Advancements and Challenges in Self-Healing Hydrogels for Wound Care
Abstract
1. Introduction
2. Self-Healing Hydrogel Polymers
3. Crosslinking in Self-Healing Hydrogels
4. Self-Healing Hydrogels Crosslinked via Dynamic Covalent Bonding
4.1. Chitosan-Based Hydrogels with Schiff Base and Other Dynamic Linkages
4.2. Carboxymethyl Chitosan (CMC) Composites and Hydrogels
4.3. Specialty Chitosan Derivatives and Crosslinking Methods
4.4. Dynamic Crosslinking in Non-Chitosan Polysaccharides and Polymers
4.5. Functionalized Polymers and Innovative Crosslinking
4.6. Composite Materials and Hybrid Hydrogels
4.7. Oxidation and Reduction Reactions
4.8. Targeted Functional Crosslinking
5. Self-Healing Hydrogels with Ionic Crosslinking and Metal Coordination
5.1. Metal Ion Coordination and Crosslinking
5.2. Ionic Crosslinking with Non-Metal Ions
5.3. Physical Crosslinking and Self-Assembly
5.4. Hybrid Crosslinking Systems Involving Ion Interactions
6. Self-Healing Hydrogels Using Enhanced Functional Polymers and Responsive Systems
7. Nanocomposite Self-Healing Hydrogels
8. Collective Outcomes, Limitations, and Future Directions
8.1. Outcomes
8.2. Limitations
8.3. Future Directions
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balitaan, J.N.I.; Luo, W.J.; Su, Y.W.; Yu, C.Y.; Wu, T.Y.; Chang, C.A.; Jia, H.W.; Lin, S.R.; Hsiao, C.D.; Yeh, J.M. Healing Wounds Efficiently with Biomimetic Soft Matter: Injectable Self-Healing Neutral Glycol Chitosan/Dibenzaldehyde-Terminated Poly(ethylene glycol) Hydrogel with Inherent Antibacterial Properties. ACS Appl. Bio Mater. 2023, 6, 552–565. [Google Scholar] [CrossRef]
- Cui, H.; Cui, B.; Chen, H.; Geng, X.; Geng, X.; Li, Z.; Cao, S.; Shen, J.; Li, J. A chitosan-based self-healing hydrogel for accelerating infected wound healing. Biomater. Sci. 2023, 11, 4226–4237. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Li, W.Y.; Wang, B.X.; Zhang, M.H.; Wu, Z.T.; Wei, J.X.; Jiang, Y.; Sheng, N.; Liang, Q.Q.; Zhang, D.; Chen, S.Y. All-natural injectable hydrogel with self-healing and antibacterial properties for wound dressing. Cellulose 2020, 27, 2637–2650. [Google Scholar] [CrossRef]
- Deng, L.; Wang, B.; Li, W.; Han, Z.; Chen, S.; Wang, H. Bacterial cellulose reinforced chitosan-based hydrogel with highly efficient self-healing and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 2022, 217, 77–87. [Google Scholar] [CrossRef]
- Wang, K.; Dong, R.; Tang, J.; Li, H.; Dang, J.; Zhang, Z.; Yu, Z.; Guo, B.; Yi, C. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem. Eng. J. 2022, 430, 132664. [Google Scholar] [CrossRef]
- Li, Q.; Liu, K.; Jiang, T.; Ren, S.; Kang, Y.; Li, W.; Yao, H.; Yang, X.; Dai, H.; Chen, Z. Injectable and self-healing chitosan-based hydrogel with MOF-loaded alpha-lipoic acid promotes diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112519. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, S.; Fan, D. A physicochemical double cross-linked multifunctional hydrogel for dynamic burn wound healing: Shape adaptability, injectable self-healing property and enhanced adhesion. Biomaterials 2021, 276, 120838. [Google Scholar] [CrossRef]
- Ding, C.; Tian, M.; Feng, R.; Dang, Y.; Zhang, M. Novel Self-Healing Hydrogel with Injectable, pH-Responsive, Strain-Sensitive, Promoting Wound-Healing, and Hemostatic Properties Based on Collagen and Chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef]
- Chanmontri, M.; Swilem, A.E.; Mutch, A.L.; Grondahl, L.; Suwantong, O. Physicochemical and in vitro biological evaluation of an injectable self-healing quaternized chitosan/oxidized pectin hydrogel for potential use as a wound dressing material. Int. J. Biol. Macromol. 2023, 242, 124984. [Google Scholar] [CrossRef]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lopez Lasaosa, F.; He, Y.; Mao, H.; et al. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Su, D.; Wu, S.; Zhou, J.; Chen, J. Injectable, self-healing and pH responsive stem cell factor loaded collagen hydrogel as a dynamic bioadhesive dressing for diabetic wound repair. J. Mater. Chem. B 2021, 9, 5887–5897. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, J.; Qu, Z.; Tao, R.; Wang, G.; Liu, W. Niacin Metal-Organic Framework-Laden Self-Healing Hydrogel for Wound Healing. J. Biomed. Nanotechnol. 2020, 16, 1719–1726. [Google Scholar] [CrossRef]
- Huang, A.; Chen, Y.; Wu, C. Wound Dressing Double-Crosslinked Quick Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Modified Nanocellulose. Polymers 2023, 15, 3389. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, K.; Li, X.; Zhang, X.; Zhang, D.; Wang, L.N.; Lee, C.S. A double-crosslinked self-healing antibacterial hydrogel with enhanced mechanical performance for wound treatment. Acta Biomater. 2021, 124, 139–152. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Z.; Cheng, X.; Zou, Z.; Chen, X.; He, C. Injectable, self-healing hydrogel adhesives with firm tissue adhesion and on-demand biodegradation for sutureless wound closure. Sci. Adv. 2023, 9, eadh4327. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ren, J.; Deng, Y.; Wu, X.; Huang, J.; Wang, G.; Zhao, Y.; Li, J. An Injectable, Wound-Adapting, Self-Healing Hydrogel for Fibroblast Growth Factor 2 Delivery System in Tissue Repair Applications. J. Biomed. Nanotechnol. 2017, 13, 1660–1672. [Google Scholar] [CrossRef]
- Chang, G.; Dang, Q.; Liu, C.; Wang, X.; Song, H.; Gao, H.; Sun, H.; Zhang, B.; Cha, D. Carboxymethyl chitosan and carboxymethyl cellulose based self-healing hydrogel for accelerating diabetic wound healing. Carbohydr. Polym. 2022, 292, 119687. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, H.; Bai, Y.; Wang, Y.; Tao, L.; Wei, Y.; Fan, Y.; Guo, X.; Liu, H. Improving Chronic Diabetic Wound Healing through an Injectable and Self-Healing Hydrogel with Platelet-Rich Plasma Release. ACS Appl. Mater. Interfaces 2020, 12, 55659–55674. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.Y.; Wang, J.H.; Wu, J.L.; Zhang, Q.Q. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Shen, J.; Chang, R.; Chang, L.; Wang, Y.; Deng, K.; Wang, D.; Qin, J. Light emitting CMC-CHO based self-healing hydrogel with injectability for in vivo wound repairing applications. Carbohydr. Polym. 2022, 281, 119052. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tian, J.; Zhang, K.; Fei, X.; Yin, F.; Xu, L.; Wang, Y.; Li, Y. Bioinspired Adhesive Antibacterial Hydrogel with Self-Healing and On-Demand Removability for Enhanced Full-Thickness Skin Wound Repair. Biomacromolecules 2023, 24, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Yang, Y.T.; He, J.H.; Li, M.; Guo, B.L. Novel supramolecular self-healing silk fibroin-based hydrogel via host-guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2021, 417, 128278. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
- Jafari, H.; Alimoradi, H.; Delporte, C.; Bernaerts, K.V.; Heidari, R.; Podstawczyk, D.; Niknezhad, S.V.; Shavandi, A. An injectable, self-healing, 3D printable, double network co-enzymatically crosslinked hydrogel using marine poly- and oligo-saccharides for wound healing application. Appl. Mater. Today 2022, 29, 101581. [Google Scholar] [CrossRef]
- Tavakolizadeh, M.; Pourjavadi, A.; Ansari, M.; Tebyanian, H.; Tabaei, S.J.S.; Atarod, M.; Rabiee, N.; Bagherzadeh, M.; Varma, R.S. An environmentally friendly wound dressing based on a self-healing, extensible and compressible antibacterial hydrogel. Green Chem. 2021, 23, 1312–1329. [Google Scholar] [CrossRef]
- Hao, Z.; Liu, G.; Ren, L.; Liu, J.; Liu, C.; Yang, T.; Wu, X.; Zhang, X.; Yang, L.; Xia, J.; et al. A Self-Healing Multifunctional Hydrogel System Accelerates Diabetic Wound Healing through Orchestrating Immunoinflammatory Microenvironment. ACS Appl. Mater. Interfaces 2023, 15, 19847–19862. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, K.T.; Zhang, Y.; Cui, Y.L.; Wang, Q. A self-healing hydrogel wound dressing based on oxidized Bletilla striata polysaccharide and cationic gelatin for skin trauma treatment. Int. J. Biol. Macromol. 2023, 253, 127189. [Google Scholar] [CrossRef]
