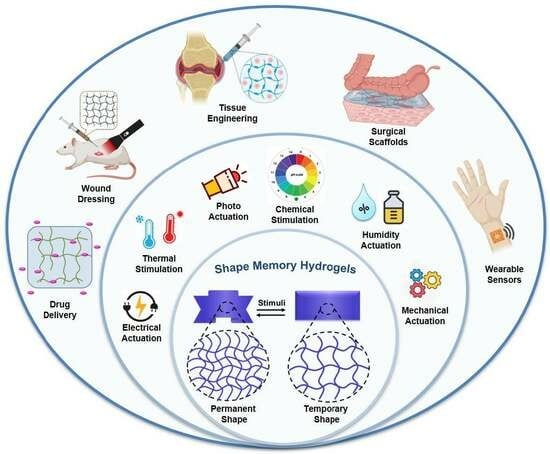
Shape Memory Hydrogels for Biomedical Applications
Abstract
:1. Introduction
2. Fundamental Mechanism and Programming of Shape Memory Hydrogels
3. Types of Shape Memory Hydrogels
3.1. Thermally Responsive SMHs
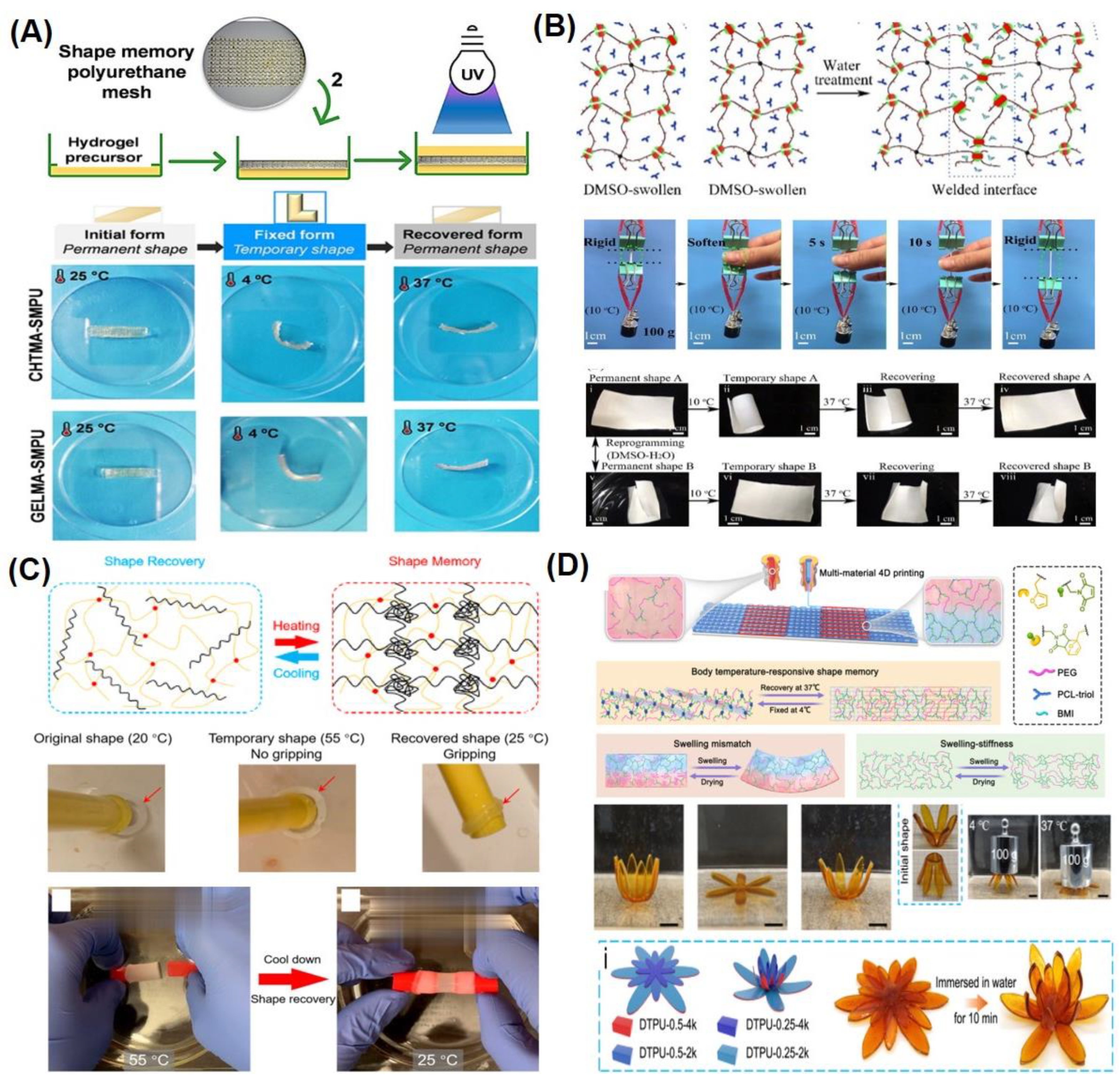
3.2. Chemically Responsive SMHs
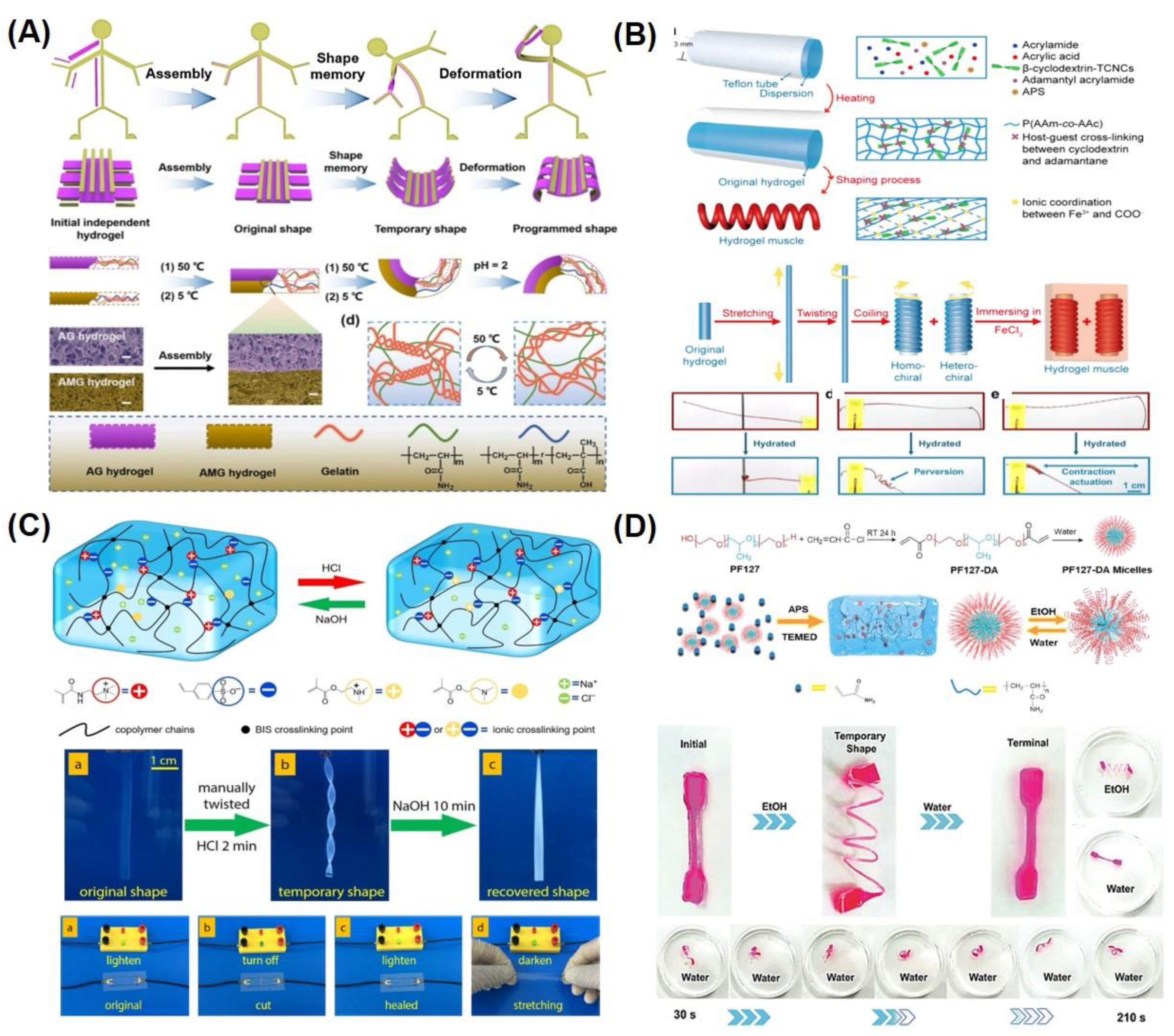
3.3. Light Responsive SMHs
3.4. Electrically Responsive SMHs
| Core Polymeric Hydrogel Network | Shape Memory Hydrogel Matrix | Influence of Additives on Properties or Function of SMHs | Type of Stimuli | Ref. |
|---|---|---|---|---|
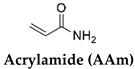 | P(AAm-co-PEA) | DMSO/Water (DMSO allowed for hydrophobic interactions for the formation of hard polymer network) | Temperature (37 °C) | [46] |
| P(AAm) and ELP | - | Temperature (20 °C) | [47] | |
| P(AAm-co-MAA) | Gelatin (Thermally induced coil-triple helix transition of gelatin facilitates the welding between copolymers) | Chemical (pH 2) | [58] | |
| P(AAm-co-AAc-co-Ad-Am) | TCNC nanocrystals and Fe3+ ions (TCNC enables the shaping process of hydrogesl while Fe3+ ions assists in the fixation of network via Fe3+-COO- coordination). | Chemical (EtOH) | [61] | |
| P(AAm) and SA | Fe3+ ions (To help in strain fixation via Fe3+-carboxylate coordination) | Light (UV = 365 nm) | [73] | |
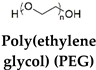 | PEG, MDI, and IU | - | Temperature (4°C to 50°C) | [45] |
| PEG, MDI, and IU | Tannic acid and Kartogenin (To promote MSC differentiation into chondrocytes) | Temperature (37°C) | [52] | |
 | - | - | Temperature (Ambient) | [53] |
| P(DA-co-AAm-co-AA) and alginate | - | Chemical (Ferric Chloride) | [57] | |
| P(AA-co-AN) | Chemical (pH) | [59] | ||
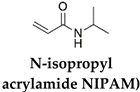 | P(NIPAM) | Nanosheets of Zr-Fc MOFs (MOFs function as a photothermal nanotransducer and enhance the actuation performance of the hydrogel actuators) | Light (NIR = 800 nm) | [70] |
| P(NIPAM-AA), PPy and alginate | Fe3+ ions (To induce the formation of the PPy pattern) | Light (NIR = 800 nm) | [71] | |
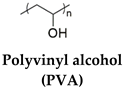 | PVA and chitosan | Graphene oxide (It crosslinked with chitosan to form a network of the hydrogel by electrostatic interactions) | Temperature (60°C) | [40] |
| PVA and cornstarch | - | Chemical (water) | [48] | |
| PVA | Tannic acid and Fe3+ ions (To endow the hydrogel with a good photothermal effect) | Light (NIR= 808) | [72] | |
| PVA and chitosan | Carbon Nanotubes (To enhance the mechanical and electrical properties of the hydrogel system) | Electricity (40 V) | [36] | |
 | - | - | Temperature (10 °C) | [43] |
| LBL assembly of PU with CHTMA, LAMMA, or HAMA, GELMA. | - | Temperature (37 °C) | [44] | |
 | P(AN-co-AAm) | BaSO4 (To equip the hydrogel coils with radiopacity) | Temperature (20 to 40 °C) | [51] |
| P(AN-co-ACG) | Temperature/Chemical (37 °C and pH 6) | [60] | ||
