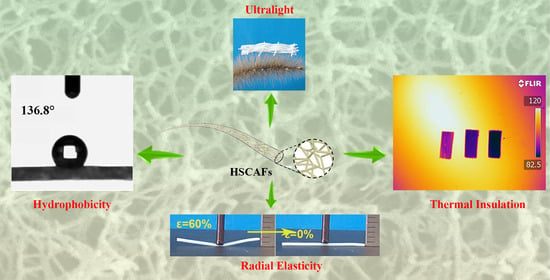
Hydrophobic Silk Fibroin–Agarose Composite Aerogel Fibers with Elasticity for Thermal Insulation Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of HSCAFs
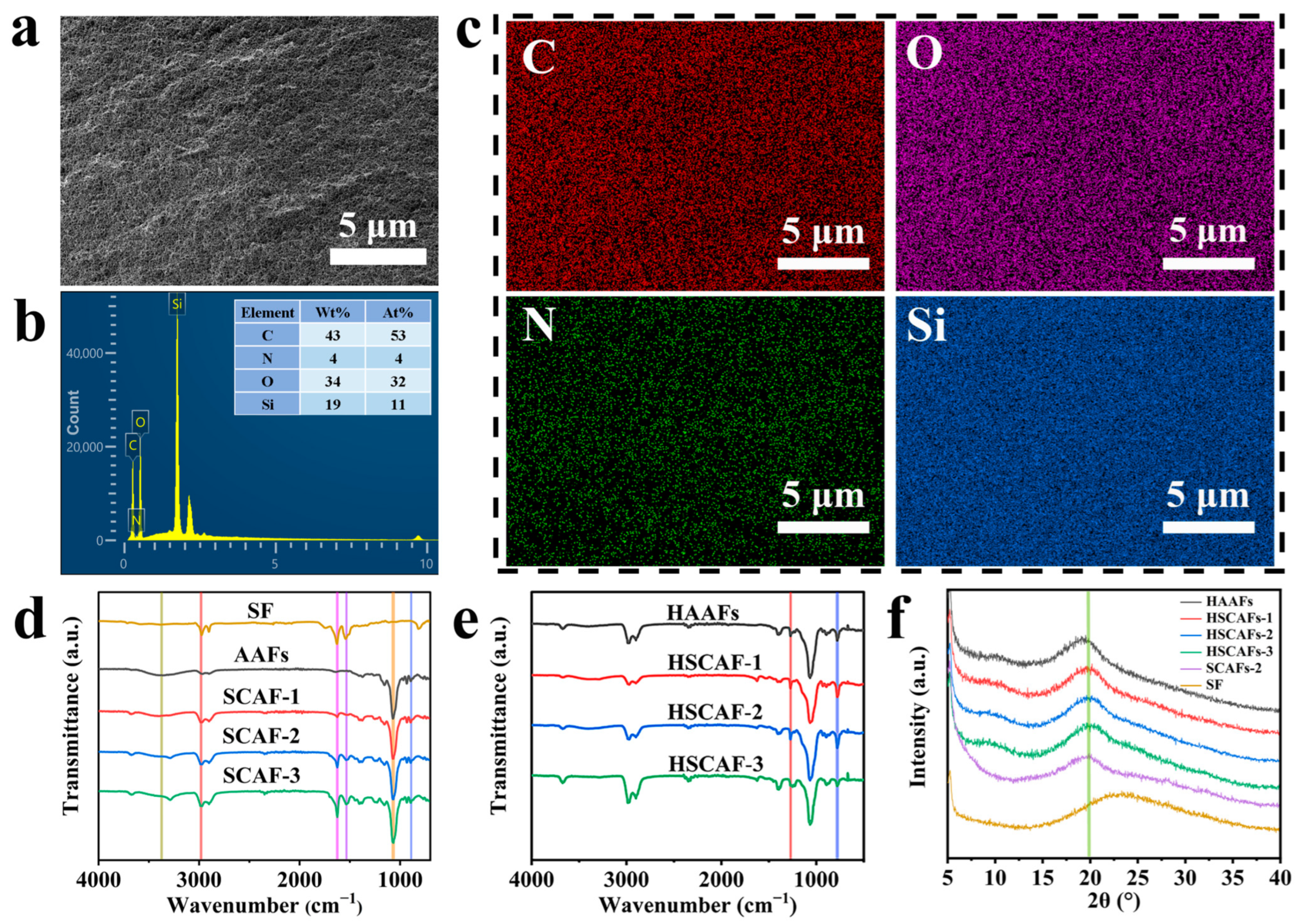
2.2. Microstructure Characterization
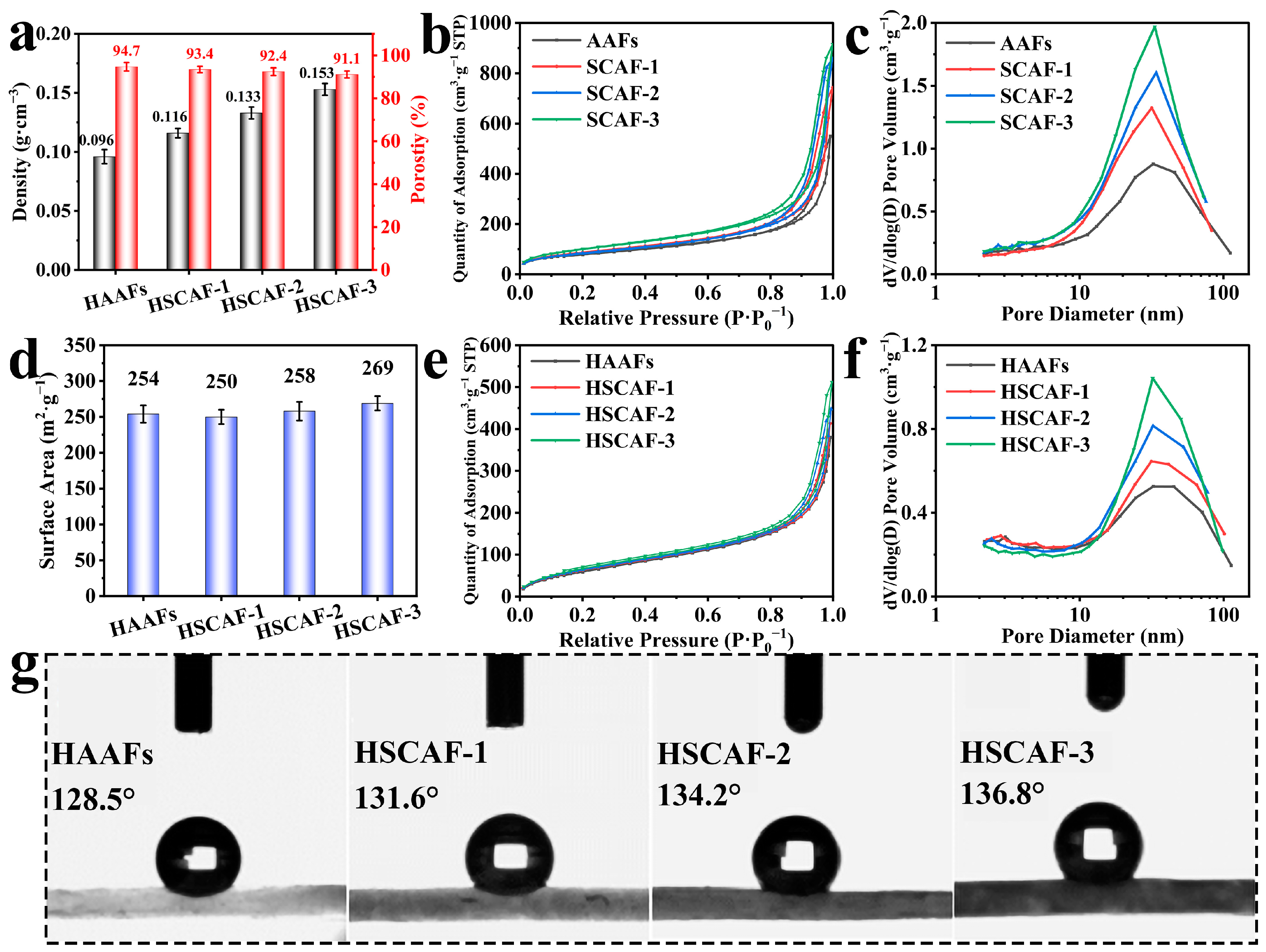
2.3. Nitrogen Adsorption–Desorption Test and Wettability
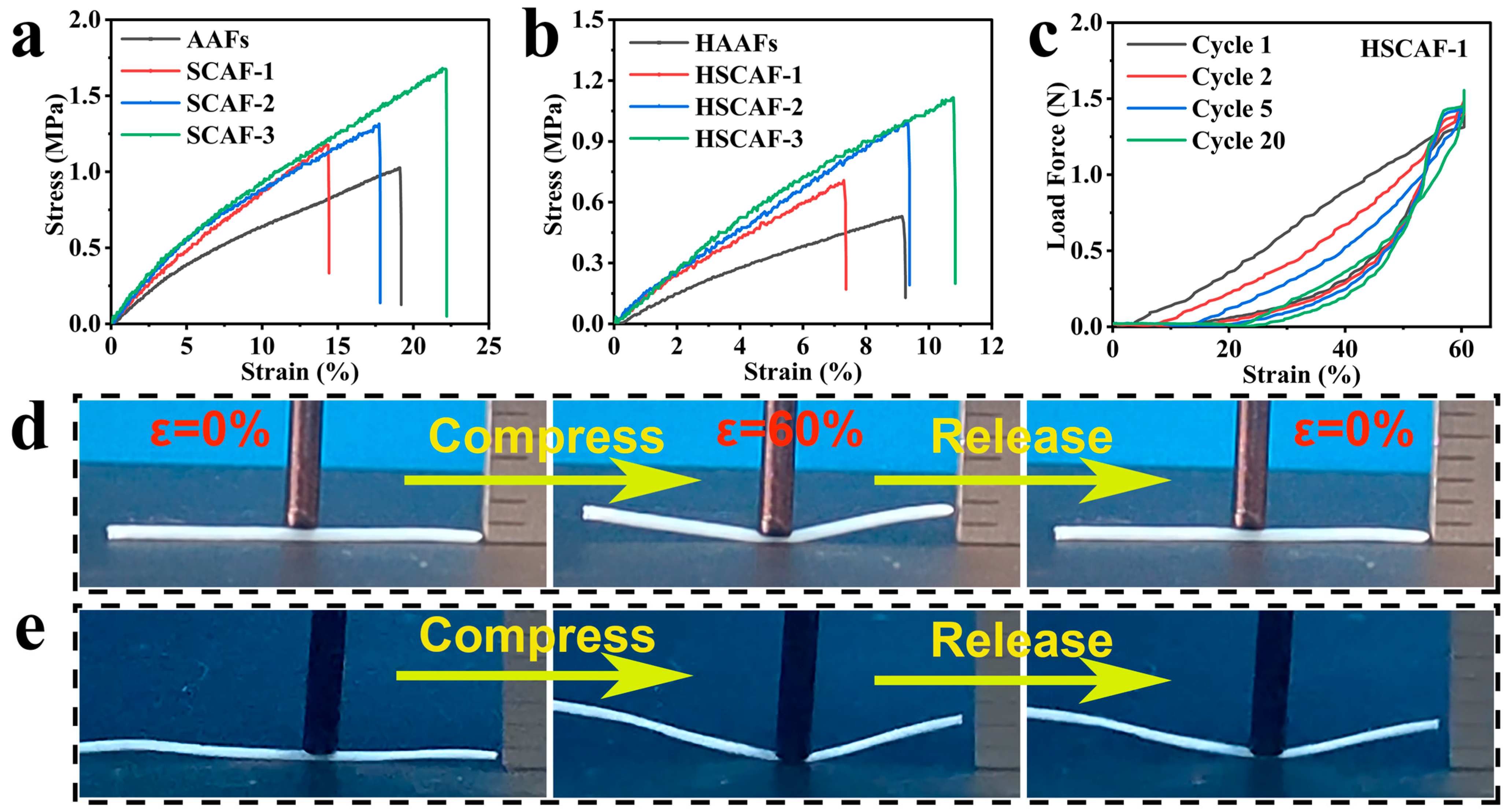
2.4. Mechanical Properties
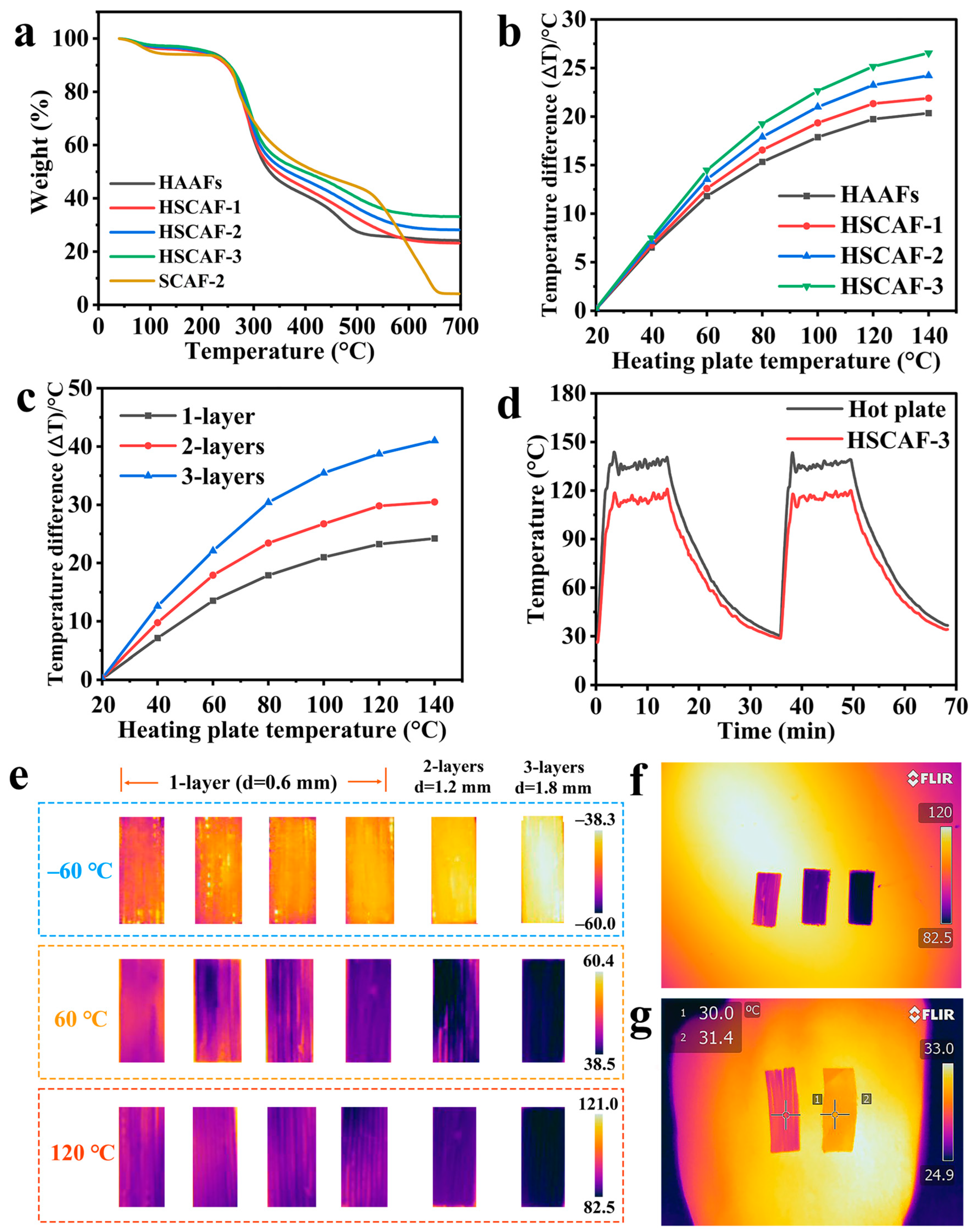
2.5. Thermal Insulation Performance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of SCAFs
4.3. Preparation of HSCAFs
4.4. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Jiang, P.J.; Xiao, R.; Fu, R.; Miao, C.Q.; Wen, L.S.; Song, Q.Q.; Liu, Y.H.; Yu, H.Q.; Gu, J.; et al. Elastic Agarose Nanowire Aerogels for Oil-Water Separation and Thermal Insulation. ACS Appl. Nano Mater. 2022, 5, 12423–12434. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Su, B.L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; E, Y.; Li, J.; Du, T.; Wang, K.; Yao, X.; Jiang, J.; Wang, M.; Yuan, S. Sustainable bacterial cellulose-based composite aerogels with excellent flame retardant and heat insulation. Cellulose 2023, 30, 9563–9574. [Google Scholar] [CrossRef]
- Sai, H.; Wang, M.; Miao, C.; Song, Q.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L.; Hao, Y. Robust Silica-Bacterial Cellulose Composite Aerogel Fibers for Thermal Insulation Textile. Gels 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Duan, S.; Wu, J.; Fu, Y.; Yang, W.; Yao, S.; Zhang, Z.; Xiao, H.; Dai, H.; Hu, C. Lignocellulosic nanofibril aerogel via gas phase coagulation and diisocyanate modification for solvent absorption. Carbohydr. Polym. 2022, 278, 119011. [Google Scholar] [CrossRef]
