Enhancing the Topical Antibacterial Activity of Fusidic Acid via Embedding into Cinnamon Oil Nano-Lipid Carrier
Abstract
1. Introduction
2. Results and Discussion
2.1. Model Fitting and Design Validation
2.2. Analysis of the Detected Response
2.2.1. Effect of Selected Factors on Particle Size
2.2.2. Effect of the Selected Factors on In Vitro Release
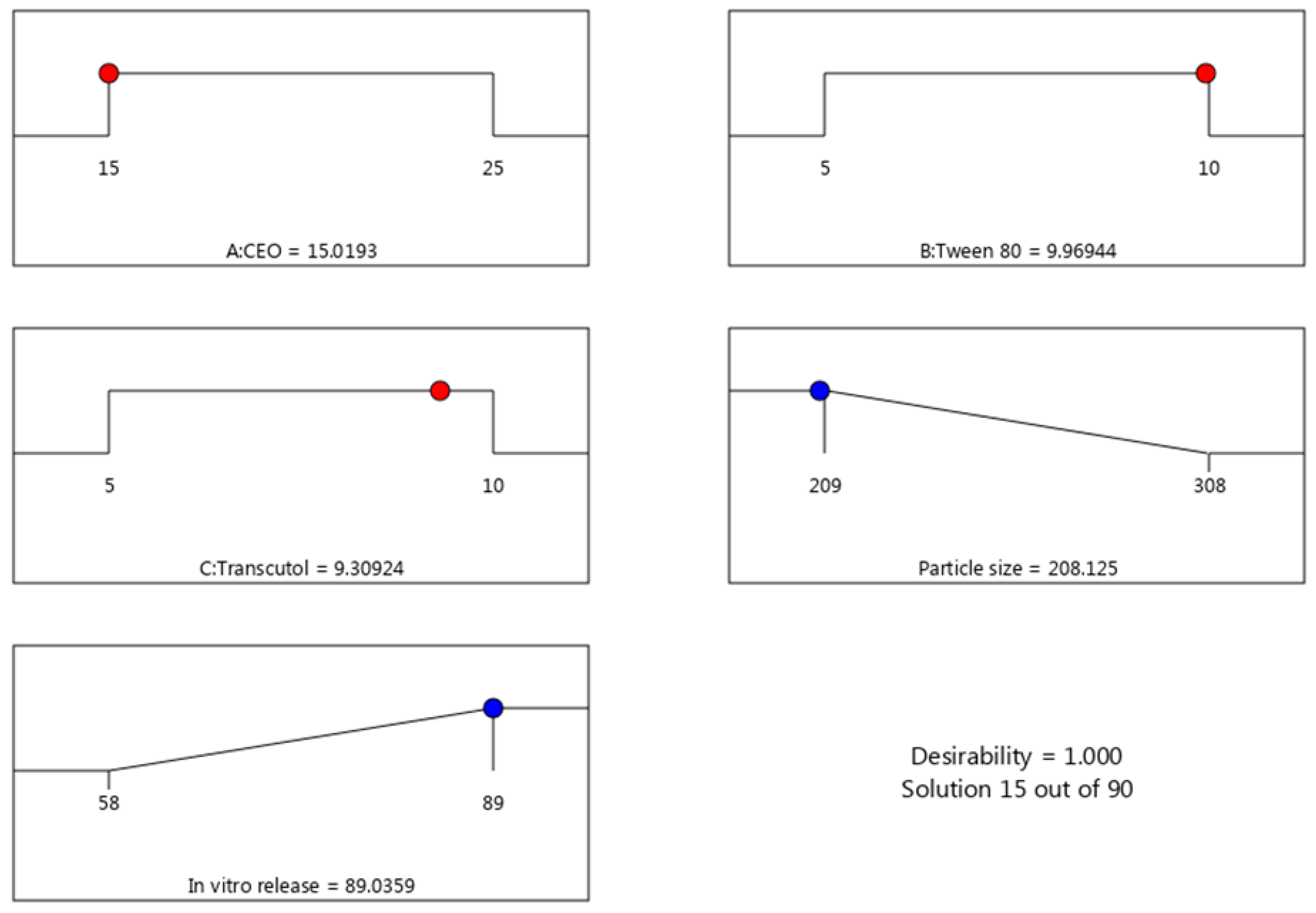
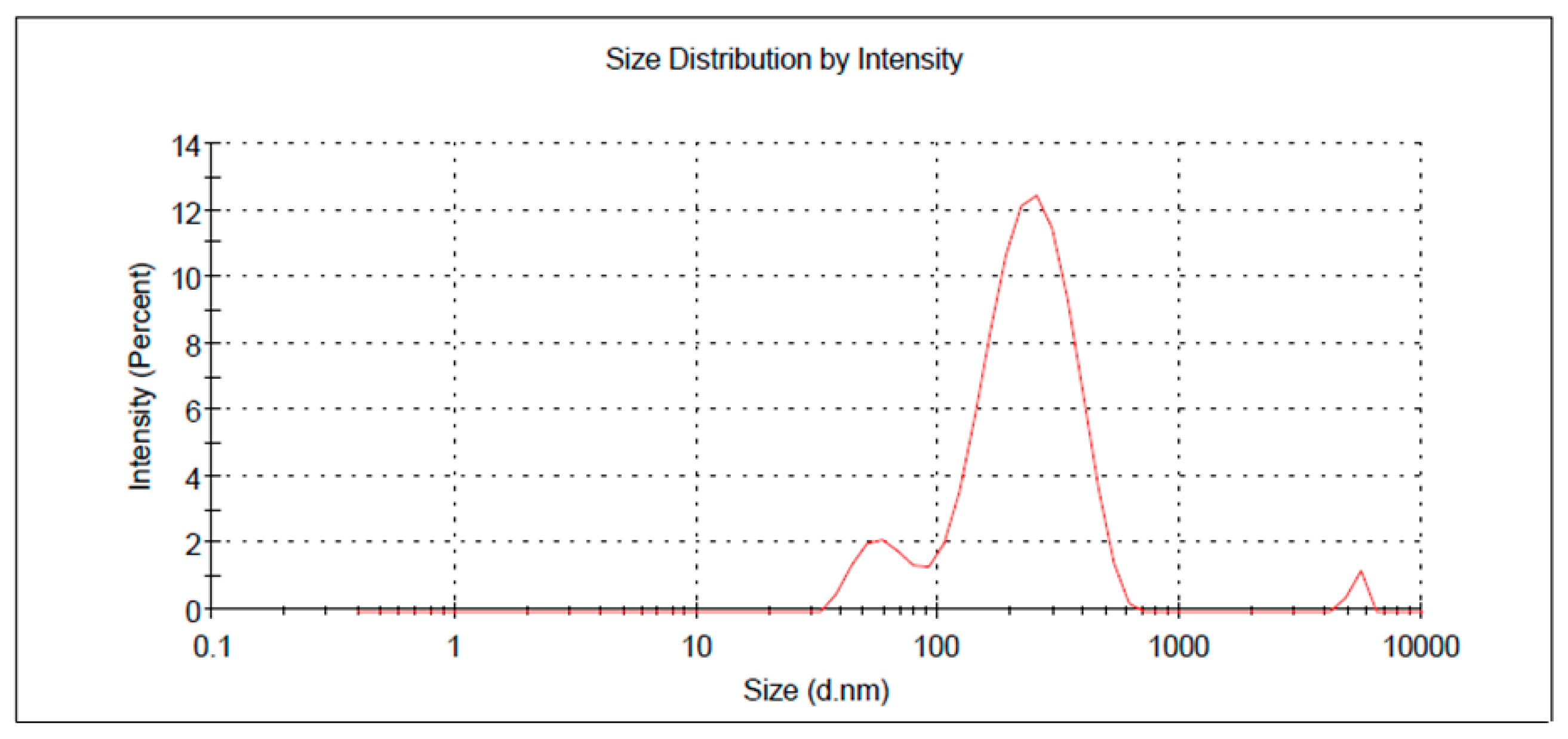
2.3. Verification of Optimization Process
2.4. Compatibility Studies (Fourier Transform Infrared Spectroscopy (FTIR) Studies)
2.5. FA-NE-Hydrogel Characterization
2.6. In Vitro Release from NE-Hydrogel Formulation
2.7. Kinetic Study
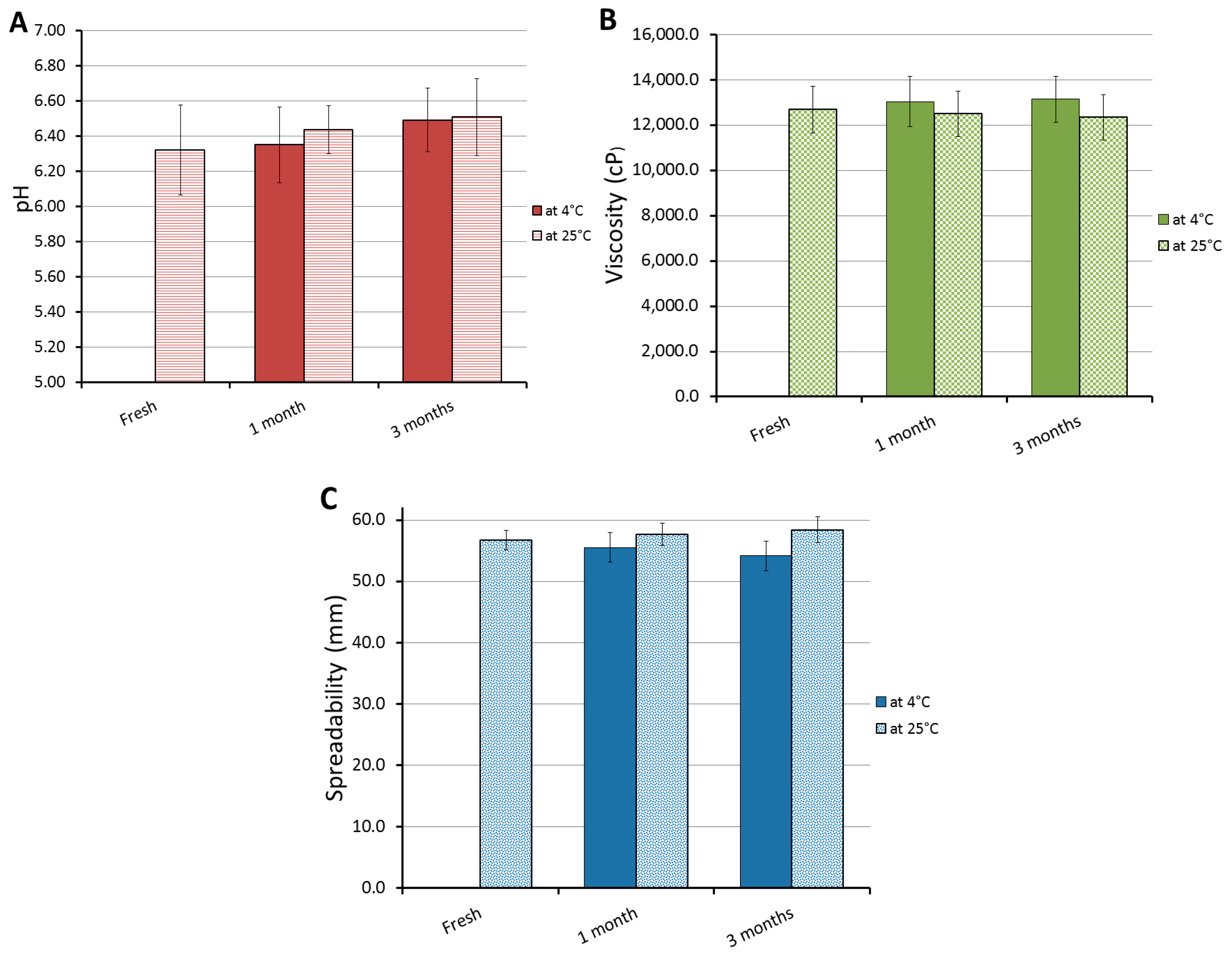
2.8. Stability Test
2.9. In Vivo Study: Skin Irritation Test
2.10. Antibacterial Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Box–Behnken Experimental Design (BBD)
4.3. Developing FA-NE
4.4. Particle Size and Size Distribution (PDI)
4.5. In Vitro Release Study
4.6. Compatibility Studies (Fourier—Transform Infrared Spectroscopy (FTIR) Studies)
4.7. Developing FA-NE-Hydrogel
4.8. FA-NE-Hydrogel Characterization
4.9. In Vitro Release from NE-Hydrogel Formulation
4.10. Kinetic Study
4.11. Stability Test
4.12. Animals
4.12.1. Animal Handling
4.12.2. Declaration of Ethical Approval
4.13. In Vivo Study
Skin Irritation Test
4.14. Antibacterial Study
4.15. Morphology of Bacterial Cells Treated with FA-NE-Hydrogel Formulation
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh Malik, D.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Kappelle, W.F.W.; Siersema, P.D.; Bogte, A.; Vleggaar, F.P. Challenges in oral drug delivery in patients with esophageal dysphagia. Expert Opin. Drug Deliv. 2016, 13, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Praestegaard, M.; Steele, F.; Crutchley, N. Polyaphron Dispersion Technology, A Novel Topical Formulation and Delivery System Combining Drug Penetration, Local Tolerability and Convenience of Application. Dermatol. Ther. 2022, 12, 2217–2231. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, W.; Jones, A.; Doherty, M. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: Meta-analysis of randomised controlled trials. BMJ 2004, 329, 324. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Wiffen, P.J.; Kalso, E.A.; Bell, R.F.; Aldington, D.; Phillips, T.; Gaskell, H.; Moore, R.A. Topical analgesics for acute and chronic pain in adults—An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 5, Cd008609. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Imran, M.; Gupta, D.K.; Akhtar, A. Formulation of Transliposomal Nanocarrier Gel Containing Strychnine for the Effective Management of Skin Cancer. Gels 2023, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Verma, A.; Pathak, K. Topical delivery of drugs for the effective treatment of fungal infections of skin. Curr. Pharm. Des. 2015, 21, 2892–2913. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Elsewedy, H.S.; Shehata, T.M.; Almostafa, M.M.; Soliman, W.E. Hypolipidemic Activity of Olive Oil-Based Nanostructured Lipid Carrier Containing Atorvastatin. Nanomaterials 2022, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Mardhiah Adib, Z.; Ghanbarzadeh, S.; Kouhsoltani, M.; Yari Khosroshahi, A.; Hamishehkar, H. The Effect of Particle Size on the Deposition of Solid Lipid Nanoparticles in Different Skin Layers: A Histological Study. Adv. Pharm. Bull. 2016, 6, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ceci, C.; Graziani, G.; Faraoni, I.; Cacciotti, I. Strategies to improve ellagic acid bioavailability: From natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology 2020, 31, 382001. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, S.M.; Yamauchi, Y.; Kim, J.H.; Horvat, J.; Dou, S.X.; Alici, G.; Ooi, L.; Martinac, B.; Shiddiky, M.J.A.; Gopalan, V.; et al. Nanotechnology and its medical applications: Revisiting public policies from a regulatory perspective in Australia. Nanotechnol. Rev. 2017, 6, 255–269. [Google Scholar] [CrossRef]
- Gray, K.A. Five Myths about Nanotechnology in the Current Public Policy Debate: A science and engineering perspective: Creating Legal Institutions for Uncertain Risks. In The Nanotechnology Challenge: Creating Legal Institutions for Uncertain Risk; Cambridge Press: Cambridge, UK, 2011; pp. 11–60. [Google Scholar]