 | PLU-DA and SA | - | Chemical (Ca2+) | [56] |
| (PLU-DA-co-AAm) | - | Chemical (Ethanol) | [64] | |
 | P(SS-DMAEMA-co-MPTC) | - | Chemical (pH) | [63] |
| P(SS-co-DEAEMA) | - | Chemical (pH) | [62] | |
 | PCL and PEG | DA-Diols (Act as a chain extender and initiate thermally driven retro Diels–Alder reaction) | Temperature (37 °C) | [50] |
| - | Liquid Ga and Zn metal (To enhance electrical conductivity and facilitate electricity induce shape deformability) | Electrical (2 V) | [84] |
| Type of SMHs | Fabrication Strategy for Development of SMHs | Applied Stimuli | Application of SMHs | Ref. |
|---|---|---|---|---|
| Thermally Responsive | Copolymer of P(AAm-co-PEA) physically stabilized by DMSO-mediated hydrophobic interactions | 37 °C | Reconfigurable Surgical Scaffold | [46] |
| Physically crosslinked dual networks of P(AAm) and ELP | 20 °C | Hydrogel Actuators | [47] | |
| Dual-network of PCL and PEG crosslinked by retro Diels–Alder reactions | 37 °C | Implantable Scaffold | [50] | |
| Copolymer of P(AN-co-AAm) physically crosslinked with BaSO4 | 40 °C | Reconfigurable Surgical scaffold | [51] | |
| Copolymer of P(AN-co-ACG) physically crosslinked by supramolecular hydrogen bonds | 37 °C | Implantable Scaffold | [60] | |
| Chemically Responsive | IPNs of PLU-DA and SA physically crosslinked by Ca+2 ions | Calcium Ions | Drug Delivery | [56] |
| IPNs of P(DA-co-AAm-co-AA) and alginate, physically crosslinked by Fe+3/COO- interactions | Ferric Ions | Hydrogel Actuators | [57] | |
| Copolymer of P(SS-co-DMAEMA-co-MPTC), physically crosslinked by polyionic interactions | pH | Soft Robotics | [63] | |
| Photo-responsive | Nanocomposite of P(NIPAM) containing Zr-Fc MOFs, which act as photothermal nanotransducers | 800 nm | Hydrogel Actuators | [70] |
| IPNs of P(NIPAM-AA), PPy and alginate, physically crosslinked by Fe+3/COO- interactions | 800 nm | Hydrogel Actuator | [71] | |
| Electrically Responsive | Dual networks of PVA and chitosan, containing CNTs to enhance electrical conductivity | 40 V | Hydrogel Actuator | [36] |
| PCL containing gallium and zinc metal to enhance electrical conductivity and responsivity | Electrical (2 V) | Hydrogel Actuator | [84] |
4. Biomedical Applications of Shape Memory Hydrogels
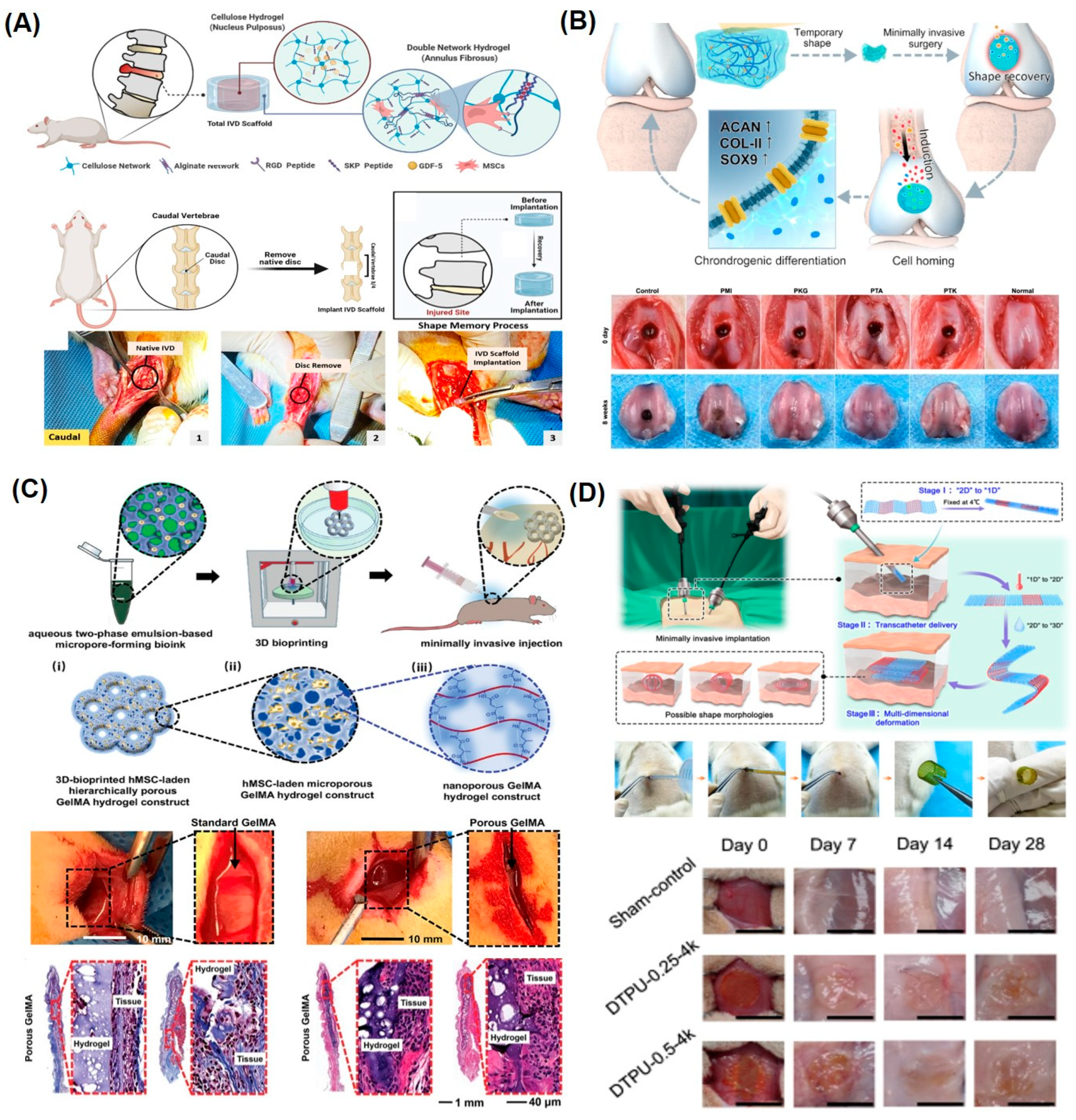
4.1. SMHs for Tissue Regeneration Therapies
4.2. SMHs for Surgical Applications
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xia, Y.; Liu, Y.; Leng, J. Nano/microstructures of shape memory polymers: From materials to applications. Nanoscale Horiz. 2020, 5, 1155–1173. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.R.; Grunlan, M.A. Smart scaffolds: Shape memory polymers (SMPs) in tissue engineering. J. Mater. Chem. B 2021, 9, 4287–4297. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Prabhakaran, M.P.; Parvin, N.; Ramakrishna, S. Thermally-induced two-way shape memory polymers: Mechanisms, structures, and applications. Chem. Eng. J. 2019, 374, 706–720. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Le, X.; Zhang, J.; Chen, T.; Theato, P. Trends in polymeric shape memory hydrogels and hydrogel actuators. Polym. Chem. 2019, 10, 1036–1055. [Google Scholar] [CrossRef]
