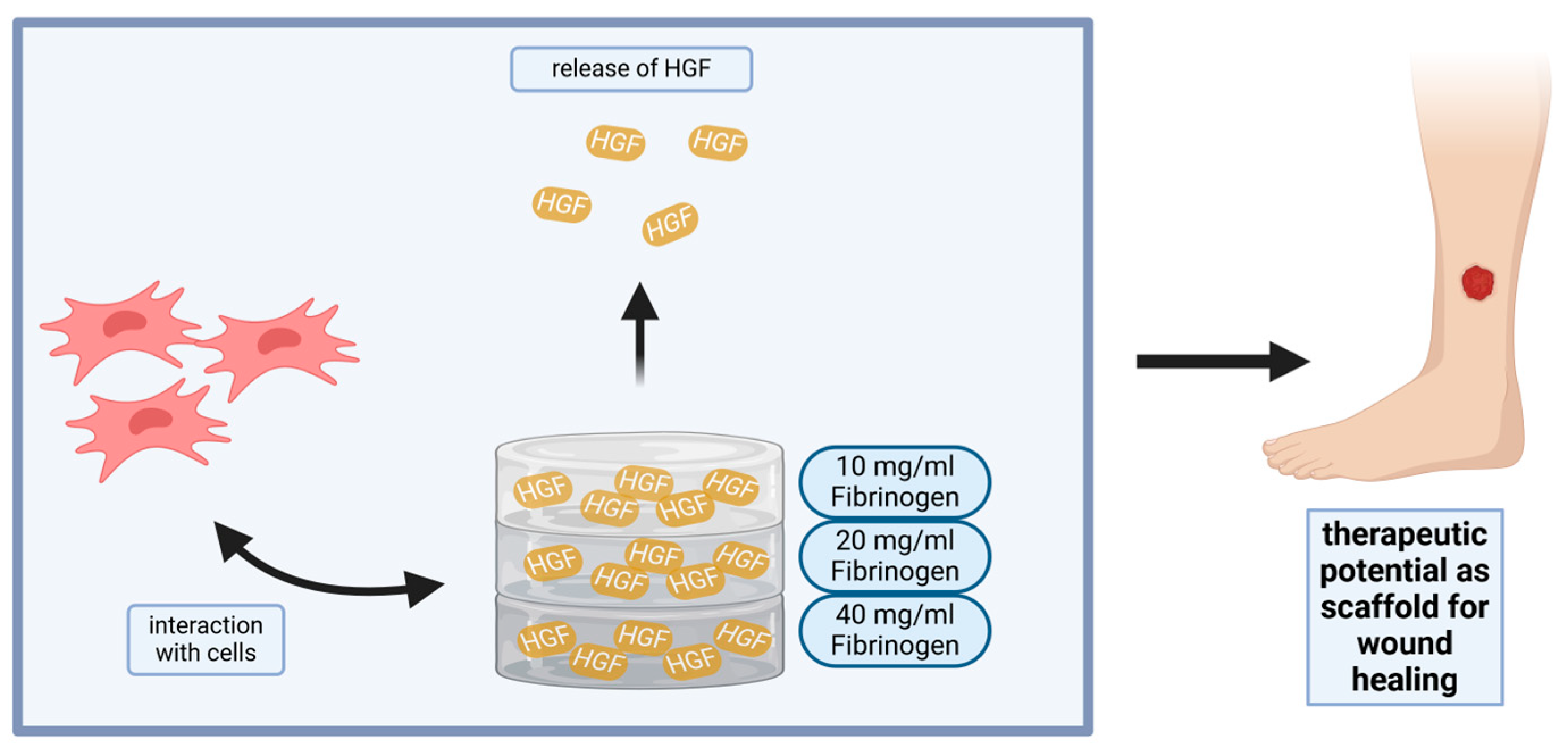
Impact of Fibrin Gel Architecture on Hepatocyte Growth Factor Release and Its Role in Modulating Cell Behavior for Tissue Regeneration
Abstract
:1. Introduction
Impact Statement
2. Results and Discussion
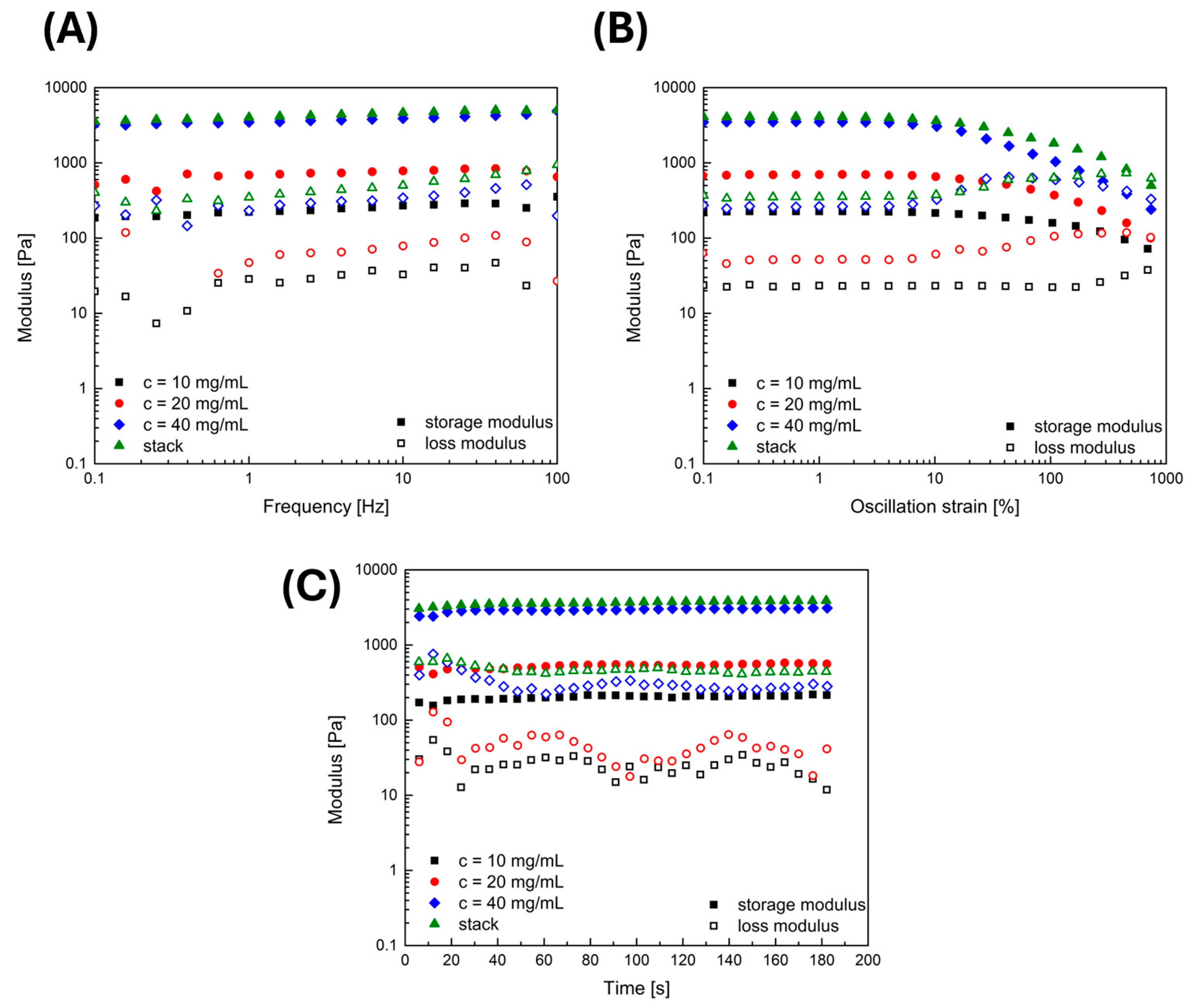
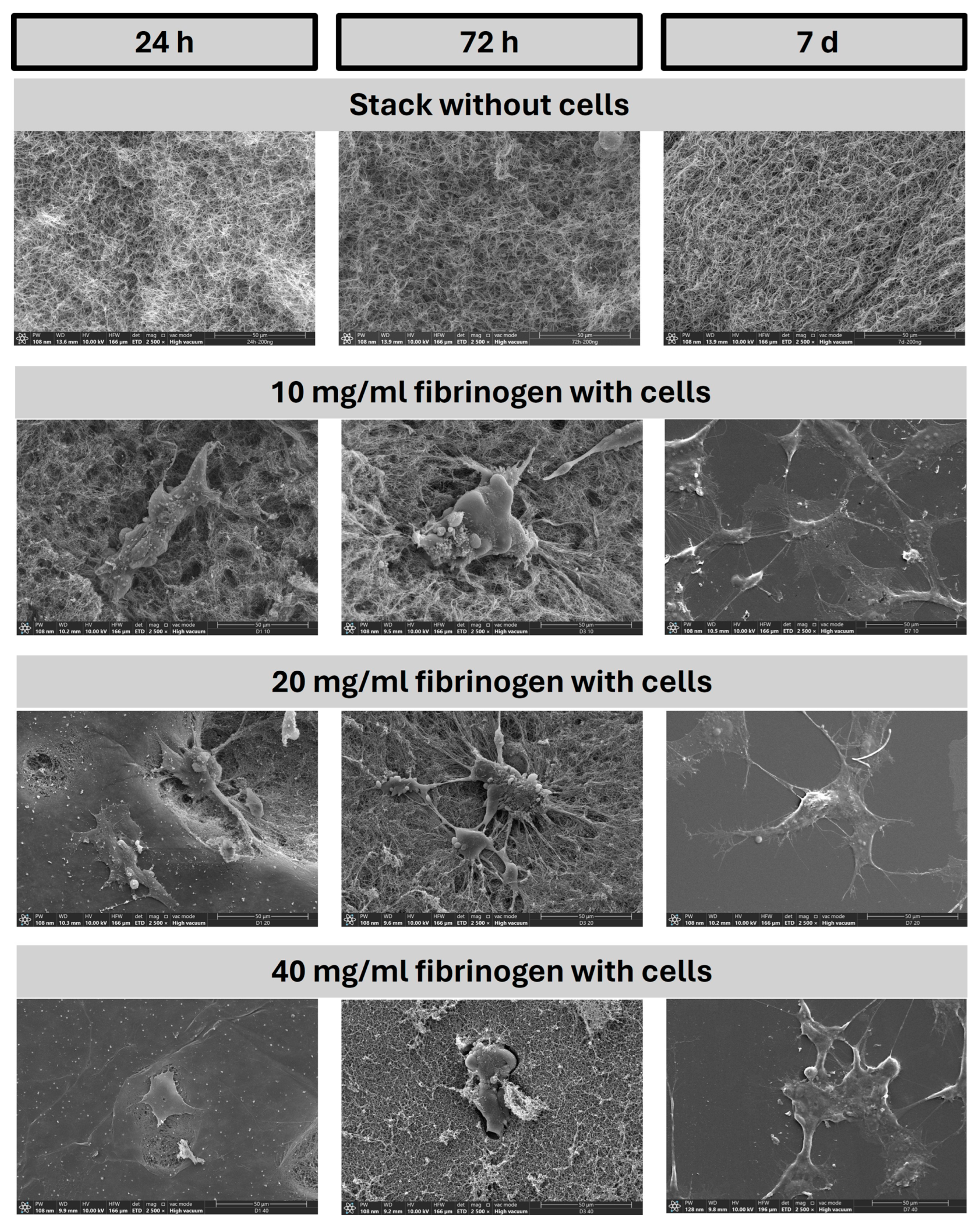
2.1. Rheological and Structural Properties of Applied Fibrin Gels: Linking Gel Characteristics to Cellular Behavior
2.1.1. Rheology
2.1.2. SEM Analysis
2.2. Decoding Fibrin Gel Degradation Dynamics and Hepatocyte Growth Factor Release
2.2.1. D-Dimer ELISA
2.2.2. HGF-Elisa
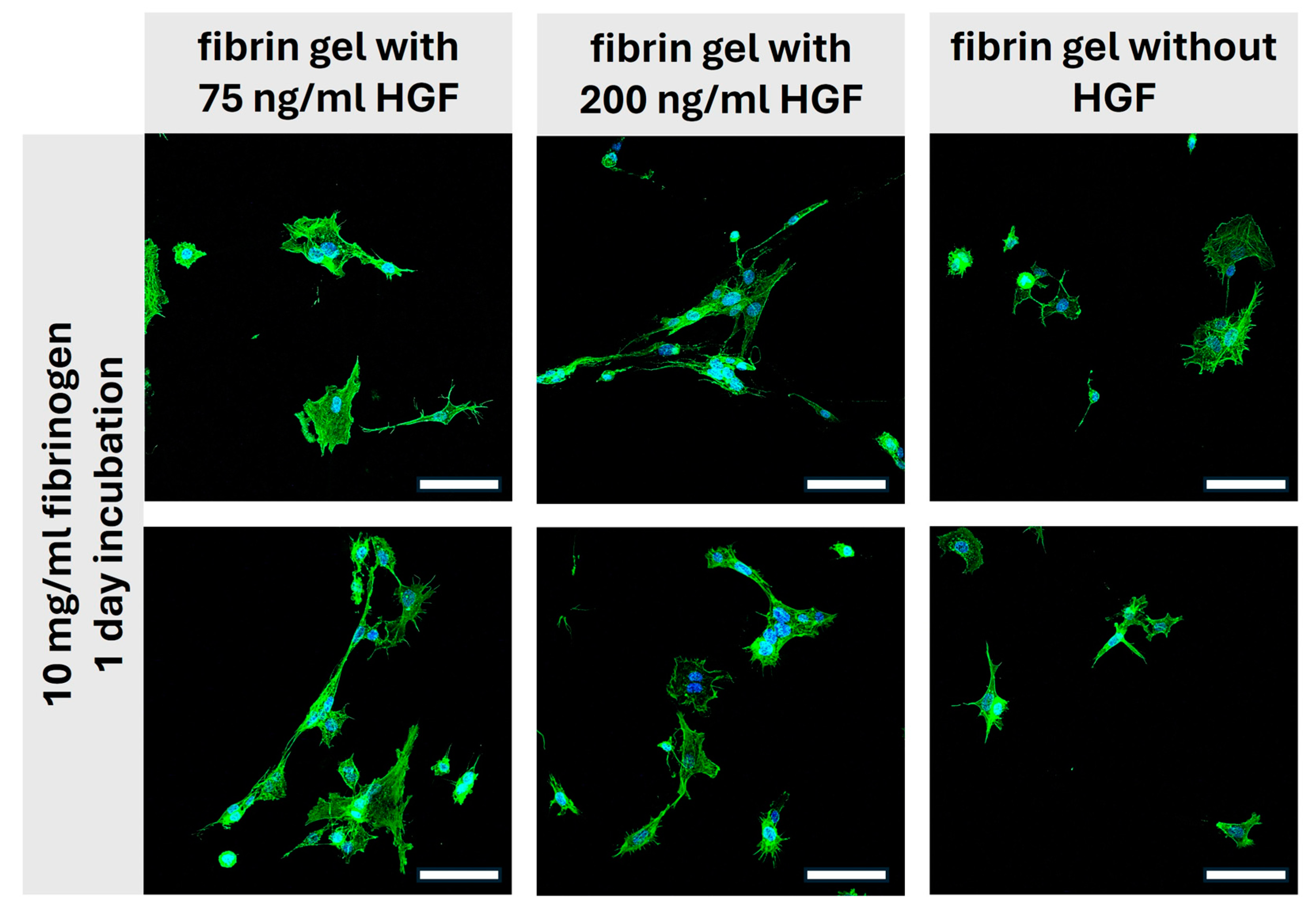
2.3. Evaluating the Bioactivity of Hepatocyte Growth Factor Released from Fibrin Gels
2.3.1. Scratch Assay
2.3.2. Scatter Assay
2.4. Assessment of Fibrin-HGF Gels as Scaffold Materials: Morphological Insights from Two-Photon Microscopy
Two-Photon-Microscopy
3. Conclusions
4. Materials and Methods
4.1. Cells
4.2. Fibrin Hydrogels with and without HGF
4.3. Mechanical Properties of Fibrin Gels: Rheology
4.4. Quantification of D-Dimer and HGF Levels via ELISA
4.5. Functional Assessment of Released HGF: Scratch and Scatter Assays
4.5.1. Experimental Implementation of the Scratch Assay
4.5.2. Experimental Implementation of the Scatter Assay
4.6. Immunofluorescence Staining and Microscopy
Scanning Electron Microscopy
4.7. Quantification and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakamura, S.; Ijima, H. Solubilized matrix derived from decellularized liver as a growth factor-immobilizable scaffold for hepatocyte culture. J. Biosci. Bioeng. 2013, 116, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Grasman, J.M.; Do, D.M.; Page, R.L.; Pins, G.D. Rapid release of growth factors regenerates force output in volumetric muscle loss injuries. Biomaterials 2015, 72, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Wang, C.-H.; Tong, Y.W. In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold. J. Biomed. Mater. Res. A 2009, 89, 411–423. [Google Scholar] [CrossRef] [PubMed]
