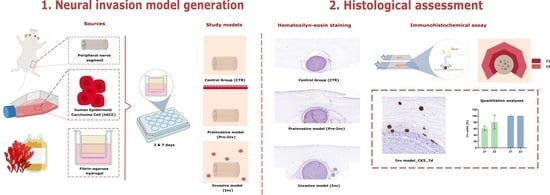
A Novel In Vitro Pathological Model for Studying Neural Invasion in Non-Melanoma Skin Cancer
Abstract
1. Introduction
2. Results and Discussion
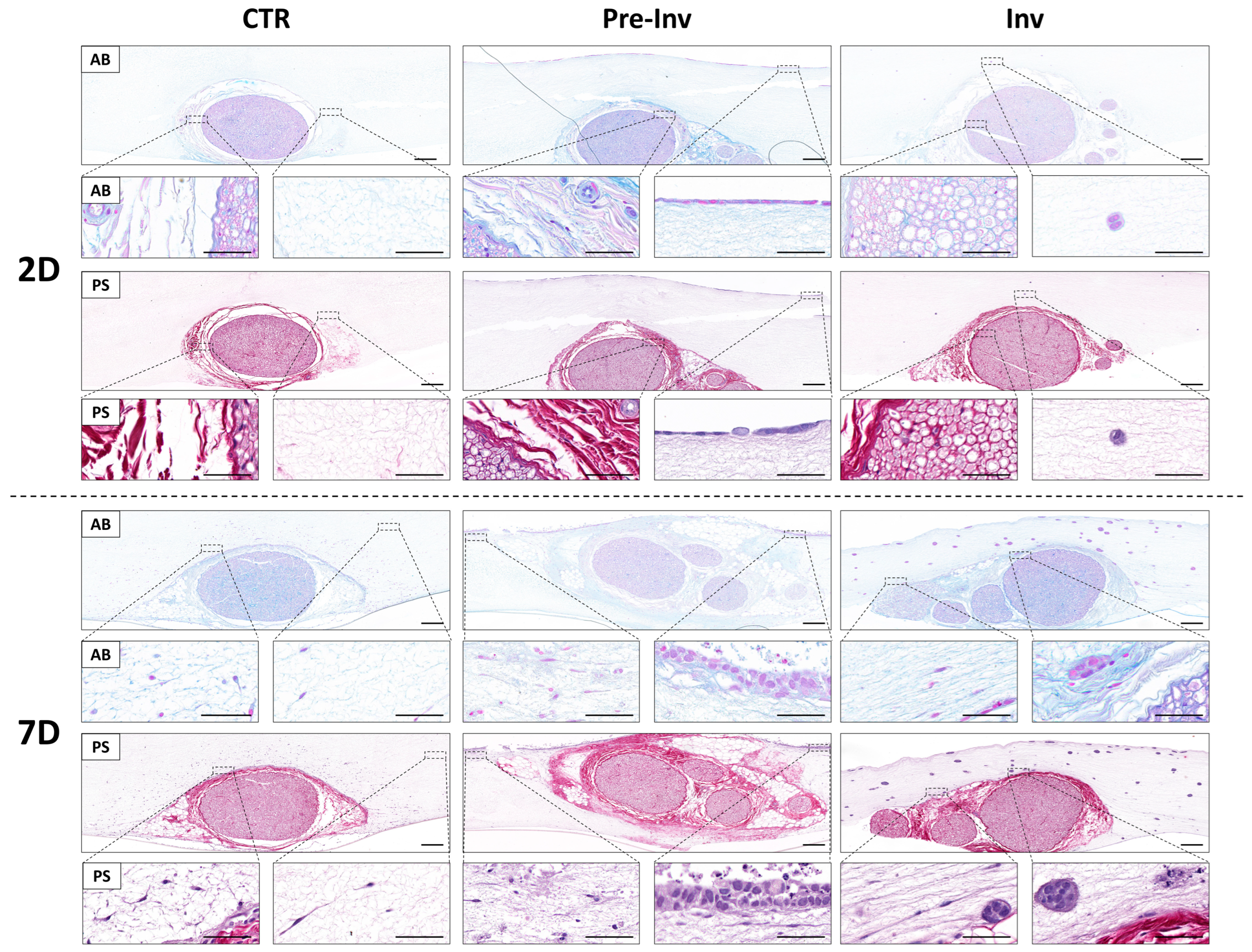
2.1. Histological Analysis of Neural Invasion Models Using Hematoxylin and Eosin Staining
2.2. Histochemical Assessment of Extracellular Matrix Components in Neural Invasion Models
2.3. Analysis of Cell Proliferation in Neural Invasion Models
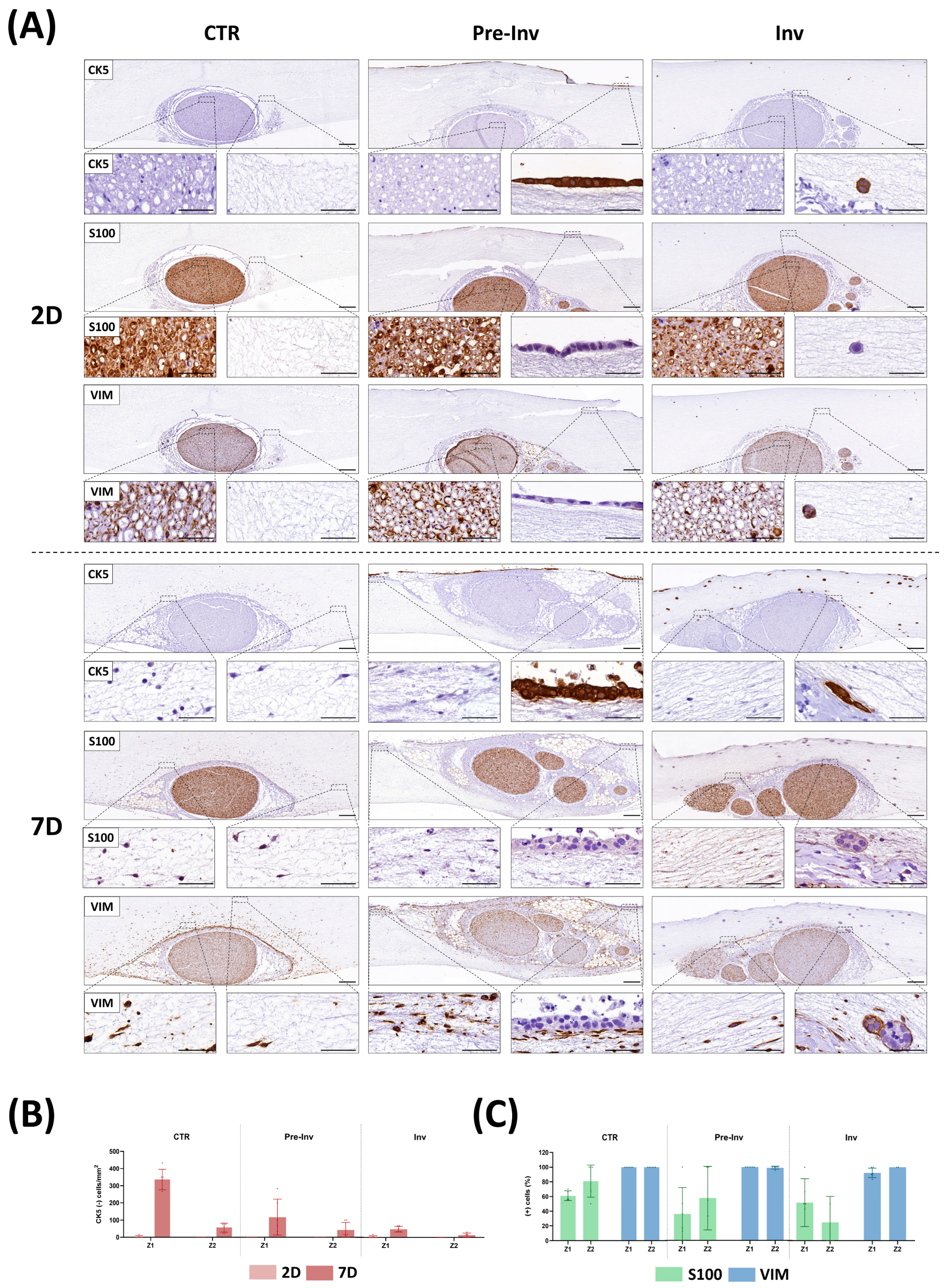
2.4. Phenotypic Characterization of Nerve-Derived Cells in Neural Invasion Models
3. Conclusions
4. Materials and Methods
4.1. Cell Sources and Sample Acquisition
4.2. Generation of Neural Invasion Models
4.3. Histological Characterization of Neural Invasion Models
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural Invasion in Cancer: A Review of the Literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of Cancer Dissemination along Nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Krishnan, A. Neural Invasion: A Scenic Trail for the Nervous Tumor and Hidden Therapeutic Opportunity. Am. J. Cancer Res. 2020, 10, 2258–2270. [Google Scholar] [PubMed]
- Kurtz, K.A.; Hoffman, H.T.; Zimmerman, M.B.; Robinson, R.A. Perineural and Vascular Invasion in Oral Cavity Squamous Carcinoma: Increased Incidence on Re-Review of Slides and by Using Immunohistochemical Enhancement. Arch. Pathol. Lab. Med. 2005, 129, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.; Ahlawat, P.; Tandon, S.; Jain, A.; Gairola, M. Challenges Seen with Peri-Neural Invasion in Head and Neck Cancer—A Review Article. Oral Oncol. Rep. 2023, 6, 100028. [Google Scholar] [CrossRef]
- Bakst, R.L.; Glastonbury, C.M.; Parvathaneni, U.; Katabi, N.; Hu, K.S.; Yom, S.S. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Schmitd, L.B.; Scanlon, C.S.; D’Silva, N.J. Perineural Invasion in Head and Neck Cancer. J. Dent. Res. 2018, 97, 742–750. [Google Scholar] [CrossRef]
- Duraker, N.; Sişman, S.; Can, G. The Significance of Perineural Invasion as a Prognostic Factor in Patients with Gastric Carcinoma. Surg. Today 2003, 33, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Zhang, B.-Y.; Zhou, B.; Zhu, C.-Z.; Sun, L.-Q.; Feng, Y.-J. Perineural Invasion of Cancer: A Complex Crosstalk between Cells and Molecules in the Perineural Niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar]
