–5066. [Google Scholar] [CrossRef]
- Tsai, F.-C.; Huang, C.-F.; Chang, C.-J.; Lu, C.-H.; Chen, J.-K. Thermo-Tunable Pores and Antibiotic Gating Properties of Bovine Skin Gelatin Gels Prepared with Poly(n-Isopropylacrylamide) Network. Polymers 2020, 12, 2156. [Google Scholar] [CrossRef] [PubMed]
- Luckanagul, J.A.; Pitakchatwong, C.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-Based Polymer Hybrids for Thermo-Responsive Nanogel Delivery of Curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Lee, W.; Kim, S.-M.; Lee, M.; Koo, J.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Ion-Conductive Self-Healing Hydrogels Based on an Interpenetrating Polymer Network for a Multimodal Sensor. Chem. Eng. J. 2019, 371, 452–460. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Wang, Z.; Liu, H.; Li, C. Preparation and Characterization of a Positive Thermoresponsive Hydrogel for Drug Loading and Release. J. Appl. Polym. Sci. 2009, 111, 1417–1425. [Google Scholar] [CrossRef]
- Yao, C.; Wang, P.; Li, X.; Hu, X.; Hou, J.; Wang, L.; Zhang, F. Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance. Adv. Mater. 2016, 28, 9341–9348. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Tomatsu, I.; Kros, A. Light Controlled Protein Release from a Supramolecular Hydrogel. Chem. Commun. 2010, 46, 4094–4096. [Google Scholar] [CrossRef]
- Li, R.; Peng, F.; Cai, J.; Yang, D.; Zhang, P. Redox Dual-Stimuli Responsive Drug Delivery Systems for Improving Tumor-Targeting Ability and Reducing Adverse Side Effects. Asian J. Pharm. Sci. 2020, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Badparvar, F.; Marjani, A.P.; Salehi, R.; Ramezani, F. pH/Redox Responsive Size-switchable Intelligent Nanovehicle for Tumor Microenvironment Targeted DOX Release. Sci. Rep. 2023, 13, 22475. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Khan, A.R.; Ji, J.; Yu, A.; Zhai, G. Redox/Enzyme Sensitive Chondroitin Sulfate-Based Self-Assembled Nanoparticles Loading Docetaxel for the Inhibition of Metastasis and Growth of Melanoma. Carbohydr. Polym. 2018, 184, 82–93. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, D.; Shen, C.; Shen, J.; Zhao, H.; He, Y. Platelet-Rich Plasma-Loaded Poly(d,l-Lactide)-Poly(Ethylene Glycol)-Poly(d,l-Lactide) Hydrogel Dressing Promotes Full-Thickness Skin Wound Healing in a Rodent Model. Int. J. Mol. Sci. 2016, 17, 1001. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Lan, Y.; Zuo, Q.; Li, C.; Zhang, Y.; Guo, R.; Xue, W. Acceleration of Skin Regeneration in Full-Thickness Burns by Incorporation of bFGF-Loaded Alginate Microspheres into a CMCS–PVA Hydrogel. J. Tissue Eng. Regen. Med. 2017, 11, 1562–1573. [Google Scholar] [CrossRef]
- Bayer, E.A.; Jordan, J.; Roy, A.; Gottardi, R.; Fedorchak, M.V.; Kumta, P.N.; Little, S.R. Programmed Platelet-Derived Growth Factor-BB and Bone Morphogenetic Protein-2 Delivery from a Hybrid Calcium Phosphate/Alginate Scaffold. Tissue Eng. Part A 2017, 23, 1382–1393. [Google Scholar] [CrossRef]
- Santoveña, A.; Monzón, C.; Delgado, A.; Evora, C.; Llabrés, M.; Fariña, J.B. Development of a Standard Method for in Vitro Evaluation of Triamcinolone and BMP-2 Diffusion Mechanism from Thermosensitive and Biocompatible Composite Hyaluronic Acid-Pluronic Hydrogels. J. Drug Deliv. Sci. Technol. 2017, 42, 284–291. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Huang, Q.; Tong, Z.; Yao, J.; Kong, X. A Composite Hydrogel System Containing Glucose-Responsive Nanocarriers for Oral Delivery of Insulin. Mater. Sci. Eng. C 2016, 69, 37–45. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, X.; Zhang, Y.; Shang, Q. Fabrication and Evaluation of a Novel Polymeric Hydrogel of Carboxymethyl Chitosan-g-Polyacrylic Acid (CMC-g-PAA) for Oral Insulin Delivery. RSC Adv. 2016, 6, 52858–52867. [Google Scholar] [CrossRef]
- Fletcher, N.A.; Babcock, L.R.; Murray, E.A.; Krebs, M.D. Controlled Delivery of Antibodies from Injectable Hydrogels. Mater. Sci. Eng. C 2016, 59, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Drapala, P.W.; Jiang, B.; Chiu, Y.-C.; Mieler, W.F.; Brey, E.M.; Kang-Mieler, J.J.; Pérez-Luna, V.H. The Effect of Glutathione as Chain Transfer Agent in PNIPAAm-Based Thermo-Responsive Hydrogels for Controlled Release of Proteins. Pharm. Res. 2014, 31, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, L.; Zhang, J.; Chiang, C.; Pan, J.; Wang, X.; Kwak, K.J.; Li, H.; Zhao, R.; Rima, X.Y.; et al. Exosomal mRNAs for Angiogenic–Osteogenic Coupled Bone Repair. Adv. Sci. 2023, 10, 2302622. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.; Islan, G.A.; de Urraza, P.J.; Castro, G.R. Novel Biopolymer Matrices for Microencapsulation of Phages: Enhanced Protection Against Acidity and Protease Activity. Macromol. Biosci. 2012, 12, 1200–1208. [Google Scholar] [CrossRef]