- Li, P.L.; Liu, S.; Yang, X.; Du, S.K.; Tang, W.T.; Cao, W.W.; Zhou, J.W.; Gong, X.D.; Xing, X.D. Low-drug resistance carbon quantum dots decorated injectable self-healing hydrogel with potent antibiofilm property and cutaneous wound healing. Chem. Eng. J. 2021, 403, 126387. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Cai, B.; Wang, Y.; Deng, D.; Wang, X. An injectable and self-healing hydrogel with antibacterial and angiogenic properties for diabetic wound healing. Biomater. Sci. 2022, 10, 3480–3492. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, P.; Cheng, Q.; He, C.; Chen, Y.; Zhou, J. Ultrafast Fabrication of Self-Healing and Injectable Carboxymethyl Chitosan Hydrogel Dressing for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 24095–24105. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liang, Y.; Chen, J.; Huang, Y.; Alsareii, S.A.; Alamri, A.M.; Harraz, F.A.; Guo, B. Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing. Bioact. Mater. 2023, 30, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Pourbadiei, B.; Monghari, M.A.A.; Khorasani, H.M.; Pourjavadi, A. A light-responsive wound dressing hydrogel: Gelatin based self-healing interpenetrated network with metal-ligand interaction by ferric citrate. J. Photochem. Photobiol. B 2023, 245, 112750. [Google Scholar] [CrossRef] [PubMed]
- Talodthaisong, C.; Patramanon, R.; Thammawithan, S.; Lapmanee, S.; Maikaeo, L.; Sricharoen, P.; Khongkow, M.; Namdee, K.; Jantimaporn, A.; Kayunkid, N.; et al. A Shear-Thinning, Self-Healing, Dual-Cross Linked Hydrogel Based on Gelatin/Vanillin/Fe3+/AGP-AgNPs: Synthesis, Antibacterial, and Wound-Healing Assessment. Macromol. Biosci. 2023, 23, e2300250. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, H.; Li, Z.; Zhangji, A.; Guo, B. Bioinspired Injectable Self-Healing Hydrogel Sealant with Fault-Tolerant and Repeated Thermo-Responsive Adhesion for Sutureless Post-Wound-Closure and Wound Healing. Nanomicro Lett. 2022, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Cheng, X.; Zhang, A.; Liu, W.; Zhang, S.; Jian, X. Tunicate inspired gelatin-based tough hydrogel wound dressing containing twisted phthalazinone with adhesive, self-healing and antibacterial properties. Int. J. Biol. Macromol. 2022, 218, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, R.Y.; Zhao, X.; Zhang, Y.H.; Tam, A.; Yan, Y.F.; Shen, H.K.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Chang, K.Y.; Chou, Y.N.; Chen, W.Y.; Chen, C.Y.; Lin, H.R. Mussel-Inspired Adhesive and Self-Healing Hydrogel as an Injectable Wound Dressing. Polymers 2022, 14, 3346. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C.; Chang, R.; He, Y.; Guan, F.; Yao, M. Ultra-stretchable, tissue-adhesive, shape-adaptive, self-healing, on-demand removable hydrogel dressings with multiple functions for infected wound healing in regions of high mobility. Acta Biomater. 2023, 166, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Chen, Y.; Zhao, L.; Feng, Z.P.; Peng, K.L.; Wei, A.L.; Wang, Y.L.; Tong, Z.R.; Cheng, B. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos. Part. B-Eng. 2020, 197, 108139. [Google Scholar] [CrossRef]
- Mou, C.; Wang, X.; Teng, J.; Xie, Z.; Zheng, M. Injectable self-healing hydrogel fabricated from antibacterial carbon dots and varepsilon-polylysine for promoting bacteria-infected wound healing. J. Nanobiotechnol. 2022, 20, 368. [Google Scholar] [CrossRef]
- Lu, S.; Chen, Z.; Tu, H.; Liu, H.; Liu, Y.; Chen, S.; Cai, D.; Liu, C.; Zhang, X.; Zou, G.; et al. Multifunctional carbon quantum dots decorated self-healing hydrogel for highly effective treatment of superbug infected wounds. Chem. Eng. J. 2024, 480, 148218. [Google Scholar] [CrossRef]
- Ling, Z.X.; Chen, Z.K.; Deng, J.; Wang, Y.F.; Yuan, B.; Yang, X.; Lin, H.; Cao, J.; Zhu, X.D.; Zhang, X.D. A novel self-healing polydopamine-functionalized chitosan-arginine hydrogel with enhanced angiogenic and antibacterial activities for accelerating skin wound healing. Chem. Eng. J. 2021, 420, 130302. [Google Scholar] [CrossRef]
- Shan, M.; Chen, X.; Zhang, X.; Zhang, S.; Zhang, L.; Chen, J.; Wang, X.; Liu, X. Injectable Conductive Hydrogel with Self-Healing, Motion Monitoring, and Bacteria Theranostics for Bioelectronic Wound Dressing. Adv. Healthc. Mater. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.P.; Liang, Y.Q.; Pan, G.Y.; Guo, B.L. Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem. Eng. J. 2022, 427, 132039. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Wang, J.; You, L.; Zhang, R.; Meng, Q.; Zhong, S.; He, W.; Cui, X. Chitosan-based mussel-inspired hydrogel for rapid self-healing and high adhesion of tissue adhesion and wound dressings. Carbohydr. Polym. 2023, 316, 121083. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.F.; Yan, J.Y.; Meng, G.H.; Cui, L.; Li, W.J.; Liu, Z.Y.; Guo, X.H. Green Reduction of Graphene Oxide by Macromolecular CMCS to Prepare Self-Healing Conductive Hydrogel Wound Dressing with Drug/Photothermal Antibacterial Activity. Macromol. Mater. Eng. 2022, 307, 2100878. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Xie, X.; Liao, J. An injectable, self-healing carboxymethylated chitosan hydrogel with mild photothermal stimulation for wound healing. Carbohydr. Polym. 2022, 293, 119722. [Google Scholar] [CrossRef]
- Zhang, Z.; Bu, J.; Li, B.; Xuan, H.; Jin, Y.; Yuan, H. Dynamic Double Cross-Linked Self-Healing Polysaccharide Hydrogel Wound Dressing Based on Schiff Base and Thiol-Alkynone Reactions. Int. J. Mol. Sci. 2022, 23, 13817. [Google Scholar] [CrossRef]
- Bai, H.; Kyu-Cheol, N.; Wang, Z.; Cui, Y.; Liu, H.; Liu, H.; Feng, Y.; Zhao, Y.; Lin, Q.; Li, Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J. Tissue Eng. 2020, 11, 2041731420947242. [Google Scholar] [CrossRef]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Chen, Z.P.; Hong, Z.P.; Zhang, L.; Liang, X.; Li, Y.; Duan, X.; Luo, H.; Peng, J.; Guo, J. Injectable thermo-sensitive and wide-crack self-healing hydrogel loaded with antibacterial anti-inflammatory dipotassium glycyrrhizate for full-thickness skin wound repair. Acta Biomater. 2022, 143, 203–215. [Google Scholar] [CrossRef]
- Li, H.; Wei, X.; Yi, X.; Tang, S.; He, J.; Huang, Y.; Cheng, F. Antibacterial, hemostasis, adhesive, self-healing polysaccharides-based composite hydrogel wound dressing for the prevention and treatment of postoperative adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111978. [Google Scholar] [CrossRef]
- Gu, H.; Li, H.; Wei, L.; Lu, J.; Wei, Q. Collagen-based injectable and self-healing hydrogel with multifunction for regenerative repairment of infected wounds. Regen. Biomater. 2023, 10, rbad018. [Google Scholar] [CrossRef]
- Jiang, H.; Xiao, Y.; Huang, H.; Yin, W.; Lang, M. An Injectable, Adhesive, and Self-Healing Hydrogel with Inherently Antibacterial Property for Wound Dressing. Macromol. Biosci. 2023, 24, e2300282. [Google Scholar] [CrossRef]
- Khodaei, T.; Nourmohammadi, J.; Ghaee, A.; Khodaii, Z. An antibacterial and self-healing hydrogel from aldehyde-carrageenan for wound healing applications. Carbohydr. Polym. 2023, 302, 120371. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, D.; Shao, H.; Hao, Y.; Zhang, T.; Zheng, W.; Ji, Y.; Ling, P.; Lu, Y.; Zhou, Q. Injectable and Self-Healing Probiotics-Loaded Hydrogel for Promoting Superbacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 20538–20550. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chang, L.; Sun, W.; Duan, W.; Qin, J. Mussel-inspired self-healing hydrogel form pectin and cellulose for hemostasis and diabetic wound repairing. Int. J. Biol. Macromol. 2023, 246, 125644. [Google Scholar] [CrossRef]
- Yi, X.T.; He, J.M.; Wei, X.J.; Li, H.B.; Liu, X.Y.; Cheng, F. A mussel-inspired multifunctional hydrogel reinforced by bacterial cellulose for wound healing: Sustained drug release, enhanced adhesion and self-healing property. Cellulose 2023, 30, 6523–6538. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, D.; Chang, L.; Duan, W.; Wang, Y.; Li, W.; Qin, J. Cellulose based self-healing hydrogel through Boronic Ester connections for wound healing and antitumor applications. Int. J. Biol. Macromol. 2023, 230, 123294. [Google Scholar] [CrossRef]
- Wang, W.Z.; Jia, B.; Xu, H.R.; Li, Z.L.; Qiao, L.P.; Zhao, Y.R.; Huang, H.Y.; Zhao, X.; Guo, B.L. Multiple bonds crosslinked antibacterial, conductive and antioxidant hydrogel adhesives with high stretchability and rapid self-healing for MRSA infected motion skin wound healing. Chem. Eng. J. 2023, 468, 143362. [Google Scholar] [CrossRef]
- Chen, Y.A.; Chang, L.M.; Zhu, J.J.; Sun, W.C.; Wang, Y.; Li, W.J.; Liu, Y.F.; Yu, X.; Qin, J.L. Antimicrobial poly(aspartic acid) based self-healing hydrogel with enhance cell migration rate for burn wound treatment. J. Drug Deliv. Sci. Tec. 2023, 89, 105062. [Google Scholar] [CrossRef]
- Li, W.; Cai, J.; Zhou, W.; Zhao, X.; Wang, M.; Zhou, X.; Ren, L. Poly(aspartic acid)-based self-healing hydrogel with precise antibacterial ability for rapid infected-wound repairing. Colloids Surf. B Biointerfaces 2023, 221, 112982. [Google Scholar] [CrossRef]
