Improving the Decay Resistance of Wood through the Fixation of Different Nanoparticles Using Silica Aerogel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Weight Percent Gain
2.2. Scanning Electron Microscopy (SEM) Imaging and Energy-Dispersive X-ray Spectroscopy (EDX) Analysis
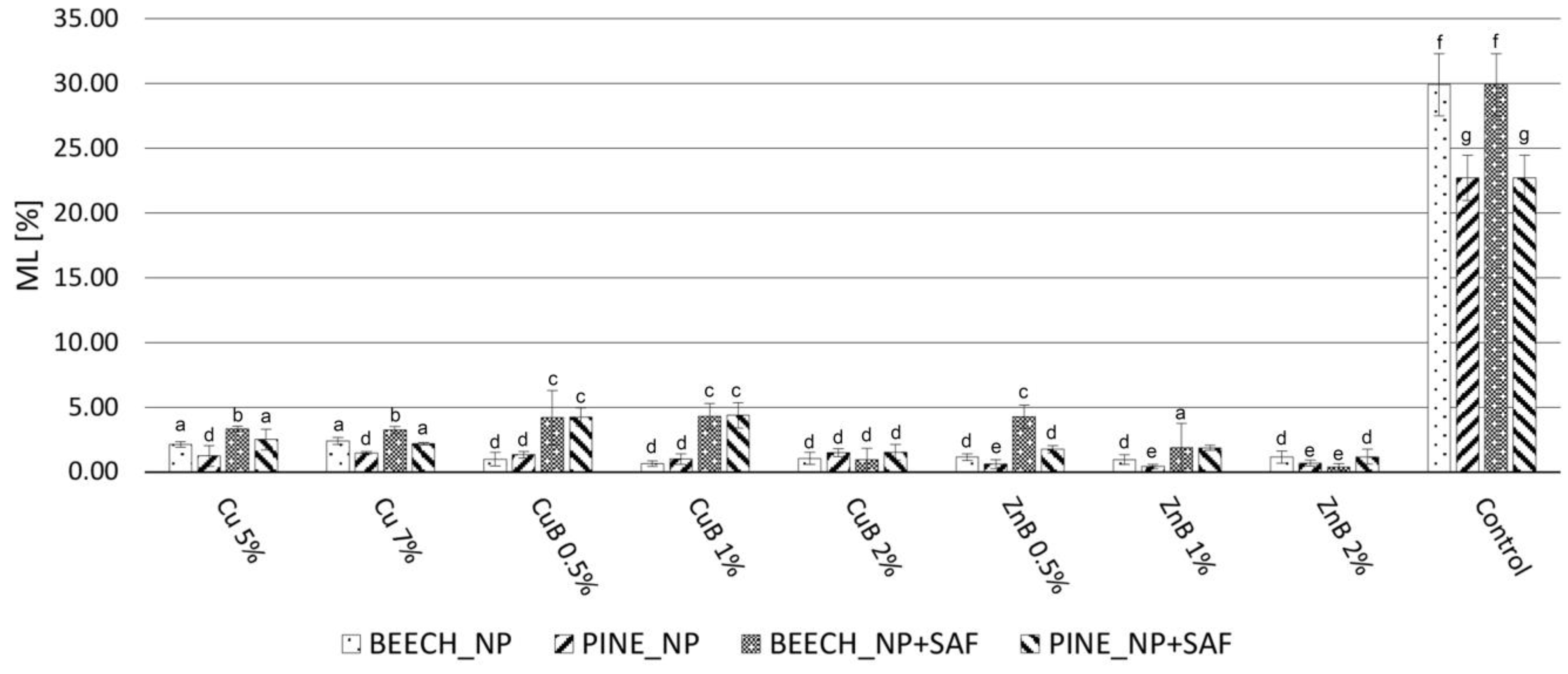
2.3. Decay Test
3. Conclusions
4. Materials and Methods
4.1. Nanoparticle Treatment of the Samples
4.2. Fixation of the Nanoparticles in Wood
4.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX) Analysis
4.4. Decay Test
- Treated test specimens: impregnated specimens subjected to attack by the wood-destroying fungi. Five treated test specimens (NP-treated, aerogel-treated, and NP + aerogel-treated) were used for each NP concentration, timber species and fungus species.