- van de Kamp, J.; Jahnen-Dechent, W.; Rath, B.; Knuechel, R.; Neuss, S. Hepatocyte growth factor-loaded biomaterials for mesenchymal stem cell recruitment. Stem Cells Int. 2013, 2013, 892065. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.C.; Chan, R.W.; Weinberger, D.G.; Efune, G.; Pawlowski, K.S. Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction. J. Biomed. Mater. Res. A 2010, 93, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef]
- Al Enezy-Ulbrich, M.A.; Malyaran, H.; de Lange, R.D.; Labude, N.; Plum, R.; Rütten, S.; Terefenko, N.; Wein, S.; Neuss, S.; Pich, A. Impact of Reactive Amphiphilic Copolymers on Mechanical Properties and Cell Responses of Fibrin-Based Hydrogels. Adv Funct Materials 2020, 30, 2003528. [Google Scholar] [CrossRef]
- van de Kamp, J.; Paefgen, V.; Wöltje, M.; Böbel, M.; Jaekel, J.; Rath, B.; Labude, N.; Knüchel, R.; Jahnen-Dechent, W.; Neuss, S. Mesenchymal stem cells can be recruited to wounded tissue via hepatocyte growth factor-loaded biomaterials. J. Tissue Eng. Regen. Med. 2017, 11, 2988–2998. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, N.; Prestwich, G.D.; Wen, X. Recruitment of endogenous stem cells for tissue repair. Macromol. Biosci. 2008, 8, 836–842. [Google Scholar] [CrossRef]
- Bhubhanil, S.; Talodthaisong, C.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Yingmema, W.; Hutchison, J.A.; Lapmanee, S.; Kulchat, S. Enhanced wound healing properties of guar gum/curcumin-stabilized silver nanoparticle hydrogels. Sci. Rep. 2021, 11, 21836. [Google Scholar] [CrossRef]