- Weiss, J.; Gaysinsky, S.; Davidson, M.; McClements, J. Nanostructured encapsulation systems: Food antimicrobials. In Global Issues in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 425–479. [Google Scholar]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy-An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, J.; Nair, A.; Kumria, R. Self-emulsifying therapeutic system: A potential approach for delivery of lipophilic drugs. Braz. J. Pharm. Sci. 2011, 47, 447–465. [Google Scholar] [CrossRef]
- Yashpal, S.; Tanuj, H.; Harsh, K. Nanoemulsions: A pharmaceutical review. Int. J. Pharma Prof. Res. 2013, 4, 928–935. [Google Scholar]
- Lovelyn, C.; Attama, A.A. Current state of nanoemulsions in drug delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626. [Google Scholar] [CrossRef]
- Fernández-García, R.; Statts, L.; de Jesus, J.A.; Dea-Ayuela, M.A.; Bautista, L.; Simão, R.; Bolás-Fernández, F.; Ballesteros, M.P.; Laurenti, M.D.; Passero, L.F.D.; et al. Ultradeformable Lipid Vesicles Localize Amphotericin B in the Dermis for the Treatment of Infectious Skin Diseases. ACS Infect. Dis. 2020, 6, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, F.; Rajabnejhad, S.; Partoazar, A.R.; Mehr, S.E.; Faridi-Majidi, R.; Sahebgharani, M.; Syedmoradi, L.; Rajabnejhad, M.R.; Amani, A. Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm. Dev. Technol. 2016, 21, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-Y.; Lee, M.-S.; Chang, E.; Kim, S.-Y.; Kang, B.; Ko, H.; Kim, I.-H.; Zhong, Q.; Jo, Y.-H.; Kim, C.-T.; et al. Formulation and Characterization of Quercetin-loaded Oil in Water Nanoemulsion and Evaluation of Hypocholesterolemic Activity in Rats. Nutrients 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Elsewedy, H.S. Paclitaxel and Myrrh oil Combination Therapy for Enhancement of Cytotoxicity against Breast Cancer; QbD Approach. Processes 2022, 10, 907. [Google Scholar] [CrossRef]
- Kumari, S.; Kumaraswamy, R.V.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef] [PubMed]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef]