- Treated and leached test specimens: impregnated specimens subjected to attack by the wood-destroying fungi, after a leaching procedure that was performed according to the relevant European standard [64]. Five treated test specimens (NP-treated, aerogel-treated, and NP + aerogel-treated) were used for each NP concentration, wood species and fungus species.
- Untreated test specimens: non-impregnated test specimens of the same wood species as those of the treated test specimens. They were placed in culture vessels next to the treated specimens and treated and leached specimens as a control.
4.5. Statistical Analysis of the Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandberg, D.; Kutnar, A.; Karlsson, O.; Jones, D. Wood Modification Technologies: Principles, Sustainability, and the Need for Innovation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-351-02822-6. [Google Scholar]
- Bansal, R.; Barshilia, H.C.; Pandey, K.K. Nanotechnology in Wood Science: Innovations and Applications. Int. J. Biol. Macromol. 2024, 262, 130025. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.C.; Tonoli, G.H.D.; Cruz, T.M.; Duarte, P.J.; Junqueira, T.A. Nanoparticles-Based Wood Preservatives: The Next Generation of Wood Protection? CERNE 2018, 24, 397–407. [Google Scholar] [CrossRef]
- Shabir Mahr, M.; Hübert, T.; Schartel, B.; Bahr, H.; Sabel, M.; Militz, H. Fire Retardancy Effects in Single and Double Layered Sol–Gel Derived TiO2 and SiO2-Wood Composites. J. Sol-Gel Sci. Technol. 2012, 64, 452–464. [Google Scholar] [CrossRef]
- Kartal, S.N.; Green, F.; Clausen, C.A. Do the Unique Properties of Nanometals Affect Leachability or Efficacy against Fungi and Termites? Int. Biodeterior. Biodegrad. 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Shirakawa, M.A.; Gaylarde, C.C.; Sahão, H.D.; Lima, J.R.B. Inhibition of Cladosporium Growth on Gypsum Panels Treated with Nanosilver Particles. Int. Biodeterior. Biodegrad. 2013, 85, 57–61. [Google Scholar] [CrossRef]
- Mantanis, G.; Terzi, E.; Kartal, S.N.; Papadopoulos, A.N. Evaluation of Mold, Decay and Termite Resistance of Pine Wood Treated with Zinc- and Copper-Based Nanocompounds. Int. Biodeterior. Biodegrad. 2014, 90, 140–144. [Google Scholar] [CrossRef]
- Terzi, E.; Kartal, S.N.; Yılgör, N.; Rautkari, L.; Yoshimura, T. Role of Various Nano-Particles in Prevention of Fungal Decay, Mold Growth and Termite Attack in Wood, and Their Effect on Weathering Properties and Water Repellency. Int. Biodeterior. Biodegrad. 2016, 107, 77–87. [Google Scholar] [CrossRef]
- Lykidis, C.; Bak, M.; Mantanis, G.; Németh, R. Biological Resistance of Pine Wood Treated with Nano-Sized Zinc Oxide and Zinc Borate against Brown-Rot Fungi. Eur. J. Wood Prod. 2016, 74, 909–911. [Google Scholar] [CrossRef]
- Weitz, I.S.; Knani, K.; Maoz, M.; Freitag, C.; Morrell, C.C. The Potential for using CuO Nanoparticles as a Wood Preservative. In Proceedings of the 42nd Annual Meeting of the International Research Group on Wood Protection, Queenstown, New Zealand, 8–12 May 2011. IRG/WP 11-30569. [Google Scholar]