- Baei, P.; Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Tafazzoli-Shadpour, M.; Baharvand, H.; Aghdami, N. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 131–141. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Dey, A.D.; Behl, T.; Chadha, S. Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sci. 2021, 268, 118932. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, H.; Su, Y.; John, J.V.; McCarthy, A.; Wong, S.L.; Xie, J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020, 108, 153–167. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, X.; Jin, L.; Bai, J.; Liu, W.; Wang, Z. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int. J. Nanomed. 2018, 13, 3897–3906. [Google Scholar] [CrossRef]
- Moreno-Arotzena, O.; Meier, J.G.; Del Amo, C.; García-Aznar, J.M. Characterization of Fibrin and Collagen Gels for Engineering Wound Healing Models. Materials 2015, 8, 1636–1651. [Google Scholar] [CrossRef] [PubMed]
- Kyomugasho, C.; Christiaens, S.; van de Walle, D.; van Loey, A.M.; Dewettinck, K.; Hendrickx, M.E. Evaluation of cation-facilitated pectin-gel properties: Cryo-SEM visualisation and rheological properties. Food Hydrocoll. 2016, 61, 172–182. [Google Scholar] [CrossRef]
- Carr, M.E.; Shen, L.L.; Hermans, J. A physical standard of fibrinogen: Measurement of the elastic modulus of dilute fibrin gels with a new elastometer. Anal. Biochem. 1976, 72, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, J.; Freemont, A.J.; Tirelli, N. Rheological and Turbidity Study of Fibrin Hydrogels. Macromol. Symp. 2013, 334, 117–125. [Google Scholar] [CrossRef]
- Wufsus, A.R.; Rana, K.; Brown, A.; Dorgan, J.R.; Liberatore, M.W.; Neeves, K.B. Elastic behavior and platelet retraction in low- and high-density fibrin gels. Biophys. J. 2015, 108, 173–183. [Google Scholar] [CrossRef]
- Linnes, M.P.; Ratner, B.D.; Giachelli, C.M. A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials 2007, 28, 5298–5306. [Google Scholar] [CrossRef]