- Loh, B.; Gondil, V.S.; Manohar, P.; Khan, F.M.; Yang, H.; Leptihn, S. Encapsulation and Delivery of Therapeutic Phages. Appl. Environ. Microbiol. 2021, 87, e01979-20. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Khaledabad, M.A.; Amiri, S.; Phimolsiripol, Y.; Mousavi Khaneghah, A. A Comprehensive Review on the Utilization of Biopolymer Hydrogels to Encapsulate and Protect Probiotics in Foods. Int. J. Biol. Macromol. 2024, 254, 127907. [Google Scholar] [CrossRef] [PubMed]
- Argin, S.; Kofinas, P.; Lo, Y.M. The Cell Release Kinetics and the Swelling Behavior of Physically Crosslinked Xanthan–Chitosan Hydrogels in Simulated Gastrointestinal Conditions. Food Hydrocoll. 2014, 40, 138–144. [Google Scholar] [CrossRef]
- Qi, C.; Yan, X.; Huang, C.; Melerzanov, A.; Du, Y. Biomaterials as Carrier, Barrier and Reactor for Cell-Based Regenerative Medicine. Protein Cell 2015, 6, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Cruise, G.M.; Hegre, O.D.; Lamberti, F.V.; Hager, S.R.; Hill, R.; Scharp, D.S.; Hubbell, J.A. In Vitro and in Vivo Performance of Porcine Islets Encapsulated in Interfacially Photopolymerized Poly(Ethylene Glycol) Diacrylate Membranes. Cell Transpl. 1999, 8, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Diniz, I.M.; Chen, C.; Aghaloo, T.; Wu, B.M.; Shi, S.; Moshaverinia, A. Alginate/Hyaluronic Acid Hydrogel Delivery System Characteristics Regulate the Differentiation of Periodontal Ligament Stem Cells toward Chondrogenic Lineage. J. Mater. Sci. Mater. Med. 2017, 28, 162. [Google Scholar] [CrossRef]
- Shi, W.; Jiang, Y.; Wu, T.; Zhang, Y.; Li, T. Advancements in Drug-Loaded Hydrogel Systems for Bone Defect Repair. Regen. Ther. 2024, 25, 174–185. [Google Scholar] [CrossRef]
- Asadikorayem, M.; Surman, F.; Weber, P.; Weber, D.; Zenobi-Wong, M. Zwitterionic Granular Hydrogel for Cartilage Tissue Engineering. Adv. Healthc. Mater. 2023, 2301831. [Google Scholar] [CrossRef]
- Zhou, Q.; Kang, H.; Bielec, M.; Wu, X.; Cheng, Q.; Wei, W.; Dai, H. Influence of Different Divalent Ions Cross-Linking Sodium Alginate-Polyacrylamide Hydrogels on Antibacterial Properties and Wound Healing. Carbohydr. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, X.; Zhao, J.; Feng, H.; Li, P.; Tong, Z.; Yang, Z.; Li, S.; Yang, J.; Jin, S. Preparation of the Chitosan/Poly(Glutamic Acid)/Alginate Polyelectrolyte Complexing Hydrogel and Study on Its Drug Releasing Property. Carbohydr. Polym. 2018, 191, 8–16. [Google Scholar] [CrossRef]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E.; Ferreira, A.M. Synthesis of Bioinspired Collagen/Alginate/Fibrin Based Hydrogels for Soft Tissue Engineering. Mater. Sci. Eng. C 2018, 91, 236–246. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Subramanian, A.; Sethuraman, S. Injectable Glycosaminoglycan–Protein Nano-Complex in Semi-Interpenetrating Networks: A Biphasic Hydrogel for Hyaline Cartilage Regeneration. Carbohydr. Polym. 2017, 175, 63–74. [Google Scholar] [CrossRef]
- Ding, H.; Li, B.; Liu, Z.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. Decoupled pH- and Thermo-Responsive Injectable Chitosan/PNIPAM Hydrogel via Thiol-Ene Click Chemistry for Potential Applications in Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 2000454. [Google Scholar] [CrossRef]
- Hu, D.-N.; Ju, X.-J.; Pu, X.-Q.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.-Y. Injectable Temperature/Glucose Dual-Responsive Hydrogels for Controlled Release of Insulin. Ind. Eng. Chem. Res. 2021, 60, 8147–8158. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Liu, B.; Xiang, J.; Cui, Z.; Qu, X.; Qiu, D.; Tian, Y.; Yang, Z. Ultra-Tough Injectable Cytocompatible Hydrogel for 3D Cell Culture and Cartilage Repair. J. Mater. Chem. B 2018, 6, 1351–1358. [Google Scholar] [CrossRef]