- Borg, A.; Pavlov, M.; Ehrenberg, M. Mechanism of fusidic acid inhibition of RRF- and EF-G-dependent splitting of the bacterial post-termination ribosome. Nucleic Acids Res. 2016, 44, 3264–3275. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Khan, D.; Ahmed, N.; Zafar, A.; Shah, K.U.; ur Rehman, A. Lipase-sensitive fusidic acid polymeric nanoparticles based hydrogel for on-demand delivery against MRSA-infected burn wounds. J. Drug Deliv. Sci. Technol. 2023, 80, 104110. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Elnahas, O.S.; Assar, N.H.; Gad, A.M.; El Hosary, R. Nanocrystals of Fusidic Acid for Dual Enhancement of Dermal Delivery and Antibacterial Activity: In Vitro, Ex Vivo and In Vivo Evaluation. Pharmaceutics 2020, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Powers, J.H.; Dudley, M.N.; Christiansen, K.; Finch, R.G. Antimicrobial drug resistance, regulation, and research. Emerg. Infect. Dis. 2006, 12, 183–190. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Mcbain, A.J.; Salgado, A.J.; Simoes, M. Combinatorial activity of flavonoids with antibiotics against drug-resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid.-Based Complement. Altern. Med. 2020, 2020, 5190603. [Google Scholar] [CrossRef] [PubMed]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Dibi, M.; Ramesh, K. Studies on the Effect of Oil and Surfactant on the Formation of Alginate-Based O/W Lidocaine Nanocarriers Using Nanoemulsion Template. Pharmaceutics 2020, 12, 1223. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, D.; Hu, J. Effect of Surfactant Molecular Structure on Emulsion Stability Investigated by Interfacial Dilatational Rheology. Polymers 2021, 13, 1127. [Google Scholar] [CrossRef] [PubMed]
- Paramashivaiah, B.; Rajashekhar, C. Studies on effect of various surfactants on stable dispersion of graphene nano particles in simarouba biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012083. [Google Scholar] [CrossRef]