- Reina, M.A.; López, A.; Villanueva, M.C.; de Andrés, J.A.; León, G.I. Morphology of peripheral nerves, their sheaths, and their vascularization. Rev. Esp. Anestesiol. Reanim. 2000, 47, 464–475. [Google Scholar] [PubMed]
- Maffioletti, S.M.; Sarcar, S.; Henderson, A.B.H.; Mannhardt, I.; Pinton, L.; Moyle, L.A.; Steele-Stallard, H.; Cappellari, O.; Wells, K.E.; Ferrari, G.; et al. Three-Dimensional Human iPSC-Derived Artificial Skeletal Muscles Model Muscular Dystrophies and Enable Multilineage Tissue Engineering. Cell Rep. 2018, 23, 899–908. [Google Scholar] [CrossRef]
- Turnbull, I.C.; Mayourian, J.; Murphy, J.F.; Stillitano, F.; Ceholski, D.K.; Costa, K.D. Cardiac Tissue Engineering Models of Inherited and Acquired Cardiomyopathies. Methods Mol. Biol. 2018, 1816, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-H.; Zhang, S.; Wang, H.; Xue, J.-L.; Zhang, Z.-G. Emerging Experimental Models for Assessing Perineural Invasion in Human Cancers. Cancer Lett. 2022, 535, 215610. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Garzón, I.; Jiménez, J.-M.; Oliveira, A.-C.-X.; Arias-Santiago, S.; Campos, A.; Sánchez-Quevedo, M.-C.; Alaminos, M. Epithelial and Stromal Developmental Patterns in a Novel Substitute of the Human Skin Generated with Fibrin-Agarose Biomaterials. Cells Tissues Organs 2012, 196, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martin-Piedra, M.A.; Carmona, G.; Campos, F.; Carriel, V.; Fernández-González, A.; Campos, A.; Cuende, N.; Garzón, I.; Gacto, P.; Alaminos, M. Histological Assessment of Nanostructured Fibrin-Agarose Skin Substitutes Grafted in Burnt Patients. A Time-Course Study. Bioeng. Transl. Med. 2023, 8, e10572. [Google Scholar] [CrossRef] [PubMed]
- Chato-Astrain, J.; Campos, F.; Roda, O.; Miralles, E.; Durand-Herrera, D.; Saez-Moreno, J.A.; Garcia-Garcia, S.; Alaminos, M.; Campos, A.; Carriel, V. In Vivo Evaluation of Nanostructured Fibrin-Agarose Hydrogels With Mesenchymal Stem Cells for Peripheral Nerve Repair. Front. Cell. Neurosci. 2018, 12, 501. [Google Scholar] [CrossRef]