- McIntyre, C.R.; Freeman, M.H. Standardized Field Trials with Micronized Copper. In Proceedings of the 42nd Annual Meeting of the International Research Group on Wood Protection, Queenstown, New Zealand, 8–12 May 2011. IRG/WP 11-30564. [Google Scholar]
- Huang, H.-L.; Lin, C.-C.; Hsu, K. Comparison of Resistance Improvement to Fungal Growth on Green and Conventional Building Materials by Nano-Metal Impregnation. Build. Environ. 2015, 93, 119–127. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Oviedo, C.; Reyes, L.; Navarrete, J.; Gómez, L.; Torres, H.; Gaviño, G.; Trollund, E. Radiata Pine Wood Treated with Copper Nanoparticles: Leaching Analysis and Fungal Degradation. Forests 2021, 12, 1606. [Google Scholar] [CrossRef]
- Clausen, C.A.; Green, F.; Nami Kartal, S. Weatherability and Leach Resistance of Wood Impregnated with Nano-Zinc Oxide. Nanoscale Res. Lett. 2010, 5, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Calgaro, L.; Semenzin, E.; Cazzagon, V.; Giubilato, E.; Marcomini, A.; Badetti, E. Leaching of Nanoparticles from Nano-Enabled Products for the Protection of Cultural Heritage Surfaces: A Review. Environ. Sci. Eur. 2021, 33, 48. [Google Scholar] [CrossRef]
- Can, A.; Palanti, S.; Sivrikaya, H.; Hazer, B.; Stefanı, F. Physical, Biological and Chemical Characterisation of Wood Treated with Silver Nanoparticles. Cellulose 2019, 26, 5075–5084. [Google Scholar] [CrossRef]
- Bak, M.; Németh, R. Effect of Different Nanoparticle Treatments on the Decay Resistance of Wood. BioResources 2018, 13, 7886–7899. [Google Scholar] [CrossRef]
- Lykidis, C.; Mantanis, G.; Adamopoulos, S.; Kalafata, K.; Arabatzis, I. Effects of Nano-Sized Zinc Oxide and Zinc Borate Impregnation on Brown Rot Resistance of Black Pine (Pinus Nigra L.) Wood. Wood Mater. Sci. Eng. 2013, 8, 242–244. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Modification of Wood with Silicon Compounds. Inorganic Silicon Compounds and Sol-gel Systems: A Review. Wood Sci. Technol. 2004, 37, 339–348. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Modification of Wood with Silicon Compounds. Treatment Systems Based on Organic Silicon Compounds—A Review. Wood Sci. Technol. 2004, 37, 453–461. [Google Scholar] [CrossRef]
- Bai, Y.; Dong, Q.; Shao, Y.; Deng, Y.; Wang, Q.; Shen, L.; Wang, D.; Wei, W.; Huang, J. Enhancing Stability and Efficiency of Perovskite Solar Cells with Crosslinkable Silane-Functionalized and Doped Fullerene. Nat. Commun. 2016, 7, 12806. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Goodier, C.I.; Austin, S.A.; Webb, J.; Glass, G.K. Long-Term Performance of Surface Impregnation of Reinforced Concrete Structures with Silane. Constr. Build. Mater. 2013, 48, 708–716. [Google Scholar] [CrossRef]
- Dey, T.; Naughton, D. Cleaning and Anti-Reflective (AR) Hydrophobic Coating of Glass Surface: A Review from Materials Science Perspective. J. Sol-Gel Sci. Technol. 2016, 77, 1–27. [Google Scholar] [CrossRef]