- Laxmi, M.; Bhardwaj, A.; Mehta, S.; Mehta, A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif. Cells Nanomed. Biotechnol. 2015, 43, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Corrie, L.; Kaur, J.; Awasthi, A.; Vishwas, S.; Gulati, M.; Saini, S.; Kumar, B.; Pandey, N.K.; Gupta, G.; Dureja, H.; et al. Multivariate Data Analysis and Central Composite Design-Oriented Optimization of Solid Carriers for Formulation of Curcumin-Loaded Solid SNEDDS: Dissolution and Bioavailability Assessment. Pharmaceutics 2022, 14, 2395. [Google Scholar] [CrossRef]
- Marian, E.; Tita, B.; Duteanu, N.; Vicas, L.; Ciocan, S.; Jurca, T.; Antal, L.; Tica, O.; Mureşan, M.; Pallag, A.; et al. Antimicrobial activity of fusidic acid inclusion complexes. Int. J. Infect. Dis. 2020, 101, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, U.A.; Ahmed, O.A.A.; Badr-Eldin, S.M.; Aldawsari, H.M.; Okbazghi, S.Z.; Awan, Z.A.; Bakhrebah, M.A.; Alomary, M.N.; Abdulaal, W.H.; Medina, C.; et al. Optimized Nanostructured Lipid Carriers Integrated into In Situ Nasal Gel for Enhancing Brain Delivery of Flibanserin. Int. J. Nanomed. 2020, 15, 5253–5264. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, M.; Winnicka, K. The Effect of Cationic Polyamidoamine Dendrimers on Physicochemical Characteristics of Hydrogels with Erythromycin. Int. J. Mol. Sci. 2015, 16, 20277–20289. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, B.; Aggarwal, G.; Harikumar, S. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int. J. Pharm. Investig. 2014, 4, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, N. Mathematical modelling of dissolution kinetics in dosage forms. Res. J. Pharm. Technol. 2020, 13, 1339–1345. [Google Scholar]
- De Oca-Ávalos, J.M.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and physical properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Tajik, H.; Yarahmadi, A.; Sanginabadi, S. Antimicrobial effect of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Health Scope 2015, 4, e21808. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Fusidic Acid: A Bacterial Elongation Factor Inhibitor for the Oral Treatment of Acute and Chronic Staphylococcal Infections. Cold Spring Harb. Perspect. Med. 2016, 6, a025437. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.K.; Serrano, D.R.