- Blanco-Elices, C.; Chato-Astrain, J.; Oyonarte, S.; Bermejo-Casares, F.; España-López, A.; Fernández-Valadés, R.; Sánchez-Quevedo, M.D.C.; Alaminos, M.; Martín-Piedra, M.A.; Garzón, I. Generation of a Novel Model of Bioengineered Human Oral Mucosa with Increased Vascularization Potential. J. Periodontal Res. 2021, 56, 1116–1131. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Elices, C.; Morales-Álvarez, C.; Chato-Astrain, J.; González-Gallardo, C.; Ávila-Fernández, P.; Campos, F.; Carmona, R.; Martín-Piedra, M.Á.; Garzón, I.; Alaminos, M. Development of Stromal Differentiation Patterns in Heterotypical Models of Artificial Corneas Generated by Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1124995. [Google Scholar] [CrossRef] [PubMed]
- Hromada, C.; Szwarc-Hofbauer, D.; Quyen Nguyen, M.; Tomasch, J.; Purtscher, M.; Hercher, D.; Teuschl-Woller, A.H. Strain-Induced Bands of Büngner Formation Promotes Axon Growth in 3D Tissue-Engineered Constructs. J. Tissue Eng. 2024, 15, 20417314231220396. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Knight, A.M.; Lu, L.; Windebank, A.J.; Yaszemski, M.J. Stimulation of Neurite Outgrowth Using Positively Charged Hydrogels. Biomaterials 2009, 30, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.L.D.; Laranjeira, S.; Evans, R.E.; Shipley, R.J.; Healy, J.; Phillips, J.B. Developing an In Vitro Model to Screen Drugs for Nerve Regeneration. Anat. Rec. 2018, 301, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Colley, H.E.; Hearnden, V.; Jones, A.V.; Weinreb, P.H.; Violette, S.M.; MacNeil, S.; Thornhill, M.H.; Murdoch, C. Development of Tissue-Engineered Models of Oral Dysplasia and Early Invasive Oral Squamous Cell Carcinoma. Br. J. Cancer 2011, 105, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Prince, E.; Cruickshank, J.; Ba-Alawi, W.; Hodgson, K.; Haight, J.; Tobin, C.; Wakeman, A.; Avoulov, A.; Topolskaia, V.; Elliott, M.J.; et al. Biomimetic Hydrogel Supports Initiation and Growth of Patient-Derived Breast Tumor Organoids. Nat. Commun. 2022, 13, 1466. [Google Scholar] [CrossRef] [PubMed]
- Nigri, J.; Gironella, M.; Bressy, C.; Vila-Navarro, E.; Roques, J.; Lac, S.; Bontemps, C.; Kozaczyk, C.; Cros, J.; Pietrasz, D.; et al. PAP/REG3A Favors Perineural Invasion in Pancreatic Adenocarcinoma and Serves as a Prognostic Marker. Cell. Mol. Life Sci. 2017, 74, 4231–4243. [Google Scholar] [CrossRef]