- Saeedi Garakani, S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2020, 143, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Navaei-Nigjeh, M.; Amoabedini, G.; Noroozi, A.; Azami, M.; Asmani, M.N.; Ebrahimi-Barough, S.; Saberi, H.; Ai, A.; Ai, J. Enhancing neuronal growth from human endometrial stem cells derived neuron-like cells in three-dimensional fibrin gel for nerve tissue engineering. J. Biomed. Mater. Res. A 2014, 102, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, M.; Ishii, T.; Hirano, Y.; Tabata, Y. Controlled release of hepatocyte growth factor from gelatin hydrogels based on hydrogel degradation. J. Drug Target. 2001, 9, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Moncion, A.; Arlotta, K.J.; O’Neill, E.G.; Lin, M.; Mohr, L.A.; Franceschi, R.T.; Kripfgans, O.D.; Putnam, A.J.; Fabiilli, M.L. In vitro and in vivo assessment of controlled release and degradation of acoustically responsive scaffolds. Acta Biomater. 2016, 46, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, J.; Malyaran, H.; Enezy-Ulbrich, M.A.A.; Jung, S.; Radermacher, C.; Buhl, E.M.; Pich, A.; Neuss, S. Assessment of Fibrin-Based Hydrogels Containing a Fibrin-Binding Peptide to Tune Mechanical Properties and Cell Responses. Macro Materials & Eng 2023, 308, 2200678. [Google Scholar] [CrossRef]
- Ellis, D.R.; Eaton, A.S.; Plank, M.C.; Butman, B.T.; Ebert, R.F. A comparative evaluation of ELISAs for D-dimer and related fibrin(ogen) degradation products. Blood Coagul. Fibrinolysis 1993, 4, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Børset, M.; Turesson, I.; Abildgaard, N.; Sundan, A.; Anders Waage for the Nordic Myeloma Study Group. Elevated Serum Concentrations of Hepatocyte Growth Factor in Patients With Multiple Myeloma. Blood 1998, 91, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Yanaga, K.; Kanematsu, T. Serum Levels of Hepatocyte Growth Factor After Hepatectomy for Living Liver Donation. Transplantation 2004, 78, 1089–1090. [Google Scholar] [CrossRef]