- Wu, B.; Lu, H.; Le, X.; Lu, W.; Zhang, J.; Théato, P.; Chen, T. Recent progress in the shape deformation of polymeric hydrogels from memory to actuation. Chem. Sci. 2021, 12, 6472–6487. [Google Scholar] [CrossRef]
- Löwenberg, C.; Balk, M.; Wischke, C.; Behl, M.; Lendlein, A. Shape-Memory Hydrogels: Evolution of Structural Principles to Enable Shape Switching of Hydrophilic Polymer Networks. Acc. Chem. Res. 2017, 50, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Korde, J.M.; Kandasubramanian, B. Naturally biomimicked smart shape memory hydrogels for biomedical functions. Chem. Eng. J. 2020, 379, 122430. [Google Scholar] [CrossRef]
- Liang, R.; Wang, L.; Yu, H.; Khan, A.; Ul Amin, B.; Khan, R.U. Molecular design, synthesis and biomedical applications of stimuli-responsive shape memory hydrogels. Eur. Polym. J. 2019, 114, 380–396. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Intelligent hydrogels and their biomedical applications. Mater. Adv. 2022, 3, 7757–7772. [Google Scholar] [CrossRef]
- Ying, G.; Jiang, N.; Parra-Cantu, C.; Tang, G.; Zhang, J.; Wang, H.; Chen, S.; Huang, N.-P.; Xie, J.; Zhang, Y.S. Bioprinted Injectable Hierarchically Porous Gelatin Methacryloyl Hydrogel Constructs with Shape-Memory Properties. Adv. Funct. Mater. 2020, 30, 2003740. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, Y.; Gao, F.; Zhai, X.; Sun, M.; Lu, W.; Cao, Z.; Liu, W. High Strength Multifunctional Multiwalled Hydrogel Tubes: Ion-Triggered Shape Memory, Antibacterial, and Anti-inflammatory Efficacies. ACS Appl. Mater. Interfaces 2015, 7, 16865–16872. [Google Scholar] [CrossRef] [PubMed]
- Gavel, P.K.; Dev, D.; Parmar, H.S.; Bhasin, S.; Das, A.K. Investigations of Peptide-Based Biocompatible Injectable Shape-Memory Hydrogels: Differential Biological Effects on Bacterial and Human Blood Cells. ACS Appl. Mater. Interfaces 2018, 10, 10729–10740. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Feng, Q.; Xu, B.; Bian, L.; Shi, P. Injectable Nanoreinforced Shape-Memory Hydrogel System for Regenerating Spinal Cord Tissue from Traumatic Injury. ACS Appl. Mater. Interfaces 2018, 10, 29299–29307. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zheng, S.; Liu, Z.; Ng, T.Y. Recent advances of the constitutive models of smart materials-Hydrogels and shape memory polymers. Int. J. Appl. Mech. 2020, 12, 2050014. [Google Scholar] [CrossRef]
- Delaey, J.; Dubruel, P.; Van Vlierberghe, S. Shape-memory polymers for biomedical applications. Adv. Funct. Mater. 2020, 30, 1909047. [Google Scholar] [CrossRef]