- Przybylak, M.; Maciejewski, H.; Dutkiewicz, A.; Dąbek, I.; Nowicki, M. Fabrication of Superhydrophobic Cotton Fabrics by a Simple Chemical Modification. Cellulose 2016, 23, 2185–2197. [Google Scholar] [CrossRef]
- Panov, D.; Terziev, N. Study on Some Alkoxysilanes Used for Hydrophobation and Protection of Wood against Decay. Int. Biodeterior. Biodegrad. 2009, 63, 456–461. [Google Scholar] [CrossRef]
- Broda, M.; Mazela, B. Application of Methyltrimethoxysilane to Increase Dimensional Stability of Waterlogged Wood. J. Cult. Herit. 2017, 25, 149–156. [Google Scholar] [CrossRef]
- Giudice, C.A.; Alfieri, P.V.; Canosa, G. Decay Resistance and Dimensional Stability of Araucaria Angustifolia Using Siloxanes Synthesized by Sol–Gel Process. Int. Biodeterior. Biodegrad. 2013, 83, 166–170. [Google Scholar] [CrossRef]
- De Vetter, L.; Stevens, M.; Van Acker, J. Fungal Decay Resistance and Durability of Organosilicon-Treated Wood. Int. Biodeterior. Biodegrad. 2009, 63, 130–134. [Google Scholar] [CrossRef]
- Ogiso, K.; Saka, S. Wood–Inorganic Composites Prepared by Sol-Gel Process. II. Effects of Ultrasonic Treatments on Preparation of Wood–Inorganic Composites. Mokuzai Gakkaishi 1993, 38, 301–307. [Google Scholar]
- Saka, S.; Sasaki, M.; Tanahashi, M. Wood–Inorganic Composites Prepared by Sol-Gel Processing. I. Wood–Inorganic Composites with Porous Structure. Mokuzai Gakkaishi 1992, 38, 1043–1049. [Google Scholar]
- Saka, S.; Miyafuji, H.; Tanno, F. Wood-Inorganic Composites Prepared by the Sol-Gel Process. J. Sol-Gel Sci. Technol. 2001, 20, 213–217. [Google Scholar] [CrossRef]
- Reim, M.; Körner, W.; Manara, J.; Korder, S.; Arduini-Schuster, M.; Ebert, H.-P.; Fricke, J. Silica Aerogel Granulate Material for Thermal Insulation and Daylighting. Sol. Energy 2005, 79, 131–139. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 23. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Demilecamps, A.; Beauger, C.; Hildenbrand, C.; Rigacci, A.; Budtova, T. Cellulose–Silica Aerogels. Carbohydr. Polym. 2015, 122, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Sèbe, G.; Wu, R.; Rivera Virtudazo, R.V.; Tingaut, P.; Koebel, M.M. Multiscale Assembly of Superinsulating Silica Aerogels Within Silylated Nanocellulosic Scaffolds: Improved Mechanical Properties Promoted by Nanoscale Chemical Compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- Sedighi Gilani, M.; Boone, M.N.; Fife, J.L.; Zhao, S.; Koebel, M.M.; Zimmermann, T.; Tingaut, P. Structure of Cellulose-Silica Hybrid Aerogel at Sub-Micron Scale, Studied by Synchrotron X-Ray Tomographic Microscopy. Compos. Sci. Technol. 2016, 124, 71–80. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, Y.; Tian, C.; Qing, Y.; Yao, C. Synergistic Effect of Nanosilica Aerogel with Phosphorus Flame Retardants on Improving Flame Retardancy and Leaching Resistance of Wood. J. Nanomater. 2014, 2014, 867106. [Google Scholar] [CrossRef]