- Yuan, Y.; Fan, D.; Shen, S.; Ma, X. An M2 Macrophage-Polarized Anti-Inflammatory Hydrogel Combined with Mild Heat Stimulation for Regulating Chronic Inflammation and Impaired Angiogenesis of Diabetic Wounds. Chem. Eng. J. 2022, 433, 133859. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, Y.; Chen, J.; Huang, Y.; Alsareii, S.A.; Alamri, A.M.; Harraz, F.A.; Guo, B. Antibacterial Conductive Self-Healing Hydrogel Wound Dressing with Dual Dynamic Bonds Promotes Infected Wound Healing. Bioact. Mater. 2023, 30, 129–141. [Google Scholar] [CrossRef]
- Zhao, L.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C.; Kim, S.Y. Hyaluronic Acid/Chondroitin Sulfate-Based Hydrogel Prepared by Gamma Irradiation Technique. Carbohydr. Polym. 2014, 102, 598–605. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Luo, Z.; Li, D.; Lu, J.; Wang, Q.; Xiao, Y.; Zhang, X. Development of an Injectable Thiolated Icariin Functionalized Collagen/Hyaluronic Hydrogel to Promote Cartilage Formation in Vitro and in Vivo. J. Mater. Chem. B 2019, 7, 2845–2854. [Google Scholar] [CrossRef]
- Na, K.; Park, J.H.; Kim, S.W.; Sun, B.K.; Woo, D.G.; Chung, H.-M.; Park, K.-H. Delivery of Dexamethasone, Ascorbate, and Growth Factor (TGF β-3) in Thermo-Reversible Hydrogel Constructs Embedded with Rabbit Chondrocytes. Biomaterials 2006, 27, 5951–5957. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, X.; Liu, Z.; Li, Z.; Li, D.; Yan, H.; Lin, Q. Development of Alginate-Chitosan Composite Scaffold Incorporation of Bacterial Cellulose for Bone Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 296–307. [Google Scholar] [CrossRef]
- Kreller, T.; Distler, T.; Heid, S.; Gerth, S.; Detsch, R.; Boccaccini, A.R. Physico-Chemical Modification of Gelatine for the Improvement of 3D Printability of Oxidized Alginate-Gelatine Hydrogels towards Cartilage Tissue Engineering. Mater. Des. 2021, 208, 109877. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Niu, D.; Wu, N.; Yun, W.; Wang, W.; Zhang, K.; Li, G.; Yan, S.; Xu, G.; et al. Mussel-Inspired Bisphosphonated Injectable Nanocomposite Hydrogels with Adhesive, Self-Healing, and Osteogenic Properties for Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z.; Chen, X.; Feng, K.; Hu, T.; Huang, B.; Tang, J.; Wang, G.; Liu, S.; Yang, G.; et al. Double-Network Cellulose-Based Hybrid Hydrogels with Favourable Biocompatibility and Antibacterial Activity for Wound Healing. Carbohydr. Polym. 2023, 319, 121193. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Folic Acid-Modified Photoluminescent Dialdehyde Carboxymethyl Cellulose Crosslinked Bionanogels for pH-Controlled and Tumor-Targeted Co-Drug Delivery. Int. J. Biol. Macromol. 2022, 200, 247–262. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Z.; Xu, Y.; Lo, W.; Wang, X.; Niu, H.; Li, X.; Xie, X.; Khan, M.; Guan, J. pH-Sensitive and Thermosensitive Hydrogels as Stem-Cell Carriers for Cardiac Therapy. ACS Appl. Mater. Interfaces 2016, 8, 10752–10760. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Truong, D.; Heffernan, J.; Cutts, J.; Brafman, D.; Sirianni, R.W.; Vernon, B.; Nikkhah, M. PNIPAAm-Based Biohybrid Injectable Hydrogel for Cardiac Tissue Engineering. Acta Biomater. 2016, 32, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, S.; Chen, D. Preparation and Characterization of Chitosan Based Injectable Hydrogels Enhanced by Chitin Nano-Whiskers. J. Mech. Behav. Biomed. Mater. 2017, 65, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual Thermo-and pH-Sensitive Injectable Hydrogels of Chitosan/(Poly(N-Isopropylacrylamide-Co-Itaconic Acid)) for Doxorubicin Delivery in Breast Cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Atoufi, Z.; Kamrava, S.K.; Davachi, S.M.; Hassanabadi, M.; Saeedi Garakani, S.; Alizadeh, R.; Farhadi, M.; Tavakol, S.; Bagher, Z.; Hashemi Motlagh, G. Injectable PNIPAM/Hyaluronic Acid Hydrogels Containing Multipurpose Modified Particles for Cartilage Tissue Engineering: Synthesis, Characterization, Drug Release and Cell Culture Study. Int. J. Biol. Macromol. 2019, 139, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Q.; Johnson, M.; Wang, X.; Lyu, J.; Li, Y.; McMahon, S.; Greiser, U.; Sigen, A.; Wang, W. A Chondroitin Sulfate Based Injectable Hydrogel for Delivery of Stem Cells in Cartilage Regeneration. Biomater. Sci. 2021, 9, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Qiao, K.; Wang, Y.; Zheng, Y.; He, W.; Xie, Y.; Yang, H. Injectable and Biodegradable Double-Network Nanocomposite Hydrogel with Regulable Sol-Gel Transition Process and Mechanical Properties. Polym. Test. 2022, 106, 107452. [Google Scholar] [CrossRef]