- Ganeson, K.; Tan Xue May, C.; Abdullah, A.A.A.; Ramakrishna, S.; Vigneswari, S. Advantages and prospective implications of smart materials in tissue engineering: Piezoelectric, shape memory, and hydrogels. Pharmaceutics 2023, 15, 2356. [Google Scholar] [CrossRef] [PubMed]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Zhou, J.; Sheiko, S.S. Reversible shape-shifting in polymeric materials. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1365–1380. [Google Scholar] [CrossRef]
- Lendlein, A.; Balk, M.; Tarazona, N.A.; Gould, O.E.C. Bioperspectives for Shape-Memory Polymers as Shape Programmable, Active Materials. Biomacromolecules 2019, 20, 3627–3640. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.M.; Ayres, N. Dynamic covalent bonds in self-healing, shape memory, and controllable stiffness hydrogels. Polym. Chem. 2020, 11, 1410–1423. [Google Scholar] [CrossRef]
- Lendlein, A.; Gould, O.E.C. Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Chen, G.; Dong, J.; Xu, X.; Zou, W.; Jin, B.; Peng, W.; Zhao, Q.; Xie, T.; Zheng, N. Converse two-way shape memory effect through a dynamic covalent network design. J. Mater. Chem. 2022, 10, 10350–10354. [Google Scholar] [CrossRef]
- Lewis, C.L.; Dell, E.M. A review of shape memory polymers bearing reversible binding groups. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1340–1364. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: A molecular platform for designing functions beyond chemical recycling and self-healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef]
- Kirillova, A.; Ionov, L. Shape-changing polymers for biomedical applications. J. Mater. Chem. B 2019, 7, 1597–1624. [Google Scholar] [CrossRef] [PubMed]
- Zende, R.; Ghase, V.; Jamdar, V. A review on shape memory polymers. Polym.-Plast. Technol. Mater. 2023, 62, 467–485. [Google Scholar] [CrossRef]
- Maiti, B.; Abramov, A.; Franco, L.; Puiggalí, J.; Enshaei, H.; Alemán, C.; Díaz, D.D. Thermoresponsive Shape-Memory Hydrogel Actuators Made by Phototriggered Click Chemistry. Adv. Funct. Mater. 2020, 30, 2001683. [Google Scholar] [CrossRef]
- Davidson-Rozenfeld, G.; Stricker, L.; Simke, J.; Fadeev, M.; Vázquez-González, M.; Ravoo, B.J.; Willner, I. Light-responsive arylazopyrazole-based hydrogels: Their applications as shape-memory materials, self-healing matrices and controlled drug release systems. Polym. Chem. 2019, 10, 4106–4115. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, J.; Chen, X. A Thermal-, Water-, and Near-Infrared Light-Induced Shape Memory Composite Based on Polyvinyl Alcohol and Polyaniline Fibers. ACS Appl. Mater. Interfaces 2018, 10, 14017–14025. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, C.; Julich-Gruner, K.K.; Neffe, A.T.; Behl, M.; Lendlein, A. Salt-Induced Shape-Memory Effect in Gelatin-Based Hydrogels. Biomacromolecules 2020, 21, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Manna, K.; Ray, P.G.; Dhara, S.; Pal, S. β-Cyclodextrin-Based Ultrahigh Stretchable, Flexible, Electro- and Pressure-Responsive, Adhesive, Transparent Hydrogel as Motion Sensor. ACS Appl. Mater. Interfaces 2022, 14, 17065–17080. [Google Scholar] [CrossRef] [PubMed]