- Wu, K.; Fang, Y.; Wu, H.; Wan, Y.; Qian, H.; Jiang, F.; Chen, S. Improving konjac glucomannan-based aerogels filtration properties by combining aerogel pieces in series with different pore size distributions. Int. J. Biol. Macromol. 2021, 166, 1499–1507. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured Electrospun Nanofibers for Air Filtration: A Review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef]
- Gia-Thien Ho, T.; Thao Truong, D.P.; Nguyen, H.B.; Long Do, B.; Dinh, T.A.; Ton-That, P.; Van Nguyen, T.T.; Ta Truong, T.B.; Ha Huynh, K.P.; Tri, N. Green synthesized nano-silver/cellulose aerogel as a robust active and recyclable catalyst towards nitrophenol hydrogenation. Chem. Eng. J. 2023, 471, 144604. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Merillas, B.; Pontinha, A.D.R. Trends on Aerogel-Based Biosensors for Medical Applications: An Overview. Int. J. Mol. Sci. 2024, 25, 1309. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhang, B.; Yu, H.; Yan, X.; Xu, H.; Li, X.; Ji, H. Si3N4 Nanofibrous Aerogel with In Situ Growth of SiOx Coating and Nanowires for Oil/Water Separation and Thermal Insulation. ACS Appl. Mater. Interfaces 2021, 13, 22765–22773. [Google Scholar] [CrossRef] [PubMed]