- Abiatari, I.; DeOliveira, T.; Kerkadze, V.; Schwager, C.; Esposito, I.; Giese, N.A.; Huber, P.; Bergman, F.; Abdollahi, A.; Friess, H.; et al. Consensus Transcriptome Signature of Perineural Invasion in Pancreatic Carcinoma. Mol. Cancer Ther. 2009, 8, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Besikcioglu, H.E.; Yurteri, Ü.; Munkhbaatar, E.; Ye, L.; Zhang, F.; Moretti, A.; Mota Reyes, C.; Özoğul, C.; Friess, H.; Ceyhan, G.O.; et al. Innervated Mouse Pancreas Organoids as an Ex Vivo Model to Study Pancreatic Neuropathy in Pancreatic Cancer. STAR Protoc. 2021, 2, 100935. [Google Scholar] [CrossRef]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann Cells at Neoplastic Cell Sites before the Onset of Cancer Invasion. J. Natl. Cancer Inst. 2014, 106, dju184. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ling, Z.; Ren, X. Extracellular Matrix Dynamics: Tracking in Biological Systems and Their Implications. J. Biol. Eng. 2022, 16, 13. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ha, P.; Wai, K.C.; Naara, S. The Role of ECM Remodeling, EMT, and Adhesion Molecules in Cancerous Neural Invasion: Changing Perspectives. Adv. Biol. 2022, 6, e2200039. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Quevedo, M.C.; Alaminos, M.; Capitan, L.M.; Moreu, G.; Garzon, I.; Crespo, P.V.; Campos, A. Histological and Histochemical Evaluation of Human Oral Mucosa Constructs Developed by Tissue Engineering. Histol. Histopathol. 2007, 22, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Martín-Piedra, M.A.; Alaminos, M.; Fernández-Valadés-Gámez, R.; España-López, A.; Liceras-Liceras, E.; Sánchez-Montesinos, I.; Martínez-Plaza, A.; Sánchez-Quevedo, M.C.; Fernández-Valadés, R.; Garzón, I. Development of a Multilayered Palate Substitute in Rabbits: A Histochemical Ex Vivo and in Vivo Analysis. Histochem. Cell Biol. 2017, 147, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Guan, X.; Ma, M.; Liang, B.; Ren, L.; Liu, Y.; Du, Y.; Jiang, S.-H.; Song, D. Stiffened Tumor Microenvironment Enhances Perineural Invasion in Breast Cancer via Integrin Signaling. Cell. Oncol. 2023. [CrossRef] [PubMed]