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Bolás-Fernández, F.; Torrado, J.J.; Molero, G. New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int. J. Pharm. 2014, 473, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Almostafa, M.M.; Elsewedy, H.S. Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect. Polymers 2022, 14, 3021. [Google Scholar] [CrossRef] [PubMed]
- Haimhoffer, Á.; Vasvári, G.; Budai, I.; Béresová, M.; Deák, Á.; Németh, N.; Váradi, J.; Sinka, D.; Bácskay, I.; Vecsernyés, M.; et al. In Vitro and In Vivo Studies of a Verapamil-Containing Gastroretentive Solid Foam Capsule. Pharmaceutics 2022, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Bujubarah, M.M.; Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Formulation by Design of an Innovative Tea Tree Oil Nanoemulgel Incorporating Mupirocin for Enhanced Wound Healing Activity. Appl. Sci. 2023, 13, 13244. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics. 2023, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Elnahas, H.M.; Elsewedy, H.S. Development, Characterization and Optimization of the Anti-Inflammatory Influence of Meloxicam Loaded into a Eucalyptus Oil-Based Nanoemulgel. Gels 2022, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Elsewedy, H.S.; Shehata, T.M.; Alqahtani, N.K.; Khalil, H.E.; Soliman, W.E. Date Palm Extract (Phoenix dactylifera) Encapsulated into Palm Oil Nanolipid Carrier for Prospective Antibacterial Influence. Plants 2023, 12, 3670. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability testing of pharmaceutical products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for improved topical delivery of retinyl palmitate: Formulation design and stability evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef] [PubMed]
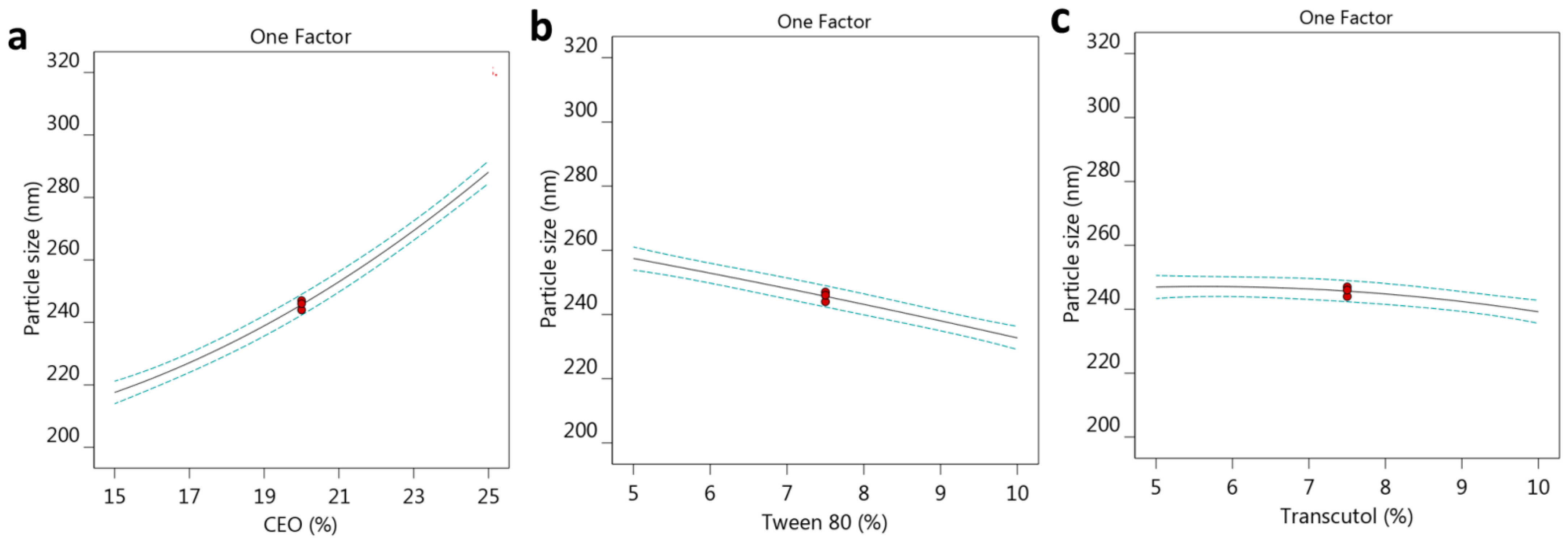
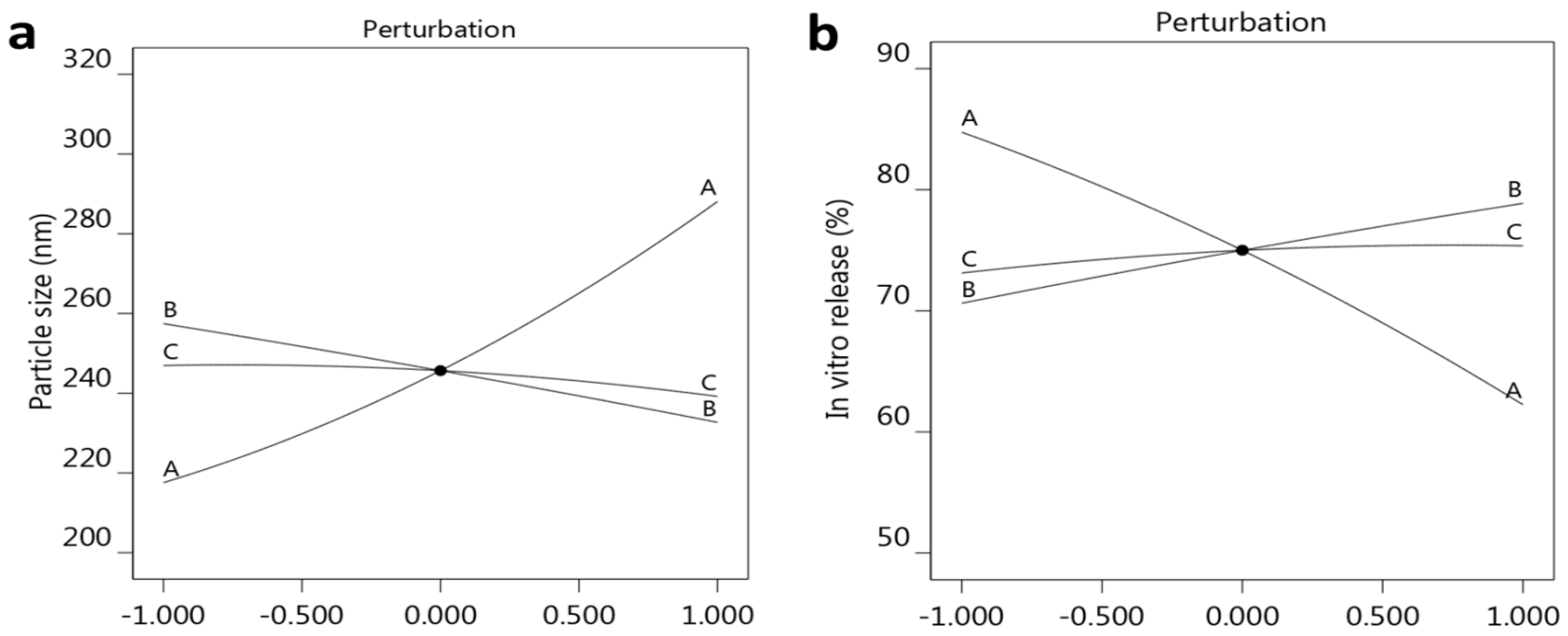
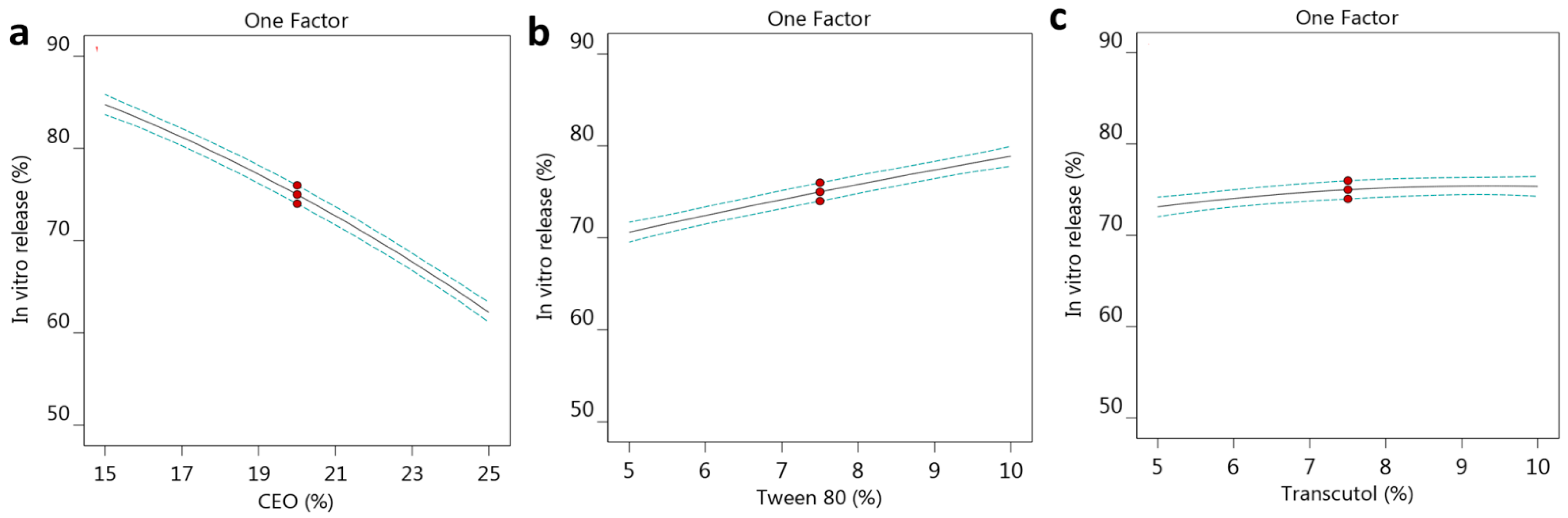




| Formula | Factors Values | Response Values | PDI | |||
|---|---|---|---|---|---|---|
| A (%) | B (%) | C (%) | R1 (nm) | R2 (%) | ||
| F1 | 20 | 5 | 5 | 257 ± 4.5 | 69 ± 3.0 | 0.355 ± 0.004 |
| F2 | 20 | 10 | 5 | 234 ± 3.6 | 77 ± 2.6 | 0.333 ± 0.02 |