- Pirahmadi, P.; Kokabi, M.; Alamdarnejad, G. Polyvinyl alcohol/chitosan/carbon nanotubes electroactive shape memory nanocomposite hydrogels. J. Appl. Polym. Sci. 2021, 138, 49995. [Google Scholar] [CrossRef]
- Tang, J.; Yin, Q.; Qiao, Y.; Wang, T. Shape Morphing of Hydrogels in Alternating Magnetic Field. ACS Appl. Mater. Interfaces 2019, 11, 21194–21200. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gao, X.; Wu, D.; Guo, Q. Advances in physiologically relevant actuation of shape memory polymers for biomedical applications. Polym. Rev. 2021, 61, 280–318. [Google Scholar] [CrossRef]
- Zhu, C.N.; Bai, T.; Wang, H.; Ling, J.; Huang, F.; Hong, W.; Zheng, Q.; Wu, Z.L. Dual-Encryption in a Shape-Memory Hydrogel with Tunable Fluorescence and Reconfigurable Architecture. Adv. Mater. 2021, 33, 2102023. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, M.; Jia, F.; Ren, X.; Gao, G. Thermo-responsive shape memory sensors based on tough, remolding and anti-freezing hydrogels. J. Mater. Chem. C 2020, 8, 2326–2335. [Google Scholar] [CrossRef]
- Tartivel, L.; Blocki, A.M.; Braune, S.; Jung, F.; Behl, M.; Lendlein, A. An Inverse Shape-Memory Hydrogel Scaffold Switching Upon Cooling in a Tissue-Tolerated Temperature Range. Adv. Mater. Interfaces 2022, 9, 2101588. [Google Scholar] [CrossRef]
- Jiao, C.; Chen, Y.; Liu, T.; Peng, X.; Zhao, Y.; Zhang, J.; Wu, Y.; Wang, H. Rigid and Strong Thermoresponsive Shape Memory Hydrogels Transformed from Poly(vinylpyrrolidone-co-acryloxy acetophenone) Organogels. ACS Appl. Mater. Interfaces 2018, 10, 32707–32716. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Z.; Jiang, S.; Yan, B.; Shi, X.; Xie, Y.; Huang, X.; Yu, Z.; Liu, H.; Weng, S.; et al. A shape-memory and spiral light-emitting device for precise multisite stimulation of nerve bundles. Nat. Commun. 2019, 10, 2790. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.S.; Costa, P.D.C.; Gomes, M.C.; Chandrakar, A.; Wieringa, P.A.; Moroni, L.; Mano, J.F. Universal Strategy for Designing Shape Memory Hydrogels. ACS Mater. Lett. 2022, 4, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Chen, X.; Cui, C.; Zhang, J.; Zhang, Q.; Zhang, Y.; Wang, S.; et al. H-Bonding Supramolecular Hydrogels with Promising Mechanical Strength and Shape Memory Properties for Postoperative Antiadhesion Application. ACS Appl. Mater. Interfaces 2020, 12, 34161–34169. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Yu, H.; Wang, L.; Lin, L.; Wang, N.; Naveed, K.-u.-R. Highly Tough Hydrogels with the Body Temperature-Responsive Shape Memory Effect. ACS Appl. Mater. Interfaces 2019, 11, 43563–43572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Desai, M.S.; Wang, T.; Lee, S.-W. Elastin-Based Thermoresponsive Shape-Memory Hydrogels. Biomacromolecules 2020, 21, 1149–1156. [Google Scholar] [CrossRef]