- Miyafuji, H.; Saka, S. Na2O-SiO2 Wood-Inorganic Composites Prepared by the Sol-Gel Process and Their Fire-Resistant Properties. J. Wood Sci. 2001, 47, 483–489. [Google Scholar] [CrossRef]
- Donath, S.; Militz, H.; Mai, C. Treatment of Wood with Aminofunctional Silanes for Protection against Wood Destroying Fungi. Holzforschung 2006, 60, 210–216. [Google Scholar] [CrossRef]
- Palanti, S.; Feci, E.; Predieri, G.; Vignali, F. A Wood Treatment Based on Siloxanes and Boric Acid against Fungal Decay and Coleopter Hylotrupes bajulus. Int. Biodeterior. Biodegrad. 2012, 75, 49–54. [Google Scholar] [CrossRef]
- Unger, B.; Bücker, M.; Reinsch, S.; Hübert, T. Chemical Aspects of Wood Modification by Sol–Gel-Derived Silica. Wood Sci. Technol. 2013, 47, 83–104. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, M.; Zhan, H. Preparation of SiO2–Wood Composites by an Ultrasonic-Assisted Sol–Gel Technique. Cellulose 2014, 21, 4393–4403. [Google Scholar] [CrossRef]
- Rosenthal, M.; Bues, C.-T. Longitudinal Penetration of Silicon Dioxide Nanosols in Wood of Pinus sylvestris. Eur. J. Wood Prod. 2010, 68, 363–366. [Google Scholar] [CrossRef]
- Wagenführ, R.; Wagenführ, A. Holzatlas, 7th ed.; Carl Hanser: Munich, Germany, 2021; ISBN 978-3-446-46838-2. [Google Scholar]
- Damay, J.; Bender, T.; Munk, C.; Jousserand, M.; Creton, M.; Fredon, E.; Rémond, R.; Meausoone, P.J.; Pfriem, A.; Gérardin, P. Properties Improvement of Seven Hardwood Species by Combination of Thermal and Chemical Modifications. Eur. J. Wood Prod. 2024, 82, 93–106. [Google Scholar] [CrossRef]
- Donath, S.; Militz, H.; Mai, C. Wood Modification with Alkoxysilanes. Wood Sci. Technol. 2004, 38, 555–566. [Google Scholar] [CrossRef]
- Bak, M.; Molnár, F.; Rákosa, R.; Németh, Z.; Németh, R. Dimensional Stabilization of Wood by Microporous Silica Aerogel Using In-Situ Polymerization. Wood Sci. Technol. 2022, 56, 1353–1375. [Google Scholar] [CrossRef]
- CEN (European Committee for Standardization). Wood Preservatives-Test Method for Determining the Protective Effectiveness against Wood Destroying Basidiomycetes-Determination of the Toxic Values, MSZ EN 113; European Committee for Standardization: Brussels, Belgium, 2001. [Google Scholar]
- Moya, R.; Rodriguez-Zuñiga, A.; Berrocal, A.; Vega-Baudrit, J. Effect of Silver Nanoparticles Synthesized with NPsAg-Ethylene Glycol (C2H6O2) on Brown Decay and White Decay Fungi of Nine Tropical Woods. J. Nanosci. Nanotechnol. 2017, 17, 5233–5240. [Google Scholar] [CrossRef]
- Pařil, P.; Baar, J.; Čermák, P.; Rademacher, P.; Prucek, R.; Sivera, M.; Panáček, A. Antifungal Effects of Copper and Silver Nanoparticles against White and Brown-Rot Fungi. J. Mater. Sci. 2017, 52, 2720–2729. [Google Scholar] [CrossRef]