- Teichner, S.J.; Nicolaon, G.A.; Vicarini, M.A.; Gardes, G.E.E. Inorganic oxide aerogels. Adv. Colloid Interface Sci. 1976, 5, 245–273. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, R.; Feng, D.; Ru, H.; Wang, W.; Zhang, C. Resorcinol formaldehyde hydrogel: Synthesis, polymerization, and application in ceramic gel-casting. Colloids Surf. A 2022, 647, 129192. [Google Scholar] [CrossRef]
- Georgi, M.; Klemmed, B.; Benad, A.; Eychmüller, A. A versatile ethanolic approach to metal aerogels (Pt, Pd, Au, Ag, Cu and Co). Mater. Chem. Front. 2019, 3, 1586–1592. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, X.; Wang, T.; Huang, X.; Dou, J.; Wu, D.; Yu, J.; Wu, S.; Chen, X. S/N-codoped carbon nanotubes and reduced graphene oxide aerogel based supercapacitors working in a wide temperature range. J. Colloid Interface Sci. 2023, 638, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, V.; Pirzada, T.; Wang, S.; Khan, S.A. Cellulose-Based Hybrid Aerogels: Strategies toward Design and Functionality. Adv. Mater. 2021, 33, 2102892. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Yang, J.; Fang, X.; Xia, Y.; Zhao, L.; Wu, H.; Guo, S. Facile fabrication of wood-inspired aerogel from chitosan for efficient removal of oil from Water. J. Hazard. Mater. 2020, 385, 121507–121516. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Y.; Guan, Y.; Luo, C.; Bao, M.; Li, Y. A solar-heated antibacterial sodium alginate aerogel for highly efficient cleanup of viscous oil spills. J. Colloid Interface Sci. 2022, 621, 241–253. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Li, Y.; Zheng, X. Robust Silk Fibroin/Graphene Oxide Aerogel Fiber for Radiative Heating Textiles. ACS Appl. Mater. Interfaces 2020, 12, 15726–15736. [Google Scholar] [CrossRef]