- Beaman, H.T.; Howes, B.; Ganesh, P.; Monroe, M.B.B. Shape memory polymer hydrogels with cell-responsive degradation mechanisms for Crohn’s fistula closure. J. Biomed. Mater. Res. Part A 2022, 110, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shin, H.; Park, J.; Lee, S. Advanced neural interface toward bioelectronic medicine enabled by micro-patterned shape memory polymer. Micromachines 2021, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, H.; Meng, F.; Xu, Z.; Hao, L.; Yao, Y.; Zhu, H.; Wang, C.; Wu, J.; Bian, S.; et al. 4D printed hydrogel scaffold with swelling-stiffening properties and programmable deformation for minimally invasive implantation. Nat. Commun. 2024, 15, 1587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, H.; Wang, H.; Xu, Z.; Chen, X.; Liu, B.; Shi, Y.; Lu, Y.; Wen, L.; Li, Y.; et al. Radiopaque Highly Stiff and Tough Shape Memory Hydrogel Microcoils for Permanent Embolization of Arteries. Adv. Funct. Mater. 2018, 28, 1705962. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Wang, S.; Zhang, Y.; Yang, A.; Cheng, Y.; Chen, X. Ultra-durable cell-free bioactive hydrogel with fast shape memory and on-demand drug release for cartilage regeneration. Nat. Commun. 2023, 14, 7771. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Chen, D.; Yin, Y.; Wen, X.; Chen, X.; Yang, C.; Chen, G.; Sun, Z.; Wen, J.; Jiao, Y.; et al. Shape memory polymer with programmable recovery onset. Nature 2023, 622, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Shiblee, M.N.I.; Ahmed, K.; Kawakami, M.; Furukawa, H. 4D Printing of Shape-Memory Hydrogels for Soft-Robotic Functions. Adv. Mater. Technol. 2019, 4, 1900071. [Google Scholar] [CrossRef]
- Chen, Q.; Li, W.; Wei, Y.; Ji, Y. Reprogrammable 3D Liquid-Crystalline Actuators with Precisely Controllable Stepwise Actuation. Adv. Intell. Syst. 2021, 3, 2000249. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Zhang, J.; Wu, J.P.; Kirk, T.B.; Xu, J.; Ma, D.; Xue, W. Three-dimensional printing of shape memory hydrogels with internal structure for drug delivery. Mater. Sci. Eng. C 2018, 84, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liu, C.; Liu, C.; Zong, L.; Gu, H.; Wang, C.; Jian, X. Self-healing, pH-sensitive and shape memory hydrogels based on acylhydrazone and hydrogen bonds. Eur. Polym. J. 2022, 162, 110838. [Google Scholar] [CrossRef]
- Zhuo, J.; Wu, B.; Zhang, J.; Peng, Y.; Lu, H.; Le, X.; Wei, S.; Chen, T. Supramolecular Assembly of Shape Memory and Actuating Hydrogels for Programmable Shape Transformation. ACS Appl. Mater. Interfaces 2022, 14, 3551–3558. [Google Scholar] [CrossRef]