- Akhtari, M.; Taghiyari, H.R.; Kokandeh, M.G. Effect of Some Metal Nanoparticles on the Spectroscopy Analysis of Paulownia Wood Exposed to White-Rot Fungus. Eur. J. Wood Prod. 2013, 71, 283–285. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute Toxicological Effects of Copper Nanoparticles in Vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.H.; McIntyre, C.R. Micronized Copper Wood Preservatives: Strong Indications of the Reservoir Effect. In Proceedings of the 44th Annual Meeting of the International Research Group on Wood Protection, Stockholm, Sweden, 16–20 June 2013. IRG/WP 13-30609. [Google Scholar]
- Xue, W.; Kennepohl, P.; Ruddick, J.N.R. Chemistry of Copper Preservative Treated Wood. In Proceedings of the 45th Annual Meeting of the International Research Group on Wood Protection, St. George, UT, USA, 11–15 May 2014. IRG/WP 14-30651. [Google Scholar]
- Green, F.; Clausen, C.A. Copper Tolerance of Brown-Rot Fungi: Oxalic Acid Production in Southern Pine Treated with Arsenic-Free Preservatives. Int. Biodeterior. Biodegrad. 2005, 56, 75–79. [Google Scholar] [CrossRef]
- Guillén, Y.; Machuca, Á. The Effect of Copper on the Growth of Wood-Rotting Fungi and a Blue-Stain Fungus. World J. Microbiol. Biotechnol. 2008, 24, 31–37. [Google Scholar] [CrossRef]
- Humar, M.; Petrič, M.; Pohleven, F. Changes of the pH Value of Impregnated Wood during Exposure to Wood-Rotting Fungi. Holz Roh Werkst. 2001, 59, 288–293. [Google Scholar] [CrossRef]
- Humar, M.; Šentjurc, M.; Amartey, S.A.; Pohleven, F. Influence of Acidification of CCB (Cu/Cr/B) Impregnated Wood on Fungal Copper Tolerance. Chemosphere 2005, 58, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Hastrup, A.C.S.; Green, F.; Clausen, C.A.; Jensen, B. Tolerance of Serpula Lacrymans to Copper-Based Wood Preservatives. Int. Biodeterior. Biodegrad. 2005, 56, 173–177. [Google Scholar] [CrossRef]
- Gadd, G.M. Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 41, pp. 47–92. ISBN 978-0-12-027741-4. [Google Scholar]
- Guggenbichler, J.-P.; Böswald, M.; Lugauer, S.; Krall, T. A New Technology of Microdispersed Silver in Polyurethane Induces Antimicrobial Activity in Central Venous Catheters. Infection 1999, 27, S16–S23. [Google Scholar] [CrossRef]
- Obanda, D.N.; Shupe, T.F.; Barnes, H.M. Reducing Leaching of Boron-Based Wood Preservatives—A Review of Research. Bioresour. Technol. 2008, 99, 7312–7322. [Google Scholar] [CrossRef]
- CEN (European Committee for Standardization). Wood Preservatives-Accelerated Ageing of Treated Wood Prior to Biological Testing -Leaching Procedure, MSZ EN 84; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]





| Treatment Type | WPGNP (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Beech | Pine | |||||||
| Normal | For Leaching | Normal | For Leaching | |||||
| Average | SD | Average | SD | Average | SD | Average | SD | |
| Ag 5% (m/m) | 4.04 a | 0.33 | 3.53 a | 0.18 | 6.53 f | 0.29 | 6.54 f | 0.27 |
| Ag 7% (m/m) | 5.05 b | 0.40 | 5.07 b | 0.15 | 9.47 g | 0.68 | 9.16 g | 0.68 |
| Cu 5% (m/m) | 3.76 a | 0.36 | 3.41 a | 0.09 | 6.74 f | 0.39 | 6.41 f | 0.20 |