- Sheen-Chen, S.-M.; Liu, Y.-W.; Eng, H.-L.; Chou, F.-F. Serum levels of hepatocyte growth factor in patients with breast cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 715–717. [Google Scholar] [CrossRef]
- Lin, X.Y.; Wang, H.; Tan, Y. Role of Hepatocyte Growth Factor in Wound Repair. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2018, 40, 822–826. [Google Scholar]
- Dally, J.; Khan, J.S.; Voisey, A.; Charalambous, C.; John, H.L.; Woods, E.L.; Steadman, R.; Moseley, R.; Midgley, A.C. Hepatocyte Growth Factor Mediates Enhanced Wound Healing Responses and Resistance to Transforming Growth Factor-β1-Driven Myofibroblast Differentiation in Oral Mucosal Fibroblasts. Int. J. Mol. Sci. 2017, 18, 1843. [Google Scholar] [CrossRef] [PubMed]
- Yamagamim, H.; Moriyama, M.; Matsumura, H.; Aoki, H.; Shimizu, T.; Saito, T.; Kaneko, M.; Shioda, A.; Tanaka, N.; Arakawa, Y. Serum concentrations of human hepatocyte growth factor is a useful indicator for predicting the occurrence of hepatocellular carcinomas in C-viral chronic liver diseases. Cancer 2002, 95, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, A.; Wright, H.; Rousseau, B. Optimal concentration of hepatocyte growth factor for treatment of the aged rat vocal fold. Laryngoscope 2011, 121, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Baldanzi, G.; Graziani, A. Physiological Signaling and Structure of the HGF Receptor MET. Biomedicines 2014, 3, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Duan, H.-F.; Wu, C.-T.; Zhang, D.-J.; Deng, Y.; Yin, H.-L.; Han, B.; Gong, H.-C.; Wang, H.-W.; Wang, Y.-L. HGF accelerates wound healing by promoting the dedifferentiation of epidermal cells through β1-integrin/ILK pathway. Biomed Res. Int. 2013, 2013, 470418. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.K.Y.; Lutterbach, B.A.; Pan, B.