- Fromont, G.; Godet, J.; Pires, C.; Yacoub, M.; Dore, B.; Irani, J. Biological Significance of Perineural Invasion (PNI) in Prostate Cancer. Prostate 2012, 72, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Gysler, S.M.; Drapkin, R. Tumor Innervation: Peripheral Nerves Take Control of the Tumor Microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, J.; Chen, B.; Wang, K.; Tang, Y.; Liang, X. Plasticity of Cancer Cell Invasion: Patterns and Mechanisms. Transl. Oncol. 2020, 14, 100899. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.G.; Vignjevic, D.M. Modes of Cancer Cell Invasion and the Role of the Microenvironment. Curr. Opin. Cell Biol. 2015, 36, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Deborde, S.; Wong, R.J. The Role of Schwann Cells in Cancer. Adv. Biol. 2022, 6, e2200089. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhu, Y.; Jiang, Y.; Han, L.; Shi, M.; Su, R.; Wang, L.; Xiong, C.; Wang, C.; Wang, T.; et al. Schwann Cells Regulate Tumor Cells and Cancer-Associated Fibroblasts in the Pancreatic Ductal Adenocarcinoma Microenvironment. Nat. Commun. 2023, 14, 4600. [Google Scholar] [CrossRef] [PubMed]
- García-García, Ó.D.; El Soury, M.; González-Quevedo, D.; Sánchez-Porras, D.; Chato-Astrain, J.; Campos, F.; Carriel, V. Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves. Int. J. Mol. Sci. 2021, 22, 674. [Google Scholar] [CrossRef] [PubMed]
- Alaminos, M.; Del Carmen Sánchez-Quevedo, M.; Muñoz-Avila, J.I.; Serrano, D.; Medialdea, S.; Carreras, I.; Campos, A. Construction of a Complete Rabbit Cornea Substitute Using a Fibrin-Agarose Scaffold. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Arrabal, O.; Irastorza-Lorenzo, A.; Campos, F.; Martín-Piedra, M.Á.; Carriel, V.; Garzón, I.; Ávila-Fernández, P.; de Frutos, M.J.; Esteban, E.; Fernández, J.; et al. Fibrin and Marine-Derived Agaroses for the Generation of Human Bioartificial Tissues: An Ex Vivo and In Vivo Study. Mar. Drugs 2023, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Garzon, I.; Alaminos, M.; Cornelissen, M. Histological Assessment in Peripheral Nerve Tissue Engineering. Neural Regen. Res. 2014, 9, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M. Histological and Histochemical Methods—Theory and Practice. Eur. J. Histochem. 2016, 60, 2639. [Google Scholar] [CrossRef]