| Cu 7% (m/m) | 5.53 b | 0.55 | 5.00 b | 0.19 | 9.35 g | 0.80 | 9.07 g | 0.48 |
| CuB 0.5% (m/m) | 0.42 c | 0.03 | 0.39 c | 0.02 | 0.68 h | 0.04 | 0.65 h | 0.03 |
| CuB 1% (m/m) | 0.79 d | 0.08 | 0.73 d | 0.02 | 1.28 i | 0.06 | 1.29 i | 0.06 |
| CuB 2% (m/m) | 1.63 e | 0.15 | 1.48 e | 0.04 | 2.69 j | 0.21 | 2.51 j | 0.16 |
| ZnB 0.5% (m/m) | 0.38 c | 0.03 | 0.41 c | 0.01 | 0.68 h | 0.03 | 0.64 h | 0.03 |
| ZnB 1% (m/m) | 0.78 d | 0.05 | 0.73 d | 0.02 | 1.39 i | 0.12 | 1.32 i | 0.05 |
| ZnB 2% (m/m) | 1.66 e | 0.08 | 1.50 e | 0.03 | 2.68 j | 0.21 | 2.52 j | 0.09 |
| Treatment Type | WPGA (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Beech | Pine | |||||||
| Normal | For Leaching | Normal | For Leaching | |||||
| Average | SD | Average | SD | Average | SD | Average | SD | |
| Ag-A 5% (m/m) | 14.80 a | 1.64 | 12.31 a | 1.55 | 18.95 b | 1.09 | 16.74 b | 0.50 |
| Ag-A 7% (m/m) | 13.71 a | 1.97 | 11.93 a | 1.69 | 18.92 b | 1.81 | 18.21 b | 2.02 |
| Cu-A 5% (m/m) | 14.22 a | 0.79 | 12.28 a | 1.07 | 17.87 b | 2.20 | 17.86 b | 1.77 |
| Cu-A 7% (m/m) | 12.34 a | 2.02 | 12.42 a | 1.36 | 19.26 b | 1.13 | 18.78 b | 1.28 |
| CuB-A 0.5% (m/m) | 14.14 a | 1.58 | 13.90 a | 1.62 | 17.65 b | 1.20 | 18.15 b | 2.33 |
| CuB-A 1% (m/m) | 14.01 a | 1.13 | 12.71 a | 1.32 | 19.17 b | 2.49 | 18.58 b | 2.37 |
| CuB-A 2% (m/m) | 12.55 a | 1.59 | 14.77 a | 2.21 | 17.58 b | 2.04 | 18.60 b | 1.83 |
| ZnB-A 0.5% (m/m) | 13.42 a | 1.19 | 13.48 a | 1.12 | 18.32 b | 2.59 | 15.97 b | 2.82 |
| ZnB-A 1% (m/m) | 14.52 a | 1.53 | 13.64 a | 0.74 | 18.23 b | 1.52 | 17.82 b | 1.44 |
| ZnB-A 2% (m/m) | 14.78 a | 1.91 | 12.30 a | 1.56 | 18.96 b | 1.80 | 17.74 b | 1.70 |
| Nanoparticle | Precursor (Supplier) | Reducing Agent (Supplier) | Protecting Agent (Supplier) | Average NP Size | NP Conc. (m/m%) |
|---|---|---|---|---|---|
| Silver | Silver nitrate a | L-ascorbic acid b | Polyvinyl- pyrrolidone c | 80–100 nm | 5% |
| 7% | |||||
| Copper | Copper (II) nitrate—anhydrous d | L-ascorbic acid b | Polyvinyl- pyrrolidone c | 2–4 nm | 5% |
| 7% | |||||
| Copper–borate | Copper (II) nitrate—anhydrous d | Borax decahydrate e | - | 100 nm | 0.5% |
| 1% | |||||
| 2% | |||||
| Zinc–borate | Zinc nitrate hexahydrate e | Borax decahydrate e | - | 100–200 nm | 0.5% |
| 1% | |||||
| 2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bak, M.; Plesér, Z.; Németh, R. Improving the Decay Resistance of Wood through the Fixation of Different Nanoparticles Using Silica Aerogel. Gels 2024, 10, 255. https://doi.org/10.3390/gels10040255
Bak M, Plesér Z, Németh R. Improving the Decay Resistance of Wood through the Fixation of Different Nanoparticles Using Silica Aerogel. Gels. 2024; 10(4):255. https://doi.org/10.3390/gels10040255
Chicago/Turabian StyleBak, Miklós, Zsófia Plesér, and Róbert Németh. 2024. "Improving the Decay Resistance of Wood through the Fixation of Different Nanoparticles Using Silica Aerogel" Gels 10, no. 4: 255. https://doi.org/10.3390/gels10040255