- Ma, X.Y.D.; Zeng, Z.; Wang, Z.; Xu, L.; Zhang, Y.; Ang, J.M.; Wan, M.P.; Ng, B.F.; Lu, X. Robust microhoneycomb-like nanofibrous aerogels derived from cellulose and lignin as highly efficient, low-resistant and anti-clogging air filters. J. Membr. Sci. 2022, 642, 119977. [Google Scholar] [CrossRef]
- Sheng, Z.; Liu, Z.; Hou, Y.; Jiang, H.; Li, Y.; Li, G.; Zhang, X. The Rising Aerogel Fibers: Status, Challenges, and Opportunities. Adv. Sci. 2023, 10, 2205762. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, C.; Yang, Y.; Ren, J.; Ling, S. The Fractal Network Structure of Silk Fibroin Molecules and Its Effect on Spinning of Silkworm Silk. ACS Nano 2023, 17, 7662–7673. [Google Scholar] [CrossRef]
- Yang, H.W.; Wang, P.; Yang, Q.L.; Wang, D.F.; Wang, Y.; Kuai, L.; Wang, Z.Q. Superelastic and multifunctional fibroin aerogels from multiscale silk micro-nanofibrils exfoliated via deep eutectic solvent. Int. J. Biol. Macromol. 2023, 224, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Tao, S.; Chai, H.; Xu, D.; Li, X.; Qi, H. High-performance smart cellulose nanohybrid aerogel fibers as a platform toward multifunctional textiles. Chem. Eng. J. 2023, 466, 143153. [Google Scholar] [CrossRef]