- Kesavan, M.P.; Ayyanaar, S.; Vijayakumar, V.; Dhaveethu Raja, J.; Annaraj, J.; Sakthipandi, K.; Rajesh, J. Magnetic Iron Oxide Nanoparticles (MIONs) Cross-Linked Natural Polymer-Based Hybrid Gel Beads: Controlled Nano Anti-TB Drug Delivery Application. J. Biomed. Mater. Res. Part A 2018, 106, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small 2022, 18, 2104368. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Zheng, Z.; Liu, Y.; Cao, S.; Xu, Y. Mussel-Inspired Biocompatible PAADOPA/PAAm Hydrogel Adhesive for Amoxicillin Delivery. Ind. Eng. Chem. Res. 2020, 59, 13556–13563. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, Y.; Wang, Q.; Li, D.; Hussain, T.; Ke, H.; Wei, Q. Mussel-Inspired Sandwich-like Nanofibers/Hydrogel Composite with Super Adhesive, Sustained Drug Release and Anti-Infection Capacity. Chem. Eng. J. 2020, 399, 125668. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Z.; Deng, M.; Chen, L.; He, C.; Zhuang, X.; Chen, X. Thermo- and pH-Responsive Microgels for Controlled Release of Insulin. Polym. Int. 2012, 61, 1151–1157. [Google Scholar] [CrossRef]
- Sun, S.; Liang, N.; Yamamoto, H.; Kawashima, Y.; Cui, F.; Yan, P. pH-Sensitive Poly(Lactide-Co-Glycolide) Nanoparticle Composite Microcapsules for Oral Delivery of Insulin. Int. J. Nanomed. 2015, 10, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Samanta, P.; Dhara, D. Temperature, pH and Redox Responsive Cellulose Based Hydrogels for Protein Delivery. Int. J. Biol. Macromol. 2016, 87, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Dahlan, N.A.; Pushpamalar, J.; Veeramachineni, A.K.; Muniyandy, S. Smart Hydrogel of Carboxymethyl Cellulose Grafted Carboxymethyl Polyvinyl Alcohol and Properties Studied for Future Material Applications. J. Polym. Environ. 2018, 26, 2061–2071. [Google Scholar] [CrossRef]
- Ahmad, N.; Amin, M.C.I.M.; Mahali, S.M.; Ismail, I.; Chuang, V.T.G. Biocompatible and Mucoadhesive Bacterial Cellulose-g-Poly(Acrylic Acid) Hydrogels for Oral Protein Delivery. Mol. Pharm. 2014, 11, 4130–4142. [Google Scholar] [CrossRef]
- Mohd Amin, M.C.I.; Ahmad, N.; Pandey, M.; Jue Xin, C. Stimuli-Responsive Bacterial Cellulose-g-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels for Oral Controlled Release Drug Delivery. Drug Dev. Ind. Pharm. 2014, 40, 1340–1349. [Google Scholar] [CrossRef]
- Massoumi, B.; Mozaffari, Z.; Jaymand, M. A Starch-Based Stimuli-Responsive Magnetite Nanohydrogel as de Novo Drug Delivery System. Int. J. Biol. Macromol. 2018, 117, 418–426. [Google Scholar] [CrossRef]
- Talebian, S.; Shim, I.K.; Kim, S.C.; Spinks, G.M.; Vine, K.L.; Foroughi, J. Coaxial Mussel-Inspired Biofibers: Making of a Robust and Efficacious Depot for Cancer Drug Delivery. J. Mater. Chem. B 2020, 8, 5064–5079. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, Q.; Kuang, G.; Xia, J.; Shang, L. Multicomponent Microspheres with Spatiotemporal Drug Release for Post-Surgical Liver Cancer Treatment and Liver Regeneration. Chem. Eng. J. 2023, 455, 140585. [Google Scholar] [CrossRef]
- He, G.; Yan, X.; Miao, Z.; Qian, H.; Ma, Y.; Xu, Y.; Gao, L.; Lu, Y.; Zha, Z. Anti-Inflammatory Catecholic Chitosan Hydrogel for Rapid Surgical Trauma Healing and Subsequent Prevention of Tumor Recurrence. Chin. Chem. Lett. 2020, 31, 1807–1811. [Google Scholar] [CrossRef]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug Release and Kinetic Models of Anticancer Drug (BTZ) from a pH-Responsive Alginate Polydopamine Hydrogel: Towards Cancer Chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef]
- Naghizadeh, S.; Hassanzadeh Nemati, N.; Hassani Najafabadi, A.; Niknejad, H.; Khani, M.-M. Controlled Release of Fluorouracil (5-FU) from Chitosan-Co-Poly(Ethylene Glycol)/Poly(Glycerol Sebacate)-Co-Poly(Ethylene Glycol)-Coated Iron Oxide. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 212–220. [Google Scholar] [CrossRef]
- Silva, J.C.; Udangawa, R.N.; Chen, J.; Mancinelli, C.D.; Garrudo, F.F.F.; Mikael, P.E.; Cabral, J.M.S.; Ferreira, F.C.; Linhardt, R.J. Kartogenin-Loaded Coaxial PGS/PCL Aligned Nanofibers for Cartilage Tissue Engineering. Mater. Sci. Eng. C 2020, 107, 110291. [Google Scholar] [CrossRef]