-S.; Kariv, I.; Szewczak, A.A. High-throughput analysis of HGF-stimulated cell scattering. J. Biomol. Screen. 2008, 13, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Vaghela, R.; Arkudas, A.; Horch, R.E.; Hessenauer, M. Actually Seeing What Is Going on-Intravital Microscopy in Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 627462. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, N.; Kajihara, T.; Arakaki, R.; Ohnishi, T.; Kazi, J.A.; Nakashima, H.; Daikuhara, Y. Involvement of oxidative stress in tumor cytotoxic activity of hepatocyte growth factor/scatter factor. J. Biol. Chem. 1999, 274, 13541–13546. [Google Scholar] [CrossRef]
- Cao, Z.; Xie, Y.; Yu, L.; Li, Y.; Wang, Y. Hepatocyte growth factor (HGF) and stem cell factor (SCF) maintained the stemness of human bone marrow mesenchymal stem cells (hBMSCs) during long-term expansion by preserving mitochondrial function via the PI3K/AKT, ERK1/2, and STAT3 signaling pathways. Stem Cell Res. Ther. 2020, 11, 329. [Google Scholar] [CrossRef]
- Umezaki, Y.; Hashimoto, Y.; Nishishita, N.; Kawamata, S.; Baba, S. Human Gingival Integration-Free iPSCs; a Source for MSC-Like Cells. Int. J. Mol. Sci. 2015, 16, 13633–13648. [Google Scholar] [CrossRef]
- Guan, N.; Liu, Z.; Zhao, Y.; Li, Q.; Wang, Y. Engineered biomaterial strategies for controlling growth factors in tissue engineering. Drug Deliv. 2020, 27, 1438–1451. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Mazaki, T.; Shiozaki, Y.; Yoshida, A.; Shinohara, K.; Nakamura, M.; Yoshida, Y.; Zhou, D.; Kitajima, T.; Tanaka, M.; et al. Collagen-Binding Hepatocyte Growth Factor (HGF) alone or with a Gelatin- furfurylamine Hydrogel Enhances Functional Recovery in Mice after Spinal Cord Injury. Sci. Rep. 2018, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Neuss, S.; Becher, E.; Wöltje, M.; Tietze, L.; Jahnen-Dechent, W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004, 22, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]