- Liu, Z.; Sheng, Z.; Bao, Y.; Cheng, Q.; Wang, P.-X.; Liu, Z.; Zhang, X. Ionic Liquid Directed Spinning of Cellulose Aerogel Fibers with Superb Toughness for Weaved Thermal Insulation and Transient Impact Protection. ACS Nano 2023, 17, 18411–18420. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shao, Z.; Zhao, N.; Zhang, R.; Yuan, G.; Tian, L.; Zhang, Z.; Gao, W.; Bai, H. Biomimetic, knittable aerogel fiber for thermal insulation textile. Science 2023, 382, 1379–1383. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Li, X.; Dong, J.; Zhao, X.; Zhang, Q. Controllable Strong and Ultralight Aramid Nanofiber-Based Aerogel Fibers for Thermal Insulation Applications. Adv. Fiber Mater. 2022, 4, 1267–1277. [Google Scholar] [CrossRef]
- Xue, T.; Zhu, C.; Feng, X.; Wali, Q.; Fan, W.; Liu, T. Polyimide Aerogel Fibers with Controllable Porous Microstructure for Super-Thermal Insulation Under Extreme Environments. Adv. Fiber Mater. 2022, 4, 1118–1128. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Liu, J.; Zhao, Y.; Jiang, Q.; Zhang, X.; Wang, J.; Wang, H.; Yu, Z. Biomass based active-cum-passive aerogel heater with enhanced thermal insulation property derived from hollow cellulose kapok fiber for personal thermal management. Cellulose 2023, 30, 7031–7045. [Google Scholar] [CrossRef]
- Cui, Y.; Gong, H.; Wang, Y.; Li, D.; Bai, H. A Thermally Insulating Textile Inspired by Polar Bear Hair. Adv. Mater. 2018, 30, 1706807. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, A.N.; Burpo, F.J.; Nguyen, C.K.; Nagelli, E.A.; Ryu, M.Y.; Wang, J.; Sims, R.K.; Woronowicz, K.; Wickiser, J.K. Noble Metal Composite Porous Silk Fibroin Aerogel Fibers. Materials 2019, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Z.; Liu, Z.; Cheng, H.; Li, C. Continuous, Strong, Porous Silk Firoin-Based Aerogel Fibers toward Textile Thermal Insulation. Polymer 2019, 11, 1899. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Zhang, C. Versatile fabrication of anisotropic and superhydrophobic aerogels for highly selective oil absorption. Carbon 2019, 155, 16–24. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering. Sci. Rep. 2017, 7, 17187–17198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, X.; Duan, D.; Xu, J.; Gao, X. Preparation and characterization of agar, agarose, and agaropectin from the red alga Ahnfeltia plicata. J. Oceanol. Limnol. 2019, 37, 815–824. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, Y.J.; Oh, H.J.; Kimura, S.; Wada, M.; Kim, U.-J. Cellulose–silk fibroin hydrogels prepared in a lithium bromide aqueous solution. Cellulose 2017, 24, 5079–5088. [Google Scholar] [CrossRef]
- Lee, K.G.; Kweon, H.; Yeo, J.H.; Woo, S.O.; Lee, J.H.; Hwan Park, Y. Structural and physical properties of silk fibroin/alginate blend sponges. J. Appl. Polym. Sci. 2004, 93, 2174–2179. [Google Scholar] [CrossRef]
- Li, N.; Chen, W.; Chen, G.; Wan, X.; Tian, J. Low-Cost, Sustainable, and Environmentally Sound Cellulose Absorbent with High Efficiency for Collecting Methane Bubbles from Seawater. ACS Sustain. Chem. Eng. 2018, 6, 6370–6377. [Google Scholar] [CrossRef]
- Chhatbar, M.U.; Godiya, C.; Siddhanta, A.K. Functional modification of agarose: A facile synthesis of an agarose-saccharate derivative. Carbohydr. Polym. 2012, 88, 1118–1123. [Google Scholar] [CrossRef]