- Ibáñez-Cortés, M.; Martín-Piedra, M.Á.; Blanco-Elices, C.; García-García, Ó.D.; España-López, A.; Fernández-Valadés, R.; Sánchez-Quevedo, M.d.C.; Alaminos, M.; Chato-Astrain, J.; Garzón, I. Histological Characterization of the Human Masticatory Oral Mucosa. A Histochemical and Immunohistochemical Study. Microsc. Res. Tech. 2023, 86, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Porras, D.; Varas, J.; Godoy-Guzmán, C.; Bermejo-Casares, F.; San Martín, S.; Carriel, V. Histochemical and Immunohistochemical Methods for the Identification of Proteoglycans. Methods Mol. Biol. 2023, 2566, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Garzon, I.; Alaminos, M.; Campos, A. Evaluation of Myelin Sheath and Collagen Reorganization Pattern in a Model of Peripheral Nerve Regeneration Using an Integrated Histochemical Approach. Histochem. Cell Biol. 2011, 136, 709–717. [Google Scholar] [CrossRef] [PubMed]
- González-Magaña, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Weiss, L.M. Expression of Cytokeratin 5/6 in Epithelial Neoplasms: An Immunohistochemical Study of 509 Cases. Mod. Pathol. 2002, 15, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Alessi, D.; Fink, D.J. S100 Is Preferentially Distributed in Myelin-Forming Schwann Cells. J. Neurocytol. 1990, 19, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Z.; Liu, S.-X.; Li, Y.-W.; He, T.; Zhao, J.; Wang, T.; Qiu, X.-X.; Wu, H.-F. Vimentin as a Potential Target for Diverse Nervous System Diseases. Neural Regen. Res. 2022, 18, 969–975. [Google Scholar] [CrossRef]
- Blanco-Elices, C.; Chato-Astrain, J.; González-González, A.; Sánchez-Porras, D.; Carriel, V.; Fernández-Valadés, R.; Sánchez-Quevedo, M.D.C.; Alaminos, M.; Garzón, I. Histological Profiling of the Human Umbilical Cord: A Potential Alternative Cell Source in Tissue Engineering. J. Pers. Med. 2022, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Chato-Astrain, J.; Roda, O.; Sánchez-Porras, D.; Miralles, E.; Alaminos, M.; Campos, F.; García-García, Ó.D.; Carriel, V. Peripheral Nerve Regeneration through Nerve Conduits Evokes Differential Expression of Growth-Associated Protein-43 in the Spinal Cord. Neural Regen. Res. 2023, 18, 1852–1856. [Google Scholar] [CrossRef] [PubMed]
- Dexter, F. Wilcoxon-Mann-Whitney Test Used for Data That Are Not Normally Distributed. Anesth. Analg. 2013, 117, 537–538. [Google Scholar] [CrossRef] [PubMed]


| Antibody/Reagent | Dilution | Pretreatment | Reference |
|---|---|---|---|