- Cai, S.; Niu, B.; Ma, X.; Wan, S.; He, X. High strength, recyclable, anti-swelling and shape-memory hydrogels based on crystal microphase crosslinking and their application as flexible sensor. Chem. Eng. J. 2022, 430, 132957. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Z.; Gao, H.; Fan, C.; Ma, G.; Zhang, D.; Xiao, M.; Zhang, B.; Yang, Y.; Cui, C.; et al. Stiffness Self-Tuned Shape Memory Hydrogels for Embolization of Aneurysms. Adv. Funct. Mater. 2020, 30, 1910197. [Google Scholar] [CrossRef]
- Cui, Y.; Li, D.; Gong, C.; Chang, C. Bioinspired Shape Memory Hydrogel Artificial Muscles Driven by Solvents. ACS Nano 2021, 15, 13712–13720. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, Y.; Wang, T.; Sun, W.; Tong, Z. pH Responsive Strong Polyion Complex Shape Memory Hydrogel with Spontaneous Shape Changing and Information Encryption. Macromol. Rapid Commun. 2021, 42, 2000747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Q.; Yang, S.; Wang, T.; Sun, W.; Tong, Z. Unique Self-Reinforcing and Rapid Self-Healing Polyampholyte Hydrogels with a pH-Induced Shape Memory Effect. Macromolecules 2021, 54, 5218–5228. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Wen, J.; Liu, J.; Zhang, D.; Li, J.; Chu, H. Ultra-Stretchable, Variable Modulus, Shape Memory Multi-Purpose Low Hysteresis Hydrogel Derived from Solvent-Induced Dynamic Micelle Sea-Island Structure. Adv. Funct. Mater. 2021, 31, 2011259. [Google Scholar] [CrossRef]
- Cera, L.; Gonzalez, G.M.; Liu, Q.; Choi, S.; Chantre, C.O.; Lee, J.; Gabardi, R.; Choi, M.C.; Shin, K.; Parker, K.K. A bioinspired and hierarchically structured shape-memory material. Nat. Mater. 2021, 20, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, H.; Yang, X.; Tian, J.; Luo, B.; Liu, M. Halloysite nanotubes@polydopamine reinforced polyacrylamide-gelatin hydrogels with NIR light triggered shape memory and self-healing capability. Compos. Sci. Technol. 2020, 191, 108071. [Google Scholar] [CrossRef]
- Dai, S.; Yue, S.; Ning, Z.; Jiang, N.; Gan, Z. Polydopamine Nanoparticle-Reinforced Near-Infrared Light-Triggered Shape Memory Polycaprolactone–Polydopamine Polyurethane for Biomedical Implant Applications. ACS Appl. Mater. Interfaces 2022, 14, 14668–14676. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhu, Y.; Wang, J.-H.; Li, X.-N.; Wu, X.-G.; Qin, Y.-X.; Chen, W.-Y. Fe3+, NIR light and thermal responsive triple network composite hydrogel with multi-shape memory effect. Compos. Sci. Technol. 2021, 206, 108653. [Google Scholar] [CrossRef]