- Gao, R.; Xiao, S.; Gan, W.; Liu, Q.; Amer, H.; Rosenau, T.; Li, J.; Lu, Y. Mussel adhesive-inspired design of superhydrophobic nanofibrillated cellulose aerogels for oil/water separation. ACS Sustain. Chem. Eng. 2018, 6, 9047–9055. [Google Scholar] [CrossRef]


| Sample | HAAFs | HSCAF-1 | HSCAF-2 | HSCAF-3 |
|---|---|---|---|---|
| SF (wt %) | 0 | 0.5 | 1 | 1.5 |
| AG (wt %) | 2 | 2 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Jiang, P.; Yang, X.; Fu, R.; Liu, L.; Miao, C.; Wang, Y.; Sai, H. Hydrophobic Silk Fibroin–Agarose Composite Aerogel Fibers with Elasticity for Thermal Insulation Applications. Gels 2024, 10, 266. https://doi.org/10.3390/gels10040266
Du Y, Jiang P, Yang X, Fu R, Liu L, Miao C, Wang Y, Sai H. Hydrophobic Silk Fibroin–Agarose Composite Aerogel Fibers with Elasticity for Thermal Insulation Applications. Gels. 2024; 10(4):266. https://doi.org/10.3390/gels10040266
Chicago/Turabian StyleDu, Yuxiang, Pengjie Jiang, Xin Yang, Rui Fu, Lipeng Liu, Changqing Miao, Yaxiong Wang, and Huazheng Sai. 2024. "Hydrophobic Silk Fibroin–Agarose Composite Aerogel Fibers with Elasticity for Thermal Insulation Applications" Gels 10, no. 4: 266. https://doi.org/10.3390/gels10040266
APA StyleDu, Y., Jiang, P., Yang, X., Fu, R., Liu, L., Miao, C., Wang, Y., & Sai, H. (2024). Hydrophobic Silk Fibroin–Agarose Composite Aerogel Fibers with Elasticity for Thermal Insulation Applications. Gels, 10(4), 266. https://doi.org/10.3390/gels10040266