- Ye, H.; Owh, C.; Jiang, S.; Ng, C.Z.Q.; Wirawan, D.; Loh, X.J. A Thixotropic Polyglycerol Sebacate-Based Supramolecular Hydrogel as an Injectable Drug Delivery Matrix. Polymers 2016, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Hemmati, A.; Namazi, H.; Heydari, A. Carboxymethylcellulose-Coated 5-fluorouracil@MOF-5 Nano-Hybrid as a Bio-Nanocomposite Carrier for the Anticancer Oral Delivery. Int. J. Biol. Macromol. 2020, 155, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Schneible, J.D.; Shi, K.; Young, A.T.; Ramesh, S.; He, N.; Dowdey, C.E.; Dubnansky, J.M.; Lilova, R.L.; Gao, W.; Santiso, E.; et al. Modified Gaphene Oxide (GO) Particles in Peptide Hydrogels: A Hybrid System Enabling Scheduled Delivery of Synergistic Combinations of Chemotherapeutics. J. Mater. Chem. B 2020, 8, 3852–3868. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Montaser, A.S.; Li, S. Effect of Cellulose Nanocrystals on Scaffolds Comprising Chitosan, Alginate and Hydroxyapatite for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 121, 814–821. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. Enhanced Mechanical, Biomineralization, and Cellular Response of Nanocomposite Hydrogels by Bioactive Glass and Halloysite Nanotubes for Bone Tissue Regeneration. Mater. Sci. Eng. C 2021, 128, 112236. [Google Scholar] [CrossRef]
- Gautam, S.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Sharma, C.; Chou, C.-F.; Mishra, N.C. Surface Modification of PCL-Gelatin-Chitosan Electrospun Scaffold by Nano-Hydroxyapatite for Bone Tissue Engineering. Mater. Today Commun. 2023, 34, 105237. [Google Scholar] [CrossRef]
- Shao, Y.-F.; Qing, X.; Peng, Y.; Wang, H.; Shao, Z.; Zhang, K.-Q. Enhancement of Mechanical and Biological Performance on Hydroxyapatite/Silk Fibroin Scaffolds Facilitated by Microwave-Assisted Mineralization Strategy. Colloids Surf. B Biointerfaces 2021, 197, 111401. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Lee, S.; Rho, S.; Lee, H.; Kim, I.S.; Hwang, D.S. Tunichrome-Inspired Pyrogallol Functionalized Chitosan for Tissue Adhesion and Hemostasis. Carbohydr. Polym. 2019, 208, 77–85. [Google Scholar] [CrossRef]
- Balitaan, J.N.I.; Hsiao, C.-D.; Yeh, J.-M.; Santiago, K.S. Innovation Inspired by Nature: Biocompatible Self-Healing Injectable Hydrogels Based on Modified-β-Chitin for Wound Healing. Int. J. Biol. Macromol. 2020, 162, 723–736. [Google Scholar] [CrossRef]
- Ayati Najafabadi, S.A.; Shirazaki, P.; Zargar Kharazi, A.; Varshosaz, J.; Tahriri, M.; Tayebi, L. Evaluation of Sustained Ciprofloxacin Release of Biodegradable Electrospun Gelatin/Poly(Glycerol Sebacate) Mat Membranes for Wound Dressing Applications. Asia-Pac. J. Chem. Eng. 2018, 13, e2255. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/Chitosan/Hyaluronic Acid—Based Injectable Hydrogels for Tissue Engineering Applications—Design, Physicochemical and Biological Characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Liu, X.; Miller, A.L.; Cheng, Y.-S.; Yeh, M.-L.; Lu, L. Strengthening Injectable Thermo-Sensitive NIPAAm-g-Chitosan Hydrogels Using Chemical Cross-Linking of Disulfide Bonds as Scaffolds for Tissue Engineering. Carbohydr. Polym. 2018, 192, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Hou, Y.; Wu, L.; Li, S.; Yu, A.; Kong, D.; Wang, L.; Niu, G. An Anti-Infective Hydrogel Adhesive with Non-Swelling and Robust Mechanical Properties for Sutureless Wound Closure. J. Mater. Chem. B 2020, 8, 5682–5693. [Google Scholar] [CrossRef]
- Obiweluozor, F.O.; Maharjan, B.; Gladys Emechebe, A.; Park, C.H.; Kim, C.S. Mussel-Inspired Elastic Interpenetrated Network Hydrogel as an Alternative for Anti-Thrombotic Stent Coating Membrane. Chem. Eng. J. 2018, 347, 932–943. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular Cartilage: Structure, Injuries and Review of Management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, S.; Feng, Q.; Dai, Q.; Yao, L.; Zhang, Y.; Gao, H.; Dong, H.; Chen, D.; Cao, X. 3D Printed Silk-Gelatin Hydrogel Scaffold with Different Porous Structure and Cell Seeding Strategy for Cartilage Regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; You, Y.; Jiang, W.; Zhai, Z.; Dai, K. 3D-Bioprinting a Genetically Inspired Cartilage Scaffold with GDF5-Conjugated BMSC-Laden Hydrogel and Polymer for Cartilage Repair. Theranostics 2019, 9, 6949–6961. [Google Scholar] [CrossRef] [PubMed]