- Yang, R.; Liu, X.; Ren, Y.; Xue, W.; Liu, S.; Wang, P.; Zhao, M.; Xu, H.; Chi, B. Injectable adaptive self-healing hyaluronic acid/poly (gamma-glutamic acid) hydrogel for cutaneous wound healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef]
- Sun, A.; Hu, D.R.; He, X.Y.; Ji, X.; Li, T.; Wei, X.W.; Qian, Z.Y. Mussel-inspired hydrogel with injectable self-healing and antibacterial properties promotes wound healing in burn wound infection. NPG Asia Mater. 2022, 14, 86. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, J.; Jin, L.; Chen, J.; Xu, R.; Zhao, Y.; Yan, T.; Wan, H. Salvianolic-Acid-B-Loaded HA Self-Healing Hydrogel Promotes Diabetic Wound Healing through Promotion of Anti-Inflammation and Angiogenesis. Int. J. Mol. Sci. 2023, 24, 6844. [Google Scholar] [CrossRef]
- Tang, L.; Dang, Y.; Wang, Y.; Zhang, Y.; Hu, T.; Ding, C.; Wu, H.; Ni, Y.; Chen, L.; Huang, L.; et al. Rapid fabrication of bionic pyrogallol-based self-adhesive hydrogel with mechanically tunable, self-healing, antibacterial, wound healing, and hemostatic properties. Biomater. Adv. 2022, 136, 212765. [Google Scholar] [CrossRef]
- Guo, H.; Huang, S.; Xu, A.; Xue, W. Injectable Adhesive Self-Healing Multiple-Dynamic-Bond Crosslinked Hydrogel with Photothermal Antibacterial Activity for Infected Wound Healing. Chem. Mater. 2022, 34, 2655–2671. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Ning, X.Q.; Chen, Y.W.; Chang, H.X.; Lu, D.H.; Pei, D.T.; Geng, Z.J.; Zeng, Z.W.; Guo, C.P.; Huang, J.; et al. Dual Glucose/ROS-Sensitive Injectable Adhesive Self-Healing Hydrogel with Photothermal Antibacterial Activity and Modulation of Macrophage Polarization for Infected Diabetic Wound Healing. ACS Mater. Lett. 2023, 5, 3142–3155. [Google Scholar] [CrossRef]
- Yin, H.; Song, P.; Chen, X.; Huang, Q.; Huang, H. A self-healing hydrogel based on oxidized microcrystalline cellulose and carboxymethyl chitosan as wound dressing material. Int. J. Biol. Macromol. 2022, 221, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, X.; Xu, L.; Lu, H.; Chen, Y.; Wu, C.; Hu, P. A self-healing hydrogel based on crosslinked hyaluronic acid and chitosan to facilitate diabetic wound healing. Int. J. Biol. Macromol. 2022, 220, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, N.; Chi, Y.; Peng, Q.; Dai, G.; Qian, L.; Xu, K.; Zhong, W.; Yue, W. Injectable and Self-Healing Hydrogel Based on Chitosan-Tannic Acid and Oxidized Hyaluronic Acid for Wound Healing. ACS Biomater. Sci. Eng. 2022, 8, 3754–3764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.L.; Deng, Z.P.; Wang, Z.L.; Song, F.; Zhu, L.L. An extracellular matrix-inspired self-healing composite hydrogel for enhanced platelet-rich plasma-mediated chronic diabetic wound treatment. Carbohydr. Polym. 2023, 315, 120973. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2019, 8, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, B.; An, Z.; Zheng, P.; Liu, Y.; Zhao, H.; Zhang, Z.; Gao, T.; Cao, Y.; Zhang, Y.; et al. MMP-Responsive Nanoparticle-Loaded, Injectable, Adhesive, Self-Healing Hydrogel Wound Dressing Based on Dynamic Covalent Bonds. Biomacromolecules 2023, 24, 5769–5779. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yan, Y.; Huang, J.; Liang, Q.; Li, J.; Wang, B.; Ma, B.; Bianco, A.; Ge, S.; Shao, J. A multifunctional chitosan-based hydrogel with self-healing, antibacterial, and immunomodulatory effects as wound dressing. Int. J. Biol. Macromol. 2023, 231, 123149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, H.; Chen, W.; Han, P.; Yao, X.; Tang, B.; Duan, W.; Li, P.; Wei, X.; Chu, P.K.; et al. An injectable, self-healing composite hydrogel with enhanced near-infrared photo-antibacterial therapeutic effects for accelerated wound healing. Chem. Eng. J. 2023, 452, 139474. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Qi, Y.; Ma, X.; Wang, Y.; Song, H.; Zhao, Y.; Li, W.; Qin, J. Poly(aspartic acid) based self-healing hydrogel with blood coagulation characteristic for rapid hemostasis and wound healing applications. Colloids Surf. B Biointerfaces 2022, 214, 112430. [Google Scholar] [CrossRef]
- Chang, L.; Chang, R.; Liu, X.; Ma, X.; Chen, D.; Wang, Y.; Li, W.; Qin, J. Self-healing hydrogel based on polyphosphate-conjugated pectin with hemostatic property for wound healing applications. Biomater. Adv. 2022, 139, 212974. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.P.; Huang, Y.; He, J.H.; Han, Y.; Guo, B.L. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zhang, X.; Tang, P.; Zheng, T.; Ran, R.; Li, G. An injectable, self-healing, and antioxidant collagen- and hyaluronic acid-based hydrogel mediated with gallic acid and dopamine for wound repair. Carbohydr. Polym. 2023, 320, 121231. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.D.; Wang, C.Y.; Yuan, W.Z.; Xie, X.Y.; Song, Y. Highly Adhesive, Conductive, and Self-Healing Hydrogel with Triple Cross-Linking Inspired by Mussel and DNA for Wound Adhesion and Human Motion Sensing. ACS Appl. Polym. Mater. 2021, 3, 6586–6597. [Google Scholar] [CrossRef]
- Deng, X.Y.; Huang, B.X.; Wang, Q.H.; Wu, W.L.; Coates, P.; Sefat, F.; Lu, C.H.; Zhang, W.; Zhang, X.M. A Mussel-Inspired Antibacterial Hydrogel with High Cell Affinity, Toughness, Self-Healing, and Recycling Properties for Wound Healing. ACS Sustain. Chem. Eng. 2021, 9, 3070–3082. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Helmy, N.M.; Kamel, S. Dual-adhesive and self-healing alginate-based hydrogel for wound healing. Chem. Pap. 2023, 78, 1021–1031. [Google Scholar] [CrossRef]
- Xue, C.; Xu, X.; Zhang, L.; Liu, Y.; Liu, S.; Liu, Z.; Wu, M.; Shuai, Q. Self-healing/pH-responsive/inherently antibacterial polysaccharide-based hydrogel for a photothermal strengthened wound dressing. Colloids Surf. B Biointerfaces 2022, 218, 112738. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Dong, Y.P.; Wang, M.D.; Li, X.R.; Yu, J.Y.; Ding, B. Hydrogel/nanofibrous membrane composites with enhanced water retention, stretchability and self-healing capability for wound healing. Compos. Part. B-Eng. 2023, 257, 110672. [Google Scholar] [CrossRef]
- Bi, S.W.; He, C.Y.; Liu, R.Q.; Zhao, X.S.; Liu, J.L.; Gu, J.; Liu, W.T.; Yan, B. On-Demand Dissociable Antibacterial Self-Healing Hydrogel with Rapid Arginine-Related Biosynthesis Capacity to Promote Full-Thickness Bacteria-Infected Wound Healing. Ind. Eng. Chem. Res. 2023, 62, 7492–7503. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Du, R.; Yang, Y.; Liu, Y.; Wan, Z.; Yang, X. Injectable Self-Healing Adhesive Natural Glycyrrhizic Acid Bioactive Hydrogel for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17562–17576. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef]
- Li, C.; Jiang, T.; Zhou, C.; Jiang, A.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable self-healing chitosan-based POSS-PEG hybrid hydrogel as wound dressing to promote diabetic wound healing. Carbohydr. Polym. 2023, 299, 120198. [Google Scholar] [CrossRef]
- Gong, X.; Luo, M.; Wang, M.; Niu, W.; Wang, Y.; Lei, B. Injectable self-healing ceria-based nanocomposite hydrogel with ROS-scavenging activity for skin wound repair. Regen. Biomater. 2022, 9, rbab074. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Qiu, W.; Han, H.; Li, M.; Li, N.; Wang, Q.; Qin, X.; Wang, X.; Yu, J.; Zhou, Y.; Li, Y.; et al. Nanofibers reinforced injectable hydrogel with self-healing, antibacterial, and hemostatic properties for chronic wound healing. J. Colloid. Interface Sci. 2021, 596, 312–323. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Ren, Y.; Wang, P.; Pu, Y.; Yang, R.; Wang, X.; Tan, X.; Ye, Z.; Maurizot, V.; et al. Mussel-Inspired Dual-Cross-linking Hyaluronic Acid/epsilon-Polylysine Hydrogel with Self-Healing and Antibacterial Properties for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 27876–27888. [Google Scholar] [CrossRef]
- Xie, D.Q.; Hu, C.; Jiang, C.; Xia, J.C.; Ye, L.; Jin, Y.; Jiang, S.C.; Ji, Y.W.; Zhang, Z.D.; Song, H.H.; et al. Incorporating copper-based nanosheets into an injectable self-healing hydrogel enables superb repair of infected diabetic wound. Chem. Eng. J. 2023, 476, 146788. [Google Scholar] [CrossRef]
- Huang, X.; Tang, L.; Xu, L.; Zhang, Y.; Li, G.; Peng, W.; Guo, X.; Zhou, L.; Liu, C.; Shen, X.C. A NIR-II light-modulated injectable self-healing hydrogel for synergistic photothermal/chemodynamic/chemo-therapy of melanoma and wound healing promotion. J. Mater. Chem. B 2022, 10, 7717–7731. [Google Scholar] [CrossRef]
- Threepopnatkul, P.; Sokjorhor, J.; Homchan, P.; Khammee, W. Preparation of hydrogel with self-healing properties based on poly(vinyl alcohol)/corn starch/zeolite/cellulose nanocrystals for wound dressing applications. Mater. Today-Proc. 2022, 52, 2467–2473. [Google Scholar] [CrossRef]