| F3 | 15 | 10 | 7.5 | 209 ± 2.6 | 89 ± 3.6 | 0.237 ± 0.022 |
| F4 | 15 | 7.5 | 10 | 213 ± 2.2 | 85 ± 3.1 | 0.242 ± 0.018 |
| F5 | 25 | 5 | 7.5 | 308 ± 4.8 | 58 ± 2.5 | 0.402 ± 0.011 |
| F6 | 20 | 7.5 | 7.5 | 247 ± 3.6 | 74 ± 2.3 | 0.318 ± 0.016 |
| F7 | 25 | 7.5 | 5 | 291 ± 3.0 | 60 ± 2.6 | 0.321 ± 0.009 |
| F8 | 20 | 10 | 10 | 228 ± 2.4 | 79 ± 3.4 | 0.345 ± 0.022 |
| F9 | 15 | 5 | 7.5 | 223 ± 2.9 | 80 ± 3.5 | 0.362 ± 0.008 |
| F10 | 25 | 7.5 | 10 | 278 ± 2.8 | 63 ± 2.6 | 0.355 ± 0.013 |
| F11 | 25 | 10 | 7.5 | 269 ± 2.2 | 66 ± 3.2 | 0.380 ± 0.016 |
| F12 | 20 | 7.5 | 7.5 | 244 ± 3.6 | 76 ± 3.6 | 0.403 ± 0.015 |
| F13 | 15 | 7.5 | 5 | 219 ± 3.0 | 83 ± 4.1 | 0.396 ± 0.012 |
| F14 | 20 | 7.5 | 7.5 | 246 ± 3.3 | 75 ± 3.2 | 0.375 ± 0.019 |
| F15 | 20 | 5 | 10 | 251 ± 3.5 | 71 ± 3.5 | 0.348 ± 0.046 |
| Source | R1 | R2 | ||
|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |
| Model | 260.50 | <0.0001 * | 288.66 | <0.0001 * |
| A | 1994.75 | <0.0001 * | 2250.00 | <0.0001 * |
| B | 245.84 | <0.0001 * | 302.50 | <0.0001 * |
| C | 24.11 | 0.0044 * | 22.50 | 0.0051 * |
| AB | 31.35 | 0.0025 * | 0.5556 | 0.4896 |
| AC | 2.46 | 0.1777 | 0.5556 | 0.4896 |
| BC | 0.0000 | 1.0000 | 0.0000 | 1.0000 |
| A2 | 38.06 | 0.0016 * | 18.46 | 0.0077 * |
| B2 | 0.2521 | 0.6369 | 0.5128 | 0.5060 |
| C2 | 4.94 | 0.0768 | 4.62 | 0.0844 |
| Lack of Fit | 2.89 | 0.2673 | 0.0833 | 0.9630 |
| R2 Analysis | R1 | R2 |
|---|---|---|
| R2 | 0.9979 | 0.9981 |
| Adjusted R2 | 0.9940 | 0.9946 |
| Predicted R2 | 0.9714 | 0.9927 |
| Adequate Precision | 52.2577 | 56.1416 |
| Model | ||
| Remark | Quadratic | Quadratic |
| Selected Factors | Symbol | Constrains |
|---|---|---|
| CEO concentration | A | In range |
| Tween 80 concentration | B | In range |
| Transcutol® P concentration | C | In range |
| Observed response | ||
| Particle size (nm) | R1 | Minimize |
| In vitro release (%) | R2 | Maximize |
| Observed response | Predicted values | Observed values |
| R1 (nm) | 208.13 ± 2.23 | 202.4 ± 3.55 |
| R2 (%) | 89.03 ± 0.67 | 90.66 ± 3.43 |
| Formulation | R2 Value | |||
|---|---|---|---|---|