- Berberich, O.; Blöhbaum, J.; Hölscher-Doht, S.; Meffert, R.H.; Teßmar, J.; Blunk, T.; Groll, J. Catechol-Modified Poly(Oxazoline)s with Tunable Degradability Facilitate Cell Invasion and Lateral Cartilage Integration. J. Ind. Eng. Chem. 2019, 80, 757–769. [Google Scholar] [CrossRef]
- Gan, D.; Xu, T.; Xing, W.; Wang, M.; Fang, J.; Wang, K.; Ge, X.; Chan, C.W.; Ren, F.; Tan, H.; et al. Mussel-Inspired Dopamine Oligomer Intercalated Tough and Resilient Gelatin Methacryloyl (GelMA) Hydrogels for Cartilage Regeneration. J. Mater. Chem. B 2019, 7, 1716–1725. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Liu, J.; Chen, Z.; Li, Y.; Hu, B.; Zhang, D.; Li, J.; Chu, H. Bio-Inspired Cellulose Reinforced Anisotropic Composite Hydrogel with Zone-Dependent Complex Mechanical Adaptability and Cell Recruitment Characteristics. Compos. Part B Eng. 2020, 202, 108418. [Google Scholar] [CrossRef]
- Ziadlou, R.; Rotman, S.; Teuschl, A.; Salzer, E.; Barbero, A.; Martin, I.; Alini, M.; Eglin, D.; Grad, S. Optimization of Hyaluronic Acid-Tyramine/Silk-Fibroin Composite Hydrogels for Cartilage Tissue Engineering and Delivery of Anti-Inflammatory and Anabolic Drugs. Mater. Sci. Eng. C 2021, 120, 111701. [Google Scholar] [CrossRef]
- Ren, B.; Chen, X.; Du, S.; Ma, Y.; Chen, H.; Yuan, G.; Li, J.; Xiong, D.; Tan, H.; Ling, Z.; et al. Injectable Polysaccharide Hydrogel Embedded with Hydroxyapatite and Calcium Carbonate for Drug Delivery and Bone Tissue Engineering. Int. J. Biol. Macromol. 2018, 118, 1257–1266. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Ouyang, X.; Yang, Y.; Chen, Y.; Luo, Q.; Zhang, Y.; Zhu, D.; Yu, X.; Li, L. Biomimetic Hybrid Hydrogel for Hemostasis, Adhesion Prevention and Promoting Regeneration after Partial Liver Resection. Bioact. Mater. 2021, 11, 41–51. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Wang, Z. Engineered Liver Tissue in Vitro to Mimic Liver Functions and Its Biomedical Applications. Mater. Adv. 2022, 3, 4132–4154. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, K.; Jeong, J.; Paik, S.S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Park, J.; Choi, D. Three-Dimensional (3D) Printing of Mouse Primary Hepatocytes to Generate 3D Hepatic Structure. Ann. Surg. Treat. Res. 2017, 92, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.-F.; Zhao, F.-Q.; Ren, Y.-Z.; Zhang, Y.; Cui, Y.-L.; Wang, Q.-S. Injectable Hydrogels Based on Glycyrrhizin, Alginate, and Calcium for Three-Dimensional Cell Culture in Liver Tissue Engineering. J. Biomed. Mater. Res. Part A 2018, 106, 3292–3302. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C. Fibrin Hydrogels for Endothelialized Liver Tissue Engineering with a Predesigned Vascular Network. Polymers 2018, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.; Soleimani, M.; Zargarian, S.S.; Haddadi-Asl, V.; Ahmadbeigi, N.; Soudi, S.; Gheisari, Y.; Hajarizadeh, A.; Mohammadi, Y. In Vitro Differentiation of Human Cord Blood-Derived Unrestricted Somatic Stem Cells into Hepatocyte-Like Cells on Poly(ε-Caprolactone) Nanofiber Scaffolds. Cells Tissues Organs 2008, 190, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Janorkar, A.V.; Rajagopalan, P.; Yarmush, M.L.; Megeed, Z. The Use of Elastin-like Polypeptide–Polyelectrolyte Complexes to Control Hepatocyte Morphology and Function in Vitro. Biomaterials 2008, 29, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.C.; Zhou, L.; Hao, J. Current Stem Cell Delivery Methods for Myocardial Repair. Biomed. Res. Int. 2013, 2013, 547902. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ferguson, M.; Kamp, T.J.; Zhao, F. Constructing Biomimetic Cardiac Tissues: A Review of Scaffold Materials for Engineering Cardiac Patches. Emergent Mater. 2019, 2, 181–191. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Hua, W.; Darabi, A.; Song, X.; Song, C.; Zhong, W.; Xing, M.M.Q.; Qiu, X. Mussel-Inspired Conductive Cryogel as Cardiac Tissue Patch to Repair Myocardial Infarction by Migration of Conductive Nanoparticles. Adv. Funct. Mater. 2016, 26, 4293–4305. [Google Scholar] [CrossRef]