- Peng, W.; Li, L.; Zhang, Y.; Su, H.; Jiang, X.; Liu, H.; Huang, X.; Zhou, L.; Shen, X.C.; Liu, C. Photothermal synergistic nitric oxide controlled release injectable self-healing adhesive hydrogel for biofilm eradication and wound healing. J. Mater. Chem. B 2023, 12, 158–175. [Google Scholar] [CrossRef]
- Ren, Z.; Duan, Z.; Zhang, Z.; Fu, R.; Zhu, C.; Fan, D. Instantaneous self-healing and strongly adhesive self-adaptive hyaluronic acid-based hydrogel for controlled drug release to promote tendon wound healing. Int. J. Biol. Macromol. 2023, 242, 125001. [Google Scholar] [CrossRef]
- Bo, Y.; Zhang, L.; Wang, Z.; Shen, J.; Zhou, Z.; Yang, Y.; Wang, Y.; Qin, J.; He, Y. Antibacterial Hydrogel with Self-Healing Property for Wound-Healing Applications. ACS Biomater. Sci. Eng. 2021, 7, 5135–5143. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Shear-thinning and self-healing chitosan-graphene oxide hydrogel for hemostasis and wound healing. Carbohydr. Polym. 2022, 294, 119824. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, Y.; Han, F.; Shen, K.; Luo, L.; Li, Y.; Jia, Y.; Zhang, J.; Cai, W.; et al. Exosome/metformin-loaded self-healing conductive hydrogel rescues microvascular dysfunction and promotes chronic diabetic wound healing by inhibiting mitochondrial fission. Bioact. Mater. 2023, 26, 323–336. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable Self-Healing Adhesive Chitosan Hydrogel with Antioxidative, Antibacterial, and Hemostatic Activities for Rapid Hemostasis and Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, A.; Zhang, Y.; Tian, Z.; Cheng, X.; Gao, Y.; Zhou, X.; Wu, X.; Chen, K.; Ning, X. A photoactive self-healing carboxymethyl chitosan-based hydrogel for accelerated infected wound healing through simultaneously modulating multiple critical tissue repair factors. Int. J. Biol. Macromol. 2023, 242, 124631. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Zhang, M.; Ma, X.; Zhao, J.; Ji, Y. Novel Injectable, Self-Healing, Long-Effective Bacteriostatic, and Healed-Promoting Hydrogel Wound Dressing and Controlled Drug Delivery Mechanisms. ACS Appl. Mater. Interfaces 2024, 16, 2140–2153. [Google Scholar] [CrossRef]
- He, J.H.; Li, Z.L.; Wang, J.X.; Li, T.Y.; Chen, J.Y.; Duan, X.L.; Guo, B.L. Photothermal antibacterial antioxidant conductive self-healing hydrogel with nitric oxide release accelerates diabetic wound healing. Compos. Part. B-Eng. 2023, 266, 110985. [Google Scholar] [CrossRef]
- Wan, Z.; He, J.; Yang, Y.; Chong, T.; Wang, J.; Guo, B.; Xue, L. Injectable adhesive self-healing biocompatible hydrogel for haemostasis, wound healing, and postoperative tissue adhesion prevention in nephron-sparing surgery. Acta Biomater. 2022, 152, 157–170. [Google Scholar] [CrossRef]
- Xu, K.Y.; Yang, Y.; Li, L.; Wu, J.L. Anti-Oxidant Bactericidal Conductive Injectable Hydrogel as Self-Healing Wound Dressing for Subcutaneous Wound Healing in Nursing Care. Sci. Adv. Mater. 2018, 10, 1714–1720. [Google Scholar] [CrossRef]
- Ma, J.; Li, T.; Luo, M.; Lei, B. Single-Component Self-Healing Antibacterial Anti-Inflammatory Intracellular-Antioxidative Poly(itaconic acid-pluronic) Hydrogel for Rapid Repair of MRSA-Impaired Wound. ACS Appl. Mater. Interfaces 2023, 15, 33413–33424. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111324. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Chen, C.; Dong, W.; Yang, X.; Wu, Y.; Kong, Q.; Yan, B. Thermoresponsive Self-Healing Zwitterionic Hydrogel as an In Situ Gelling Wound Dressing for Rapid Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 55342–55353. [Google Scholar] [CrossRef]
- Sun, Z.; Xiong, H.; Lou, T.; Liu, W.; Xu, Y.; Yu, S.; Wang, H.; Liu, W.; Yang, L.; Zhou, C.; et al. Multifunctional Extracellular Matrix Hydrogel with Self-Healing Properties and Promoting Angiogenesis as an Immunoregulation Platform for Diabetic Wound Healing. Gels 2023, 9, 381. [Google Scholar] [CrossRef]
- Liu, K.; Gong, B.; Li, T.; Lei, H.; Li, J.; Tang, J.; Peng, Y.; Li, S.; Zheng, Y.; Wei, G. Bioactive self-healing umbilical cord blood exosomes hydrogel for promoting chronic diabetic wound healing. Biochem. Biophys. Res. Commun. 2024, 690, 149241. [Google Scholar] [CrossRef]
- Wang, L.; Duan, L.; Liu, G.; Sun, J.; Shahbazi, M.A.; Kundu, S.C.; Reis, R.L.; Xiao, B.; Yang, X. Bioinspired Polyacrylic Acid-Based Dressing: Wet Adhesive, Self-Healing, and Multi-Biofunctional Coacervate Hydrogel Accelerates Wound Healing. Adv. Sci. 2023, 10, e2207352. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhang, H.; Zhang, H.; Dong, W.; Sun, W.; Zhao, Y. Responsive and self-healing structural color supramolecular hydrogel patch for diabetic wound treatment. Bioact. Mater. 2022, 15, 194–202. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Liu, S.J.; Wang, Z.; Xing, J.F. A tunable multifunctional hydrogel with balanced adhesion, toughness and self-healing ability prepared by photopolymerization under green LED irradiation for wound dressing. Eur. Polym. J. 2022, 168, 111119. [Google Scholar] [CrossRef]
- Ge, S.J.; Zhou, X.J.; Liu, S.L.; Xu, M.; Shi, Y.; Geng, J.; Li, J.J.; Jia, R.P.; Gu, Z.Z.; Xu, H. Multifunctional all hydrogel-based smart dressing system fabricated by a self-healing cross-linking strategy for real-time monitoring of wound temperature, strain and on-demand drug delivery. J. Mater. Chem. C 2022, 10, 17084–17098. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Liu, K.; Ji, X.; Fatehi, P.; Chen, J. A cellulose nanofibril-reinforced hydrogel with robust mechanical, self-healing, pH-responsive and antibacterial characteristics for wound dressing applications. J. Nanobiotechnol. 2022, 20, 312. [Google Scholar] [CrossRef]
- He, J.H.; Shi, M.T.; Liang, Y.P.; Guo, B.L. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, C.; Peng, X.; Zhang, K.; Yu, X. A self-healing and injectable oxidized quaternized guar gum/carboxymethyl chitosan hydrogel with efficient hemostatic and antibacterial properties for wound dressing. Colloids Surf. B Biointerfaces 2022, 209, 112207. [Google Scholar] [CrossRef] [PubMed]






| Polymer Base and Polymerization Technique | Tests Conducted on Hydrogel | Ref.# |
|---|---|---|
| Chitosan-Based Hydrogels with Schiff Base and Other Dynamic Linkages | ||
| Neutral glycol chitosan/dibenzaldehyde-terminated poly(ethylene glycol) via imine bonds | High-low strain exposure in continuous step strain rheology; inhibition against E. coli, P. aeruginosa, S. aureus; LC50 values in vivo using zebrafish embryos; wound contraction measurement at 30-dpw | [1] |
| Quaternized chitosan via imine bonds | Evaluation in a rat skin wound infection model | [2] |
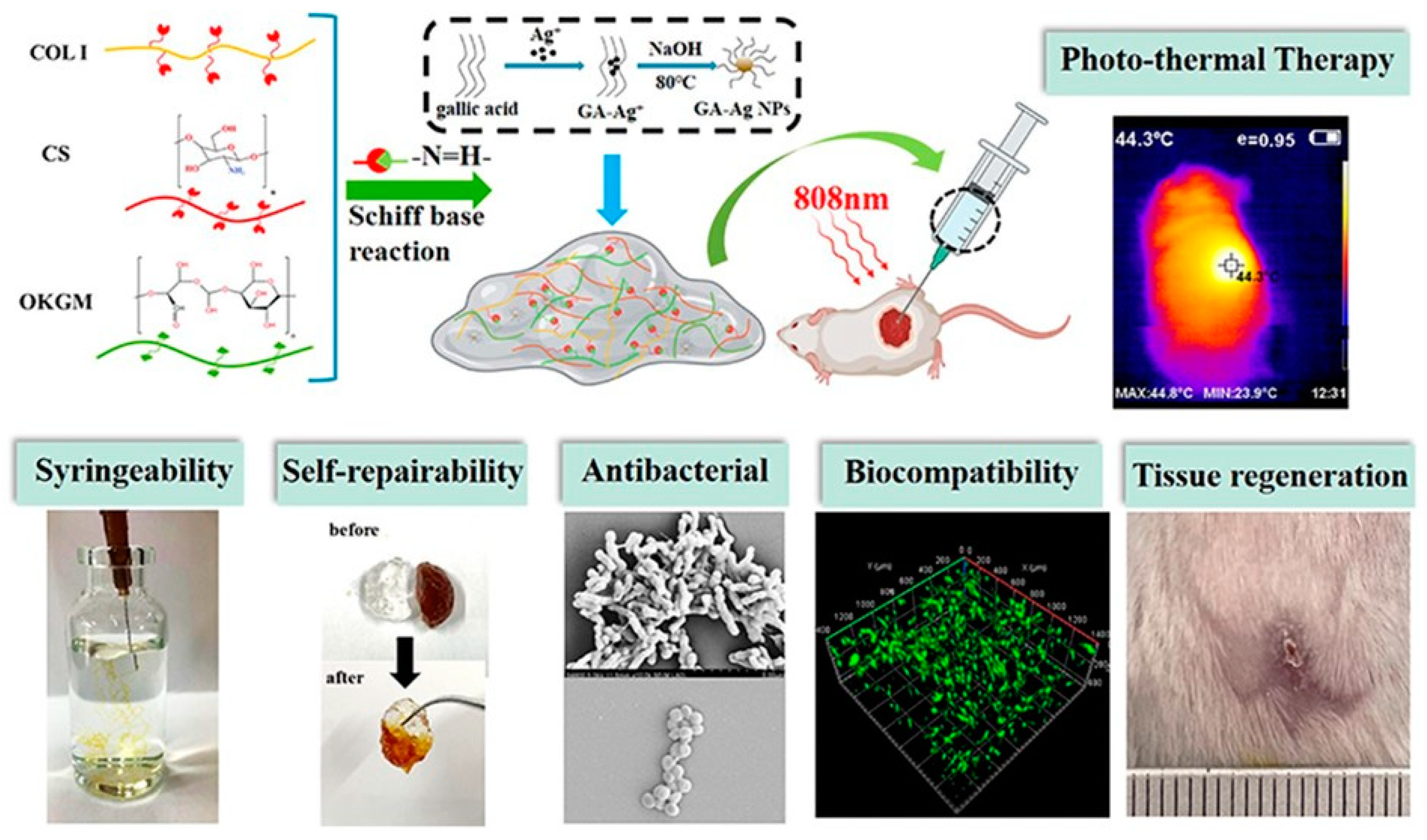
| Chitosan and oxidized konjac glucomannan via Schiff base reactions | Microscopy and rheological analyses for self-healing, inhibition rates against Staphylococcus aureus and Escherichia coli, wound recovery time in a full-thickness skin defect model | [3] |
| Chitosan with dialdehyde bacterial cellulose nanofibers via crosslinking | Not specified beyond being described as injectable, self-healing, with antibacterial properties | [4] |
| Quaternized chitosan and dialdehyde bacterial cellulose via Schiff base | Rapid self-healing performance, injectable behaviors, superior antibacterial activity, compressive property, water retention, cell proliferation and spreading | [5] |
| Chitosan-graft-aniline tetramer and dibenzaldehyde-terminated poly(ethylene glycol) | Enhanced diabetic wound healing; promotion of wound healing efficiency; collagen deposition; angiogenesis, M2 macrophage polarization; promotion of HUVEC proliferation, migration, and tube formation | [6] |
| Chitosan, hyaluronic acid, kalium gamma-cyclodextrin metal organic frameworks loaded with alpha-lipoic acid | Cell proliferation and migration (CCK-8 assay, Transwell experiments), reversal of oxidative stress-induced cell damage, in vivo wound healing in diabetic rats | [7] |
| Carboxymethyl chitosan and dialdehyde-modified cellulose nanocrystal | Dynamic Schiff base crosslinking, fluid uptake capacity, cytotoxicity assay, three-dimensional cell culture | [8] |
| Schiff base and catechol-Fe3+ chelation double crosslinked hydrogel | Gelation time, mechanical property, adhesive strength, self-healing property, antibacterial activity, hemostatic property | [9] |
| Collagen, chitosan, and dibenzaldehyde-modified poly(ethylene glycol) (PEG2000) via dynamic imine bonds | Thermal stability, injectability, pH sensitivity, promoting wound-healing and hemostatic ability, strain sensitivity | [10] |