| Gel without HGF | Gel with 75 ng/mL HGF | Gel with 200 ng/mL HGF | |
|---|---|---|---|
| Component | µL per Well | ||
| Fibrinogen (10/20/40 mg/mL) | 156 | 156 | 156 |
| CaCl2 (50 mM) | 10 | 10 | 10 |
| GBSH5 incomplete | 24 | 8.25 | 3 |
| HGF in GBSH5 | 0 | 15.75 Stock: 1000 ng/mL | 21 Stock: 2000 ng/mL |
| Thrombin (10 U) | 20 | 20 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wein, S.; Jung, S.A.; Al Enezy-Ulbrich, M.A.; Reicher, L.; Rütten, S.; Kühnel, M.; Jonigk, D.; Jahnen-Dechent, W.; Pich, A.; Neuss, S. Impact of Fibrin Gel Architecture on Hepatocyte Growth Factor Release and Its Role in Modulating Cell Behavior for Tissue Regeneration. Gels 2024, 10, 402. https://doi.org/10.3390/gels10060402
Wein S, Jung SA, Al Enezy-Ulbrich MA, Reicher L, Rütten S, Kühnel M, Jonigk D, Jahnen-Dechent W, Pich A, Neuss S. Impact of Fibrin Gel Architecture on Hepatocyte Growth Factor Release and Its Role in Modulating Cell Behavior for Tissue Regeneration. Gels. 2024; 10(6):402. https://doi.org/10.3390/gels10060402
Chicago/Turabian StyleWein, Svenja, Shannon Anna Jung, Miriam Aischa Al Enezy-Ulbrich, Luca Reicher, Stephan Rütten, Mark Kühnel, Danny Jonigk, Wilhelm Jahnen-Dechent, Andrij Pich, and Sabine Neuss. 2024. "Impact of Fibrin Gel Architecture on Hepatocyte Growth Factor Release and Its Role in Modulating Cell Behavior for Tissue Regeneration" Gels 10, no. 6: 402. https://doi.org/10.3390/gels10060402
APA StyleWein, S., Jung, S. A., Al Enezy-Ulbrich, M. A., Reicher, L., Rütten, S., Kühnel, M., Jonigk, D., Jahnen-Dechent, W., Pich, A., & Neuss, S. (2024). Impact of Fibrin Gel Architecture on Hepatocyte Growth Factor Release and Its Role in Modulating Cell Behavior for Tissue Regeneration. Gels, 10(6), 402. https://doi.org/10.3390/gels10060402