| Zero Order Kinetic | First Order Kinetic | Higuchi Kinetic | Korsmeyer-Peppas Kinetic | |
| Optimized FA-NE | 0.8147 | 0.4314 | 0.9772 | 0.9717 |
| FA-NE-hydrogel | 0.9461 | 0.5477 | 0.9843 | 0.9741 |
| Bacterial Strain | Inhibition Zone (cm) | ||
|---|---|---|---|
| FA-NE-Hydrogel (a) | Blank NE (b) | Marketed FA (c) | |
| Staphylococcus aureus | 4.43 ± 0.12 *# | 1.97 ± 0.15 * | 4.11 ± 0.11 # |
| E-coli | 2.40 ± 0.11 *# | 2.10 ± 0.10 * | Negative |
| Independent Variable | Symbol | Level of Variation | ||
|---|---|---|---|---|
| Lowest (−1) | Central (0) | Highest (+1) | ||
| CEO concentration (%) | A | 15 | 20 | 25 |
| Tween 80 concentration (%) | B | 5 | 7.5 | 10 |
| Transcutol ® P concentration (%) | C | 5 | 7.5 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsewedy, H.S.; Shehata, T.M.; Genedy, S.M.; Siddiq, K.M.; Asiri, B.Y.; Alshammari, R.A.; Bukhari, S.I.; Kola-Mustapha, A.T.; Ramadan, H.A.; Soliman, W.E. Enhancing the Topical Antibacterial Activity of Fusidic Acid via Embedding into Cinnamon Oil Nano-Lipid Carrier. Gels 2024, 10, 268. https://doi.org/10.3390/gels10040268
Elsewedy HS, Shehata TM, Genedy SM, Siddiq KM, Asiri BY, Alshammari RA, Bukhari SI, Kola-Mustapha AT, Ramadan HA, Soliman WE. Enhancing the Topical Antibacterial Activity of Fusidic Acid via Embedding into Cinnamon Oil Nano-Lipid Carrier. Gels. 2024; 10(4):268. https://doi.org/10.3390/gels10040268
Chicago/Turabian StyleElsewedy, Heba S., Tamer M. Shehata, Shaymaa M. Genedy, Khuzama M. Siddiq, Bushra Y. Asiri, Rehab A. Alshammari, Sarah I. Bukhari, Adeola T. Kola-Mustapha, Heba A. Ramadan, and Wafaa E. Soliman. 2024. "Enhancing the Topical Antibacterial Activity of Fusidic Acid via Embedding into Cinnamon Oil Nano-Lipid Carrier" Gels 10, no. 4: 268. https://doi.org/10.3390/gels10040268
APA StyleElsewedy, H. S., Shehata, T. M., Genedy, S. M., Siddiq, K. M., Asiri, B. Y., Alshammari, R. A., Bukhari, S. I., Kola-Mustapha, A. T., Ramadan, H. A., & Soliman, W. E. (2024). Enhancing the Topical Antibacterial Activity of Fusidic Acid via Embedding into Cinnamon Oil Nano-Lipid Carrier. Gels, 10(4), 268. https://doi.org/10.3390/gels10040268