| Quaternized chitosan/oxidized pectin via Schiff base reactions and ionic interactions | Continuous step strain analysis for self-healing; rapid gelation time; measurements of storage modulus, hardness, compressibility, and adhesiveness; cytotoxicity test on NCTC clone 929 cells; cell migration test; MIC(50) against E. coli and S. aureus | [11] |
| Niacin metal-organic frameworks (NOFs) with four-armed benzaldehyde-terminated polyethylene glycol and carboxymethyl chitosan | Self-healing ability through reversible Schiff base reaction, antibacterial and antioxidant activities, in vivo wound closure, tissue formation studies | [14] |
| Carboxymethyl chitosan, hydrazide-modified carboxymethyl cellulose nanofibers, and cellulose nanocrystals modified by dialdehyde | Fast self-healing, strength reinforcement, liquid absorption capacity | [15] |
| Chitosan with lysine and 3,4-dihydroxyphenylacetic acid (DOPAC) grafting | Adhesion to the wound surface, promotion of wound healing, antibacterial properties, reduction of inflammatory markers, promotion of angiogenesis | [48] |
| Collagen, chitosan, and oxidized konjac glucomannan with silver nanoparticles via dynamic Schiff base bonds | Enhanced antibacterial activity, syringeability, self-healing behavior, in vitro and in vivo wound-healing evaluations, hemostatic performance | [56] |
| Carboxymethyl Chitosan (CMC) Composites and Hydrogels | ||
| Carboxymethyl chitosan and oxidized pectin with reduced graphene oxide | Schiff base condensation, cell viability, conductivity, and mechanical strength, photothermal antibacterial activity | [49] |
| Carboxymethylated chitosan with GO-BPEI (graphene oxide-branched polyethyleneimine) and aldehyde terminated polyethylene glycol | Injectable and self-healing via dynamic Schiff bonds, mechanical strength, photothermal therapy, in vitro cell proliferation, in vivo wound healing | [50] |
| Specialty Chitosan Derivatives and Crosslinking Methods | ||
| Cysteine-modified carboxymethyl chitosan, sodium oxidized alginate, and but-3-yn-2-one based double crosslinking | Scanning electron microscopy, Fourier-transform infrared spectroscopy, rheological testing, antibacterial effect, in vivo biocompatibility | [51] |
| N-carboxyethyl chitosan, adipic acid dihydrazide, and hyaluronic acid-aldehyde in situ crosslinking | Swelling properties, stability, mechanical properties, regulation of inflammatory environment | [52] |
| Carboxyethyl chitosan-dialdehyde carboxymethyl cellulose with bone marrow mesenchymal stem cell-derived exosomes | Crosslinking through Schiff base reactions, antibacterial activity, promotion of angiogenesis, transformation of macrophages | [53] |
| Hydroxypropyl chitosan and poly(N-isopropylacrylamide) via beta-cyclodextrin and adamantyl pre-assembly | Injectable, thermosensitive, highly ductile, self-healable, biocompatible, antimicrobial activity against Staphylococcus aureus, in vivo wound healing evaluations on mouse model | [54] |
| N, O-carboxymethyl chitosan and oxidized dextran without chemical crosslinking agents | Gelation time, cytocompatibility and hemocompatibility, antibacterial activity, biodegradability and biocompatibility, efficacy in preventing post-operative peritoneal adhesion in rat model | [55] |
| Poly(glycerol sebacate)-co-poly(ethylene glycol)-g-catechol/Zn2+/(3-acrylamidophenyl) boronic acid and 2-hydroxyethyl acrylate/ionic liquids via multiple bonds crosslinking | In vitro antibacterial tests for MRSA and E. coli, cyto-compatibility and antioxidation tests, healing effect demonstration on MRSA-infected mouse model | [63] |
| Dynamic Crosslinking in Non-Chitosan Polysaccharides and Polymers | ||
| Dopamine-functionalized oxidized hyaluronic acid, adipic acid dihydrazide-modified hyaluronic acid, and aldehyde-terminated Pluronic F127 via Schiff base dynamic covalent bonds | Injection abilities and mechanical performance testing, self-healing faster than single-network hydrogels, adhesion properties testing | [12] |
| Collagen and polyethylene glycol with umbilical cord stem cell factor | Rheology, self-shaping and self-healing testing, adhesion strength enhancement, biocompatibility, cellular response, collagen deposition, neovascularization in diabetic rat model | [13] |
| Dynamic Schiff base bonds and non-dynamic photo-induced crosslinking | Mechanical performance (elastic recovery and tensile modulus), antibacterial capability, tissue adhesion, healing of infectious cutaneous wounds | [16] |
| Mixed charged polypeptides and oxidized dextran via Schiff base reaction | Injectable and self-healing performances, antibacterial activity tests against E. coli and S. aureus, hemolysis rate, and cytotoxicity assessment | [57] |
| Aldehyde-carrageenan with dopamine and zinc ions | Fourier transform infrared spectroscopy (FTIR) analysis, rheological tests, antibacterial properties against E. coli, collagen secretion and cell attachment, fibroblast viability | [58] |
| Hyaluronate-adipic dihydrazide/aldehyde-terminated Pluronic F127/fucoidan with lactobacillus rhamnosus via dynamic Schiff base reaction | Gelation time, mechanical strength, self-healing, and liquid-absorption abilities, anti-super bacteria effect, cytocompatibility and blood compatibility, in vivo wound healing efficacy | [59] |
| Poly(aspartic acid) with hydrazide functional groups crosslinked with dialdehyde functionalized polyethylene oxide | Antibacterial property, biocompatibility, in vivo burn wound-repairing experiment | [64] |
| Thiol-modified poly(gamma-glutamic acid) and oxidized hyaluronic acid via thiol-aldehyde addition reaction | Gelation time, rheological behavior, mechanical property, porous structure, degradation process, in vivo wound-healing process in full-thickness skin defect model | [66] |
| Oxidized konjac glucomannan, gamma-poly(glutamic acid) modified with dopamine and bcysteine, and epsilon-polylysine via thiol-aldehyde addition and Schiff base reactions | Antibacterial effects on Pseudomonas aeruginosa and Staphylococcus aureus, antioxidant effects, adhesion tests, burn wound infection model healing promotion | [67] |
| Functionalized Polymers and Innovative Crosslinking | ||
| Dopamine coupled pectin hydrazide and oxidized carboxymethyl cellulose for mEGF loading via Schiff base crosslinking | Fast forming and covering of irregular wounds, tissue adhesion for hemostasis, reactive oxygen species scavenging ability, in vivo diabetic wound healing in mice model | [60] |
| Bacterial cellulose with polydopamine, polyvinyl alcohol via dynamic borate ester bond crosslinking | Tissue adhesiveness, self-healing mechanical property, hemostatic ability, antibacterial activity, swelling ratio, biocompatibility, wound closure in a mouse model | [61] |
| Carboxyethyl cellulose with boronic acid and polyvinyl alcohol via boronic ester bond crosslinking | Biocompatibility, degradation by cellulase and in vivo, localized injection to cover irregular wounds, drug release carrier for antitumor drugs, in vivo wound repair, and airtight adhesion to the wound site | [62] |
| Quaternary ammonium/boronic acid modified poly(aspartic acid) and poly (vinyl alcohol) polymers with targeted peptide MP196-conjugated polydopamine | Biocompatibility enhancement, antibacterial efficacy by pH-triggering, photothermal treatment, in vitro synergistic antibacterial efficiency, in vivo healing rate and bacterial survival rate, self-healing property | [65] |
| Hyaluronic acid with salvianolic acid B via covalent crosslinking of aldehyde groups in oxidized hyaluronic acid and hydrazide groups in adipic dihydrazide-modified hyaluronic acid | Porous structures, self-healing properties, sustainable release capacity, cytocompatibility, full-thickness skin defect model in diabetic rats for wound closure and regeneration | [68] |
| Gallic acid-modified epsilon-poly-L-lysine and acrylic acid via rapid polymerization under blue light irradiation | Adhesive strength in humid environments, mechanical and self-healing properties, antibacterial ability, wound healing, scar suppression, hemostatic effect on liver bleeding | [69] |
| Multiple-dynamic-bond crosslinked network of dynamic borate/didiol interactions, hydrogen bonding, and Schiff base bond with polydopamine nanoparticles | Mechanical and adhesive properties, photothermal antibacterial activity, cytocompatibility, and hemocompatibility, in vivo bacteria-infected wound healing | [70] |
| Glucose/ROS-sensitive dynamic phenylboronester bond crosslinking network with mussel-like super adhesion catechol groups and TP@Ag NPs | Injectable, self-healing, tissue adhesion properties, glucose/ROS-sensitive drug release, photothermal synergistic antibacterial, in vivo infected diabetic wound healing | [71] |