- Tallawi, M.; Dippold, D.; Rai, R.; D’Atri, D.; Roether, J.A.; Schubert, D.W.; Rosellini, E.; Engel, F.B.; Boccaccini, A.R. Novel PGS/PCL Electrospun Fiber Mats with Patterned Topographical Features for Cardiac Patch Applications. Mater. Sci. Eng. C 2016, 69, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Tallawi, M.; Frati, C.; Falco, A.; Gervasi, A.; Quaini, F.; Roether, J.A.; Hochburger, T.; Schubert, D.W.; Seik, L.; et al. Bioactive Electrospun Fibers of Poly(Glycerol Sebacate) and Poly(ε-Caprolactone) for Cardiac Patch Application. Adv. Healthc. Mater. 2015, 4, 2012–2025. [Google Scholar] [CrossRef] [PubMed]
- Obiweluozor, F.O.; GhavamiNejad, A.; Maharjan, B.; Kim, J.; Park, C.H.; Kim, C.S. A Mussel Inspired Self-Expandable Tubular Hydrogel with Shape Memory under NIR for Potential Biomedical Applications. J. Mater. Chem. B 2017, 5, 5373–5379. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cheng, C.; Teng, Y.; Nie, C.; Zhao, C. Mussel-Inspired Post-Heparinization of a Stretchable Hollow Hydrogel Tube and Its Potential Application as an Artificial Blood Vessel. Polym. Chem. 2017, 8, 2266–2275. [Google Scholar] [CrossRef]
- Ferrari, P.F.; Aliakbarian, B.; Lagazzo, A.; Tamayol, A.; Palombo, D.; Perego, P. Tailored Electrospun Small-Diameter Graft for Vascular Prosthesis. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 635–643. [Google Scholar] [CrossRef]
- Wang, Z.; Lü, S.; Liu, Y.; Li, T.; Yan, J.; Bai, X.; Ni, B.; Yang, J.; Liu, M. Noncovalent Muscle-Inspired Hydrogel with Rapid Recovery and Antifatigue Property under Cyclic Stress. ACS Appl. Mater. Interfaces 2019, 11, 31393–31401. [Google Scholar] [CrossRef]
- Zanna, N.; Tomasini, C. Peptide-Based Physical Gels Endowed with Thixotropic Behaviour. Gels 2017, 3, 39. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, J.H.; Do, M.J.; Namkoong, E.; Lee, H.; Park, K. NiCHE Platform: Nature-Inspired Catechol-Conjugated Hyaluronic Acid Environment Platform for Salivary Gland Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 4285–4294. [Google Scholar] [CrossRef]
- Lipke, E.A.; Kerscher, P.; Hodge, A.J. Encapsulation and Cardiac Differentiation of hiPSCs in 3D PEG-Fibrinogen Hydrogels. U.S. Patent 9,587,221 B2, 7 March 2017. [Google Scholar]
- Xiaojun, S.; Jian, M.; Fuxing, P. Fibrin-HA Hydrogel Carrying G-CSF Sustained Release System and Preparation Method and Application Thereof. CN Patent 106,581,772 B, 18 February 2020. [Google Scholar]
- Jabbari, E. Keratin-Based Hydrogels. U.S. Patent 10,723,774 B2, 28 July 2020. [Google Scholar]
- KIM, S.; Yang, Y.P. Crosslinked Chitosan-Lactide Hydrogels. WO Patent 2014/169045 A1, 16 October 2017. [Google Scholar]
- Kim, I.S.; Kim, H.G.; Hong, C.A.; Lee, S.Y. Temperature Sensitive Hydrogel Composition Including Nucleic Acid and Chitosan. U.S. Patent 2019/0054015 A1, 21 February 2019. [Google Scholar]
- Yi, H.; Jung, S.; Abel, J.H. Macroporous Chitosan-Polyacrylamide Hydrogel Microspheres and Preparation Thereof. U.S. Patent 11,161,958B2, 22 July 2016. [Google Scholar]
- Madrazo, A.O.; David, L.; Montembault, A.; Viguier, E.; Cachon, T. Hydrogel Composites Comprising Chitosan and Cellulose Nanofibers. EP Patent 3765104B1, 13 March 2018. [Google Scholar]
- Yu, X.; Messina, D.J.; Pavlovic, E.; Cui, C.; SMITHER, K.M. Co-Crosslinked Hyaluronic Acid-Silk Fibroin Hydrogels for Improving Tissue Graft Viability and for Soft Tissue Augmentation. U.S. Patent 10,300,169B2, 24 August 2016. [Google Scholar]

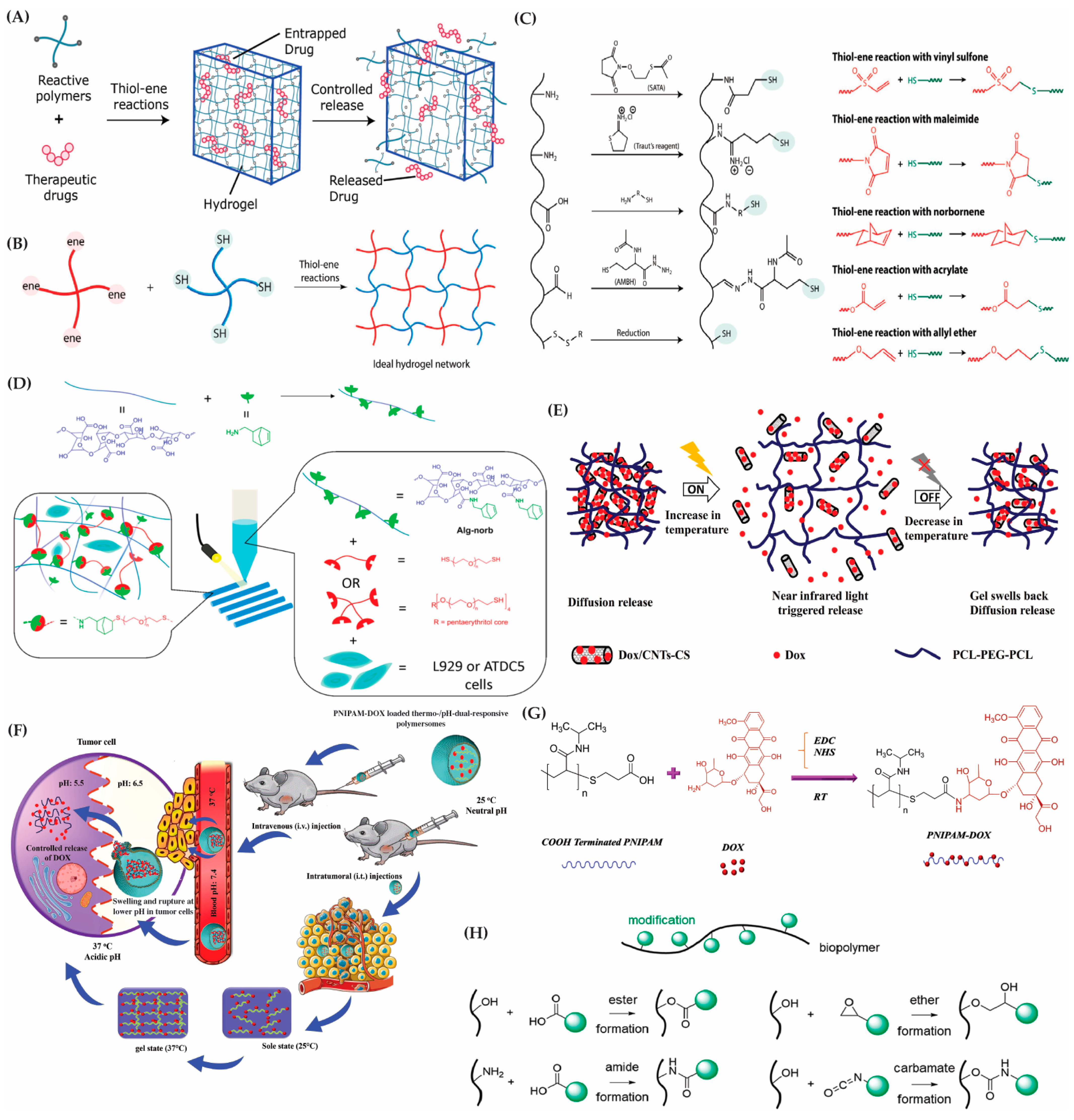
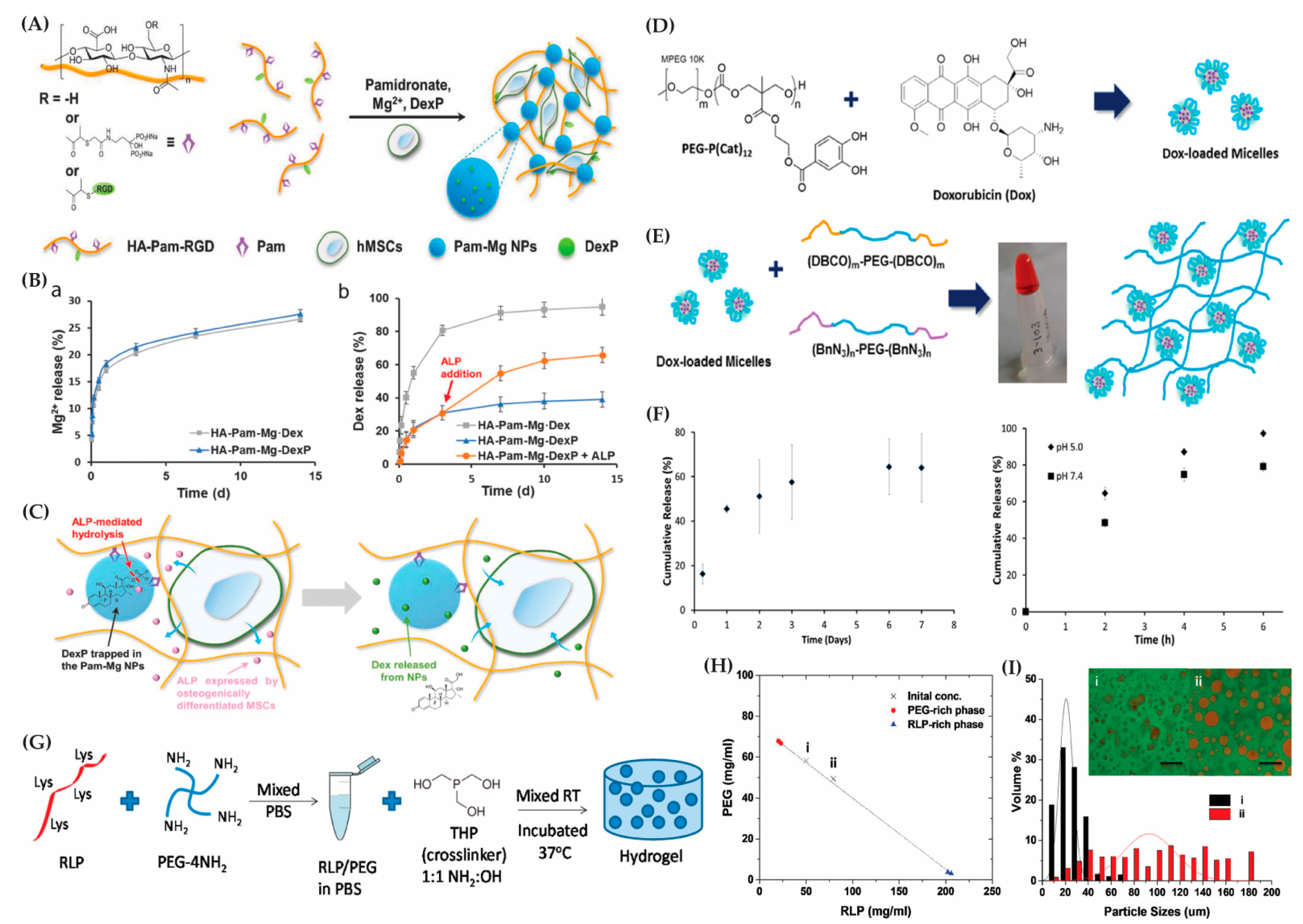
| Hybrid Hydrogels | Biocomponents | Stimuli-Response | Features | Applications | Ref. |
|---|---|---|---|---|---|
| SA-PAM | Divalent ions (Zinc) | - | The zinc crosslinked hydrogel exhibits outstanding mechanical strength | Tissue engineering and wound healing | [263] |
| Chitosan/poly(glutamic acid)/alginate polyelectrolyte complex hydrogels | Piroxicam (PXC) | - | Reduce gastrointestinal side effects of drugs (e.g., piroxicam) | Drug delivery system | [264] |
| Collagen/alginate/fibrin-based hydrogels | Murine fibroblasts | Temperature | Native soft tissue-like mechanical strength and thermosensitivity | Bone tissue engineering | [265] |
| PVA/alginate semi-IPN hydrogels | Chondroitin sulfate | - | Enhance chondrogenesis | Cartilage tissue engineering | [266] |
| Chitosan-PNIPAm hydrogels | hMSCs | Temperature | Sol–gel transition at pH 7.4 and LCST = 32–37 °C | Drug delivery, tissue engineering, and bioadhesive applications | [267] |