| Mouse anti-human PCNA (Clone PC10) | Ready to use | EDTA, pH = 8, 95 °C for 25 min | Master Diagnostica, Granada, Spain (ref. MAD-000903QD) |
| Rabbit anti-cytokeratin 5 | Ready to use | Citrate buffer pH = 6 95 °C for 25 min | Master Diagnostica, Granada, Spain (MAD-000491QD) |
| Rabbit polyclonal anti-S100 antibody (Z0311) | 1: 400 | Citrate buffer pH = 6 30 min at 95 °C | DakoCytomation, Glostrup, Denmark (ref. Z0311) |
| Mouse anti-vimentin monoclonal clone V9 | 1:200 | Citrate buffer pH = 6 25 min at 95 °C | Merck. St. Louis, MO, USA (ref. V6389) |
| ImmPRESS® HRP Anti-Mouse IgG (Peroxidase) | Ready to use | - | Vector Laboratories. Burlingame, USA (ref. MP-7402) |
| ImmPRESS® HRP Anti-Rabbit IgG (Peroxidase) | Ready to use | - | Vector Laboratories. Burlingame, USA (ref. MP-7401) |
| Chromogen: Diaminobenzidine ready to use kit | - | - | Vector Laboratories. Burlingame, USA (ref. SK-4100) |
| Counterstaining: Harris Hematoxylin | 30 s | - | Thermo Scientific. Runcorn, UK (ref. 6765004) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Fernández, P.; Etayo-Escanilla, M.; Sánchez-Porras, D.; Blanco-Elices, C.; Campos, F.; Carriel, V.; García-García, Ó.D.; Chato-Astrain, J. A Novel In Vitro Pathological Model for Studying Neural Invasion in Non-Melanoma Skin Cancer. Gels 2024, 10, 252. https://doi.org/10.3390/gels10040252
Ávila-Fernández P, Etayo-Escanilla M, Sánchez-Porras D, Blanco-Elices C, Campos F, Carriel V, García-García ÓD, Chato-Astrain J. A Novel In Vitro Pathological Model for Studying Neural Invasion in Non-Melanoma Skin Cancer. Gels. 2024; 10(4):252. https://doi.org/10.3390/gels10040252
Chicago/Turabian StyleÁvila-Fernández, Paula, Miguel Etayo-Escanilla, David Sánchez-Porras, Cristina Blanco-Elices, Fernando Campos, Víctor Carriel, Óscar Darío García-García, and Jesús Chato-Astrain. 2024. "A Novel In Vitro Pathological Model for Studying Neural Invasion in Non-Melanoma Skin Cancer" Gels 10, no. 4: 252. https://doi.org/10.3390/gels10040252
APA StyleÁvila-Fernández, P., Etayo-Escanilla, M., Sánchez-Porras, D., Blanco-Elices, C., Campos, F., Carriel, V., García-García, Ó. D., & Chato-Astrain, J. (2024). A Novel In Vitro Pathological Model for Studying Neural Invasion in Non-Melanoma Skin Cancer. Gels, 10(4), 252. https://doi.org/10.3390/gels10040252