| Composite Materials and Hybrid Hydrogels | ||
| Catalyst-free o-Phthalaldehyde/amine (hydrazide) crosslinking | Rapid and firm adhesion to tissues, controlled degradation profiles in vivo, efficacy in liver and blood vessel injury sealing, rat and rabbit models of full-thickness skin incision healing | [17] |
| Benzaldehyde-terminated polyethylene glycol and N-succinyl-chitosan via reversible Schiff base reaction | Injectable and self-healing properties, drug encapsulation and implantation, promotion of angiogenesis, collagen deposition, granulation tissue formation | [18] |
| N, O-carboxymethyl chitosan-heparin and carboxymethyl cellulose-aldehyde via Schiff base crosslinking | Biodegradation, injectable and self-healing properties, drug delivery for moderating microenvironment and promoting cell migration, proliferation, and collagen fiber deposition | [19] |
| Phenylboronic acid and benzaldehyde bifunctional polyethylene glycol-co-poly(glycerol sebacic acid)/dihydrocaffeic acid and L-arginine cografted chitosan via Schiff base and phenylboronate ester bonding | pH/glucose dual-responsive drug release; adhesion, self-healing, antibacterial, antioxidant, and hemostasis properties; promotion of wound healing in rat diabetic foot model | [20] |
| Chitosan, silk fibroin, and platelet-rich plasma | PRP protection from enzymatic hydrolysis, sustained PRP release, chemotaxis of mesenchymal stem cells, in vitro proliferation of repair cells, collagen deposition, angiogenesis, and nerve repair in diabetic rat model | [21] |
| Oxidized microcrystalline cellulose and carboxymethyl chitosan via Schiff base crosslinking | Characterization of hydrogel structures; blood clotting activity; physical properties including gel time, swelling rate, mechanical, and self-healing characteristics; controlled drug release | [72] |
| Carboxymethyl chitosan and oxidized hyaluronic acid via Schiff base reaction | Physicochemical characterization, biocompatibility, enhancement of cell migration, inhibition of inflammatory cytokines, diabetic rat wound-healing efficacy | [73] |
| Chitosan modified with tannic acid and oxidized hyaluronic acid via Schiff base reaction | Gel formation process, self-healing capability, biocompatibility, hemostatic performance, anti-inflammatory properties, cell growth promotion, in vivo wound-healing acceleration | [74] |
| Sodium alginate and hyaluronic acid crosslinked with oxidized sodium alginate | Cell migration, wound-healing promotion, antibacterial property assessment, swelling ratio, biocompatibility, controlled drug release mechanisms | [107] |
| Oxidation and Reduction Reactions | ||
| Aminated gelatin, adipic acid dihydrazide, and oxidized dextran via dynamic covalent reaction | Self-healing capability, antibacterial and sequential drug release, biodegradability, swelling behavior, sequential release of antibacterial agent and growth factor, rat wound-healing effectiveness | [22] |
| Oxidized carboxymethyl cellulose and polyethylene glycol dinaphthoate hydrazide via acylhydrazone bonding | Gelation time, biocompatibility, in vitro and in vivo drug release study, hemostatic activity, wound-repairing efficacy | [23] |
| Targeted Functional Crosslinking | ||
| Dynamic host-guest interaction-based hydrogel | Strong adhesion property testing, on-demand separation, self-healing properties, protein adsorption properties, mechanical properties, antibacterial properties, biocompatibility, full-thickness rat-skin defect model healing and histomorphological evaluation | [24] |
| Silk Fibroin, acryloyl-beta-cyclodextrin, and 2-hydroxyethyl acrylate via photo-polymerization | Mechanical properties, long-term stability, rapid self-healing behavior, injectability, biocompatibility, controlled and sustained drug release profile, full-thickness skin defect model healing efficacy | [25] |
| Dopamine-grafted oxidized sodium alginate and polyacrylamide via hydrogen bonds and dynamic Schiff crosslinking | Self-healing ability, mechanical strength and stretchability, cell affinity and tissue adhesiveness, in vivo and in vitro utility demonstration | [26] |
| Dual enzymatic co-crosslinking using glucose oxidase/horseradish peroxidase and electrostatic interaction | Toughness, self-healing, moldability, injectability, 3D printability, swelling ratio, biodegradation, antioxidant properties, antibacterial activity, cytotoxicity, cell migration enhancement, blood vessel formation, in vivo wound healing in rat model | [27] |
| Salep/poly(vinyl alcohol) with ethylene diamine-modified salep and oxidized salep via hydrogen bonds and Schiff base crosslinking | Mechanical strength, tissue adhesiveness, therapeutic properties (cell viability, wound healing), macroscopic evaluation of wound healing | [28] |
| Multiple-dynamic-bond crosslinked hydrogel with 3,4-dihydroxybenzaldehyde, chitosan, and Fe3+ coordinate bonds | Autonomous healing, antibacterial activity, immunomodulation, anti-inflammation, neovascularization, antioxidant activity, diabetic wound-healing promotion | [29] |
| Oxidized bletilla striata polysaccharide and cationic gelatin via Schiff base reaction | Rheological properties, mechanical behavior, porous structure, degradation performance, hemostatic performance, wound-healing facilitation | [30] |
| Oxidized chondroitin sulfate and carboxymethyl chitosan with platelet-rich plasma | Gelation, viscoelasticity, inhibition of PRP enzymolysis, sustained growth factor release, cell proliferation and migration, diabetic skin wound-healing acceleration | [75] |
| Polymer Base and Polymerization Technique | Tests Conducted on Hydrogel | Ref.# |
|---|---|---|
| Metal Ion Coordination and Crosslinking | ||
| Carboxymethyl chitosan, carbon quantum dots, and oxidized dextran via Schiff base linkage | Self-healing, injectable, stretchable, compressive property, pH-dependent drug release, biofilm inhibition, cytocompatibility, in vivo antibacterial and wound-healing efficacy | [31] |
| Chitosan crosslinked with silver and copper ions | Antibacterial capability, adhesive ability, water absorption ability, biocompatibility, diabetic wound healing in rat models | [32] |
| Carboxymethyl chitosan crosslinked with Fe3+ and Al(3+) ions | Gelation process, self-healing, self-adaption, thermo-responsive ability, skin tissue regeneration, wound closure | [33] |
| Oxidized sodium alginate-grafted dopamine/carboxymethyl chitosan/Fe3+ | Self-healing properties, conductivity, photothermal antibacterial properties, rheological properties, mechanical properties, antioxidant properties, tissue adhesion properties, hemostatic properties, in vivo infected wound healing | [34] |
| Gelatin and copolymer of dimethyl aminoethyl methacrylate with 2-acrylamido-2-methylpropane sulfonic acid crosslinked with ferric ions | Mechanical strength, pore size via SEM, biodegradation rate under visible light, toxicity (MTT assay for L-929 cell line), histological studies in vivo | [35] |
| Gelatin/vanillin/Fe3+ crosslinked with andrographolide-modified silver nanoparticles | Self-healing capability; swelling degree; antibacterial activity against E. coli, S. aureus, and B. pseudomallei; wound closure in animal models | [36] |
| Sodium alginate, gelatin, and protocatechualdehyde with ferric ions | Mechanical and adhesive strength, injectability and self-healing capacity, biocompatibility, photothermal antibacterial activity, antioxidation, hemostatic effect, in vivo incision closure evaluation | [37] |
| Gelatin conjugated with 2-(4’-aldehydephenyl)-4-(2’,3’,4’-trihydroxyphenyl)-2,3-phthalazine-1(2h)-one and Fe3+ ions | Mechanical property, tissue adhesion, self-healing capability, biocompatibility, hemostatic and antibacterial activity, wound healing in skin infection rat model | [38] |
| Multi-Arm thiolated polyethylene glycol crosslinked with silver nitrate | Injectable and self-healing properties, antibacterial and angiogenic in vitro, diabetic skin wound repair, enhancement of angiogenic activity | [39] |
| Hydrophobic association structure composed of surfactant SDS, stearyl methacrylate, and NIPAAm crosslinked with dopamine acrylate and ferric chloride | Rheological behavior, swelling rate, compression test, adhesiveness on pig skin, self-healing tests, antibacterial tests, in vitro transdermal absorption | [40] |
| Polyvinyl alcohol, borax, oligomeric procyanidin, and ferric ion | Ultra-stretchability, tissue-adhesive strength, shape adaptability, self-healing feature, on-demand removability, antioxidative, antibacterial, hemostasis, photothermal antibacterial ability, wound healing in mice nape model | [41] |