| Alginate-g-P(NIPAM-co-AAPBA) | Insulin | Temperature, glucose | Self-regulating, with the potential for a swift sol–gel transition | Injectable for controlled insulin release | [268] |
| CPBA-PVA | ATDC5 cells | - | High tensile strain and compressive fracture | Cartilage regeneration | [269] |
| Hyaluronic acid/collagen/deferoxamine-loaded polydopamine nanoparticles | Deferoxamine (DFO) | - | Desirable mechanical property; enhanced tissue adhesion and injectable properties | Wound healing | [270] |
| Alginate/dopamine/carboxymethyl chitosan | Fe3+ | - | Self-healing hydrogel with antimicrobial, adhesive, and conductive properties | Vascular regeneration | [271] |
| HA/CS/PVA hydrogels | Cefazoline, Theophylline, HaCaT cells | - | 85–88% degree of gelation under 15 kGy radiation | Skin tissue engineering | [272] |
| Collagen–hyaluronic acid | Icariin (Ica) | - | Injectable hydrogel to maintain chondrocyte phenotype | Cartilage repair | [273] |
| PNIPAm-co-AAC | Chondrocytes, Dexamethasone, TGF β3 | Temperature | An injectable hydrogel for promoting chondrogenesis and neocartilage formation | Cartilage tissue engineering | [274] |
| Alginate–chitosan incorporated with bacterial cellulose | Bovine serum albumin (BSA) | - | Promote osteogenic differentiation | Bone tissue engineering | [275] |
| Oxidized alginate–gelatin | Chondrocytes | - | Anti-inflammatory property to enhanced 3D printability; promote chondrogenic differentiation | Cartilage tissue regeneration | [276] |
| Nano-hydroxyapatite/PLGA/Dex | MC3T3-E1 Cells | - | Injectable hydrogel with tissue adhesive properties | Bone tissue engineering | [277] |
| Epsilon-polylysine-modified cellulose/γ-PGA double network hydrogel | ε-Polylysine (ε-PL) | - | Antibacterial property with excellent biocompatibility | Tissue engineering | [278] |
| Carbon dots/gelatin/carboxymethyl cellulose | Curcumin, doxorubicin | pH | Superior anticancer effect | Drug delivery system for cancer treatment | [279] |
| P(NIPAAm-co-PAA-co-MA-PEG-co-HEMA-oTMC) | Cardiosphere-derived cells | Temperature, pH | Sol–gel transition at pH 7.4 and LCST = 37 °C | Cell carriers for cardiac cell therapy, ocular drug delivery | [280] |
| PNIPAAm-gelatin | Cardiomyocytes, cardiac fibroblasts | Temperature | Sol–gel transition at pH 7.4 and LCST = 55 °C | Cardiac cell delivery and tissue engineering | [281] |
| Chitosan–ghitin nano-whiskers | Aspirin (ASA) | Temperature | Extracellular matrix imitation ability | Tissue regeneration | [282] |
| (PNIPAAm-co-IA)-CS | Doxorubicin | Temperature, pH | Sol–gel transition at pH 6.5 and LCST = 37 °C | Anticancer drug delivery | [283] |
| PNIPAm/HA hydrogels (containing CS-g-AA-coated PLGA or PLGA-ACH microparticles) | Melatonin, MSCs | Temperature | PLGA-ACH microparticles act as carriers of melatonin and reduce PNIPAm/HA syneresis | Drug delivery system and cartilage tissue engineering | [284] |
| CS/hyperbranched PEG | Adipose-derived MSCs | - | Injectable hydrogel with excellent mechanical feature, rapid gelation, and extended degradation profile | Cartilage tissue engineering | [285] |
| Sodium alginate/carboxymethyl bacterial cellulose (SA-CMBC) | Fibroblasts | - | Double-network injectable hydrogel with excellent mechanical property | Bone tissue engineering | [286] |
| Fe3O4 nanoparticles crosslinked polyethylene glycol hybrid chitosan (mCS-PEG) gel beads | Nanosized Rifampicin (nano-RIF) | pH, magnetic | Hybrid gel beads with dual-responsive assets in nanodrug delivery | Drug delivery (e.g., Rifampicin) application | [287] |
| Alginate/MXene-based hydrogels | Ag NPs | Photo, magnetic | Excellent antimicrobial feature and precise control release of encapsulated substances | Drug delivery and wound healing | [288] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. https://doi.org/10.3390/gels10040216
Rana MM, De la Hoz Siegler H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels. 2024; 10(4):216. https://doi.org/10.3390/gels10040216
Chicago/Turabian StyleRana, Md Mohosin, and Hector De la Hoz Siegler. 2024. "Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering" Gels 10, no. 4: 216. https://doi.org/10.3390/gels10040216
APA StyleRana, M. M., & De la Hoz Siegler, H. (2024). Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels, 10(4), 216. https://doi.org/10.3390/gels10040216