| Ionic Crosslinking with Non-metal Ions | ||
| Poly(aspartic acid) hydrazide and PEO dialdehyde crosslinked with polyphosphate | Hemostatic performance, biocompatibility, tissue adhesion, in vivo hemostasis model, antibacterial activity, wound repair rate in mouse model | [80] |
| Pectin conjugated with polyphosphate | Self-healing property, sustained release performance, coagulation characteristic, blood loss reduction in hemorrhage model, wound repair rate acceleration | [81] |
| Physical Crosslinking and Self-Assembly | ||
| Methylcellulose-chitosan hydrogel loaded with exosomes | Cell proliferation, skin remodeling, vascular formation induction, self-healing and adhesion properties, severe wound healing in diabetic conditions | [76] |
| Double-network hydrogel adhesive based on poly(glycerol sebacate)-co-poly(ethylene glycol)-g-catechol and ureido-pyrimidinone modified gelatin | Shape adaptability, self-healing, tissue adhesion, antibacterial activity, in vivo experiments for wound closure and healing, inflammation regulation, collagen deposition | [82] |
| Hybrid Crosslinking Systems Involving Ion Interactions | ||
| Dopamine-functionalized oxidized hyaluronic acid/carboxymethyl chitosan/collagen hydrogel with curcumin-loaded gelatin nanoparticles | Injectable and self-healing properties, tissue adhesion, biocompatibility, cell proliferation promotion, MMP-responsive curcumin release, animal wound-healing efficiency | [77] |
| Chitosan and poly[2-(methacryloyloxy)ethyl] trimethyl ammonium chloride | Self-healing ability, biocompatibility, promotion of macrophage M2 phenotype polarization, antibacterial activity, in vivo wound regeneration acceleration | [78] |
| CuS-grafted-curcumin and carboxymethyl cellulose modified with aldehyde groups and hydroxypropyl trimethyl ammonium chloride chitosan | Injectable and self-healing characteristics, near-infrared photosensitivity, photocatalytic activity, uniform distribution of CuS@C, enhanced photodynamic and photothermal antibacterial effects, accelerated infected-wound healing | [79] |
| Collagen and hyaluronic acid mediated with gallic acid and dopamine | Injectability and self-healing properties, tissue adhesion, biocompatibility and cell migration, antioxidant and free radical scavenging assays, in vivo wound healing studies | [83] |
| Calcium ions, sodium alginate, acrylated guanine, acrylamide, and acrylated dopamine | Strong adhesion and peeling properties on various surfaces, high toughness, self-healing property, calcium ions loading for wound monitoring, application in wound bonding on pork stomach | [84] |
| Aluminum ions, alginate-dopamine, acrylamide, and acrylic acid copolymer | Outstanding mechanical properties, self-healing ability, recycling in pollution-free ways, in vitro antibacterial test, cell affinity, in vivo wound healing | [85] |
| Hyaluronic acid, epsilon-polylysine, horseradish peroxidase enzymatic crosslinking | Inherent antibacterial properties, gram (+) and (−) bacteria killing, sol-gel transition, recovery from destruction, infected rat wound model efficacy, histological studies comparing with commercial fibrin glue | [96] |
| Methacrylic anhydride-modified gelatin, cationic guar gum, and borax with copper-tannic acid nanosheets | Hemostasis and adhesion, responsive decomposition for CuTA release, antibacterial efficiency, macrophage polarization and angiogenesis promotion, in vivo re-epithelialization acceleration | [97] |
| Iron ion-doped polyaniline tethered with guar gum | Injectability, rapid self-healing ability, thermal- or pH-stimuli responsive gel-sol transformations, second near-infrared (NIR-II) responsive photothermal conversion, photothermal-enhanced cytotoxic OH generation, in vitro and in vivo synergistic therapy effects, antibacterial activity, fibroblast cell proliferation, angiogenesis promotion for wound repair | [98] |
| Poly(vinyl alcohol), corn starch, zeolite, cellulose nanocrystals | Mechanical properties, boric acid and glycerol effects on hydrogel properties, zeolite and cellulose nanocrystals content effects, self-healing tests in wet conditions | [99] |
| Carboxymethyl chitosan, 2,3,4-trihydroxybenzaldehyde, copper chloride, graphene oxide-N,N’-di-sec-butyl-N,N’-dinitroso-1,4-phenylenediamine | Stability and mechanical properties, conductivity, biocompatibility, photothermal properties and antibacterial activity in vitro, nitric oxide release under NIR, in vivo wound contraction and angiogenesis | [108] |
| Acryloyl-6-aminocaproic acid (AA) and N-acryloyl 2-glycine (NAG) with calcium chloride (CaCl2) | Mechanical properties, swelling and adhesion properties, flexibility, in vitro blood-clotting ability, cytocompatibility, liver injury and nephron-sparing surgery models for hemostasis performance and wound healing, abdomen-caecum adhesion model for antiadhesion properties | [109] |
| Polymer Base and Polymerization Technique | Tests Conducted on Hydrogel | Ref.# |
|---|---|---|
| Poly(beta-carboxyethyl acrylate-co-acrylamide) grafted onto sodium alginate loaded with 9-aminoacridine and kanamycin sulfate | Biocompatibility, antibacterial impact on various bacterial strains, rheological properties | [86] |
| Polysaccharide-based hydrogel with quaternized chitosan (QCS) and oxidized hyaluronic acid (OHA) | Self-healing through reversible Schiff base bonds, NIR irradiation improved antibacterial effect, pH-sensitive drug release, promotion of wound healing in full-thickness skin defect model | [87] |
| Polyurethane/polydimethylsiloxane nanofibrous membrane and double-crosslinked chitosan-based hydrogel with Schiff base bonds and non-dynamic photo-crosslinking bonds | Water retention capability, stretchable and compressive performance, self-healing and cyclic resilience, in vivo full-thickness skin wound healing | [88] |
| Poly(N-isopropylacrylamide)-derived ABA triblock copolymer (TNOTN) and aldehyde beta-cyclodextrin (ACD) | Thermal responsiveness and sol-gel transition, self-healing properties, antibacterial properties, biocompatibility and hemocompatibility, in vivo spatial metabolomics for angiogenesis and collagen deposition | [89] |
| Glycyrrhizic acid (GA)-based hybrid hydrogel with aldehyde-contained GA and carboxymethyl chitosan (CMC) | Injectable, shape adaptation and remodeling, self-healing, antibacterial ability, anti-inflammation effects, in vivo skin wound healing, S. aureus-infected skin wound healing | [90] |
| Quaternized chitosan (QCS), oxidized dextran (OD), tobramycin (TOB), and polydopamine-coated polypyrrole nanowires (PPY@PDA NWs) | Electrical conductivity, antioxidant activity, slow and pH-responsive TOB release, in vitro and in vivo antibacterial activity, NIR irradiation assisted bactericidal activity, PA-infected burn wound inflammation control and healing promotion | [91] |
| Quaternization chitosan derivatives with phenylboronic acid and catechol-like moieties, BNN6-loaded mesoporous polydopamine (MPDA@BNN6 NPs) | Injectability, flexibility, rapid self-healing, bacterial affinity, tissue adhesion, antioxidant stress ability, in vivo S. aureus biofilm eradication, infected wound repair acceleration | [100] |
| Polyvinyl alcohol (PVA) and hyaluronic acid grafted with phenylboronic acid (BA-HA), polydopamine, and gelatin microspheres containing basic fibroblast growth factor (GMs@bFGF) | Shape-adaptation, strong adhesion, instantaneous self-healing, controlled bFGF release, promotion of tendon wound healing through alleviation of inflammation and collagen I secretion | [101] |
| Quaternized N-[3-(dimethylamino)propyl] methacrylamide with dithiodipropionic acid dihydrazide | Efficient self-healing capability, pH and redox-triggered gel-sol-gel transition, good antibacterial activity, biocompatibility, degradability, sustained release ability, in vivo experiments for wound closure and cutaneous regeneration | [102] |
| Quaternary ammonium chitosan (QCS)/tannic acid (TA) hydrogel | Injectability, self-healing, adhesive properties, reactive oxygen species scavenging, broad-spectrum antibacterial ability, rapid hemostatic capability, in vivo hemostasis, wound-healing acceleration | [105] |
| Poly(ethylene glycol)-co-poly(sorbitol sebacate) conjugated with chitosan-g-poly tetraaniline (QCS-g-PTA) | Antibacterial activity, conductivity, free radical hunt capability, swelling ratio, cytocompatibility, in vivo wound curing development in a full-thickness skin defect rat model | [110] |
| Itaconic acid-pluronic-itaconic acid (FIA) | Temperature-responsive sol-gel behavior, injectability, broad-spectrum antibacterial activity, hemocompatibility and cell compatibility, intracellular reactive oxygen species scavenging, decrease of inflammation factors, promotion of endotheliocyte migration and blood tube formation, MRSA-infected wound healing, rapid formation of the epithelial layer and skin appendages | [111] |
| N, O-carboxymethyl chitosan (N, O-CMC) and oxidized chondroitin sulfate (OCS) | Long gelation time and stable performances, cell viability test with NIH/3T3 cells and endothelial cells, antibacterial properties, tissue adhesion, in vivo hemostatic performance | [112] |
| Poly[(N-isopropyl acrylamide)-co-(butyl acrylate)-co-(sulfobetaine methacrylate)]-b-poly(ethylene glycol)-b-poly[(N-isopropyl acrylamide)-co-(butyl acrylate)-co-(sulfobetaine methacrylate)] (PZOPZ) | Self-healing property, cytocompatibility, antibacterial adhesion, CCK-8, and 2D/3D cell culture experiments, in vivo angiogenesis, dermal collagen synthesis | [113] |
| Small intestinal submucosa (SIS) hydrogel loaded with Quercetin (QCT) | Self-healing properties, water absorption, immunomodulatory effects, in vivo wound repair rate, promotion of granulation tissue thickness and vascularization, histological analyses of vital organs, biochemical index levels in serum | [114] |
| ABA-type amphiphilic hydrogel with human umbilical cord blood-derived exosomes (UCB-Exos) | Direct application efficacy, faster wound closure, enhanced collagen deposition, accelerated re-epithelialization, enhanced neo-vascularization | [115] |
| Polyacrylic acid (PAA) based coacervate hydrogel with isoprenyl oxy poly(ethylene glycol) ether and tannic acid (TA) | Strong wet adhesion; self-healing; extensible properties; antibacterial activity; facilitated fibroblast migration; modulated M1/M2 macrophage polarization; in vivo hemorrhage control; promoted collagen deposition, angiogenesis, and epithelialization | [116] |
| N-acryloyl glycinamide (NAGA) and 1-vinyl-1,2,4-triazole (VTZ) mixed with supramolecular hydrogel | Superior mechanical properties, self-healing, antibacterial, thermal responsiveness, enhanced wound healing in diabetic rats, color-sensing for wound monitoring | [117] |
| Injectable, self-healing, and antibacterial polypeptide-based FHE hydrogel (F127/OHA-EPL) with adipose-derived mesenchymal stem cells exosomes (AMSCs-exo) | Materials characterization; antibacterial activity; stimulated cellular behavior; in vivo full-thickness diabetic wound healing; enhanced wound closure rates; fast angiogenesis, re-epithelization, and collagen deposition | [118] |
| Poly(N-[tris(hydroxymethyl)methyl]acrylamide-co-acrylamide) (P(THMA-co-AAM)) hydrogel with quaternary chitosan | Rapid photopolymerization under green LED, mechanical properties, self-healing, adhesion ability, biocompatibility, antibacterial properties of the tunable multifunctional hydrogel (P-QCS) | [119] |
| Polymer Base and Polymerization Technique | Tests Conducted on Hydrogel | Ref.# |
|---|---|---|
| Chitosan/carboxymethyl chitosan/silver nanoparticles (CTS/CMCTS/AgNPs) polyelectrolyte composite hydrogel | Mechanical strength, self-healing ability, broad-spectrum antibacterial activities, promoting effects on P. aeruginosa infected wounds healing | [42] |
| Antibacterial carbon dots and epsilon-polylysine (CD-Plys) | Broad-spectrum antibacterial activity, wound-healing acceleration, epithelization, enhanced angiogenesis, biocompatibility | [43] |
| Carbon quantum dots (CQDs) decorated self-healing hydrogel | Antimicrobial against MRSA, ROS scavenging, protection of HUVECs migration and angiogenesis, downregulation of NO and pro-inflammatory cytokines in macrophages, in vivo MRSA elimination, anti-inflammation, promotion of angiogenesis and wound healing | [44] |
| Polydopamine-functionalized chitosan-arginine hydrogel (CA-pDA) with polydopamine nanoparticles (pDA-NPs) | Self-healing, enhanced mechanical property and pore size, angiogenic and antibacterial activities, acceleration of wound healing with reduced scar formation in rat model | [45] |
| Polysaccharide biopolymer, poly(vinyl alcohol), and hydroxylated graphene-based conductive hydrogel | Rapid self-healing, injectability, conductivity, motion monitoring, in situ bacterial sensing and killing, in vivo wound-healing promotion, real-time monitoring of joint movements | [46] |
| Adipic dihydrazide modified hyaluronic acid, benzaldehyde group functionalized poly(ethylene glycol)-co-poly(glycerol sebacate) and cuttlefish melanin nanoparticles | Tissue adhesion, stretchability, self-healing, photothermal antibacterial therapy, antioxidation, hemostasis, exudate absorption, sustained release property, in vivo wound healing | [47] |
| Chitosan-based POSS-PEG hybrid hydrogel via Schiff base reaction between HACC and POSS-PEG-CHO | Mechanical strength, injectability, self-healing efficiency, cytocompatibility, antibacterial properties, in vivo diabetic wound-healing acceleration | [92] |
| Ceria-based nanocomposite hydrogel (FVEC) via dynamic Schiff base reaction | Thermosensitivity, injectability, self-healing ability, ROS scavenging activity, in vivo biocompatibility and biodegradability, full-thickness skin wound healing enhancement | [93] |
| Quaternized chitosan-g-polyaniline and benzaldehyde group functionalized poly(ethylene glycol)-co-poly(glycerol sebacate) | Self-healing, electroactivity, free radical scavenging, antibacterial activity, adhesiveness, conductivity, swelling ratio, biocompatibility, in vivo blood clotting capacity, wound-healing process enhancement | [94] |
| Peptide-modified nanofibers and hydrogel via Schiff base dynamic crosslinking | Stability, mechanical strength, injectable, self-healing, antibacterial, hemostatic properties, chronic wound-healing acceleration | [95] |
| Chitosan and graphene oxide via one-pot heating | Injectability, self-healing, mechanical and rheological properties control, adhesiveness, hemocompatibility, in vivo hemostatic and wound-healing capability | [103] |
| Exosome/metformin-loaded PEG/Ag/CNT hydrogel with dynamic Schiff base bonds | Tissue adhesiveness, antioxidant, self-healing, electrical conductivity, cell proliferation and angiogenesis promotion, peritraumatic inflammation and vascular injury relief, ROS reduction, mitochondrial fission interference | [104] |
| Carboxymethyl chitosan, tannic acid, and graphitic carbon nitride | Self-healing capability, mechanical property, tissue adhesiveness, cell affinity, visible light-induced antibacterial activity, collagen synthesis and re-epithelization acceleration, bacteria-infected wound healing | [106] |
| PNAGA/AuNRs, PNAGA/PNIPAm, and PNAGA/AgNW hydrogels integrated by self-healing crosslinking | In vitro antibacterial experiments, in situ rat models for wound monitoring and healing, real-time monitoring of wound temperature and strain, on-demand drug delivery | [120] |
| Poly(vinyl alcohol)-borax and resveratrol grafted cellulose nanofibrils | Mechanical properties, self-healing efficiency, adhesion performance, pH-responsive drug release, biocompatibility, antioxidant effect, in vitro and in vivo antibacterial, skin tissue regeneration, wound closure capabilities | [121] |
| N-carboxyethyl chitosan (CEC) and benzaldehyde-terminated Pluronic F127/carbon nanotubes (PF127/CNT) | Gelation time, mechanical properties, hemostatic properties, water absorbency, biodegradability, pH-responsive antibiotic release, antibacterial activity, photothermal antimicrobial activity, conductivity, in vivo healing in infected wounds | [122] |
| Oxidized quaternized guar gum (OQGG) and carboxymethyl chitosan (CMCS) | Structural characterization, antibacterial, hemostatic, self-repairing, injectable properties, cytocompatibility, in vivo wound-healing promotion in an S. aureus-infected rat model | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Wilson, R.L.; Gill, E.J. Advancements and Challenges in Self-Healing Hydrogels for Wound Care. Gels 2024, 10, 241. https://doi.org/10.3390/gels10040241
Omidian H, Wilson RL, Gill EJ. Advancements and Challenges in Self-Healing Hydrogels for Wound Care. Gels. 2024; 10(4):241. https://doi.org/10.3390/gels10040241
Chicago/Turabian StyleOmidian, Hossein, Renae L. Wilson, and Erma J. Gill. 2024. "Advancements and Challenges in Self-Healing Hydrogels for Wound Care" Gels 10, no. 4: 241. https://doi.org/10.3390/gels10040241
APA StyleOmidian, H., Wilson, R. L., & Gill, E. J. (2024). Advancements and Challenges in Self-Healing Hydrogels for Wound Care. Gels, 10(4), 241. https://doi.org/10.3390/gels10040241