- Dai, W.; Guo, H.; Gao, B.; Ruan, M.; Xu, L.; Wu, J.; Brett Kirk, T.; Xu, J.; Ma, D.; Xue, W. Double network shape memory hydrogels activated by near-infrared with high mechanical toughness, nontoxicity, and 3D printability. Chem. Eng. J. 2019, 356, 934–949. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, P.; Yang, X.; Valenzuela, C.; Chen, Y.; Lv, P.; Wang, Z.; Wang, L.; Xu, X. Near-Infrared Light-Driven Shape-Programmable Hydrogel Actuators Loaded with Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2022, 14, 11834–11841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, J.; Liu, Z.; Liu, Z.; Li, G. Thermal and Near-Infrared Light-Responsive Hydrogel Actuators with Spatiotemporally Developed Polypyrrole Patterns. ACS Appl. Mater. Interfaces 2024, 16, 9286–9292. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, P.; Fang, S.; Li, H.; Yu, Y.; Liu, Y.; Zhang, H.; Guo, J. Near-infrared light-responsive shape memory hydrogels with remolding and excellent mechanical performance. New J. Chem. 2022, 46, 7223–7229. [Google Scholar] [CrossRef]
- Li, G.; Gao, T.; Fan, G.; Liu, Z.; Liu, Z.; Jiang, J.; Zhao, Y. Photoresponsive Shape Memory Hydrogels for Complex Deformation and Solvent-Driven Actuation. ACS Appl. Mater. Interfaces 2020, 12, 6407–6418. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, M.; Wu, S.; Lian, Q.; Wang, W.; Adlam, D.; Hoyland, J.A.; Saunders, B.R. Programmed Multiresponsive Hydrogel Assemblies with Light-Tunable Mechanical Properties, Actuation, and Fluorescence. Adv. Funct. Mater. 2020, 30, 1909359. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Fadeev, M.; Li, Z.; Wulf, V.; Tian, H.; Willner, I. Chemical and photochemical DNA “gears” reversibly control stiffness, shape-memory, self-healing and controlled release properties of polyacrylamide hydrogels. Chem. Sci. 2019, 10, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 2023, 14, 759. [Google Scholar] [CrossRef]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Qiao, L.; Qiao, B.; Guo, B. Conductive hydrogels for tissue repair. Chem. Sci. 2023, 14, 3091–3116. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yu, R.; Guo, B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- González-Jiménez, A.; Bernal-Ortega, P.; Salamanca, F.M.; Valentin, J.L. Shape-Memory Composites Based on Ionic Elastomers. Polymers 2022, 14, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-x.; Wei, X.; Yang, J.-h.; Zhang, N.; Huang, T.; Wang, Y.; Gao, X.-l. Triple-Shape Memory Materials Based on Cross-Linked Poly(ethylene vinyl acetate) and Poly(ε-caprolactone). Ind. Eng. Chem. Res. 2016, 55, 12232–12241. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Q.; Wu, R.; Meng, Z.; Patil, A.; Yu, R.; Yang, Y.; Zhu, S.; Fan, X.; Hou, C.; et al. From Molecular Reconstruction of Mesoscopic Functional Conductive Silk Fibrous Materials to Remote Respiration Monitoring. Small 2020, 16, 2000203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Ji, Y.-E.; Wang, T.; Fu, Y.; Li, X.; Li, G.; Zheng, T.; Wu, L.; Han, Q. Silk-protein-based gradient hydrogels with multimode reprogrammable shape changes for biointegrated devices. Proc. Natl. Acad. Sci. USA 2023, 120, 2305704120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, J.; Wu, P.; Zhang, J. Liquid metal based electrical driven shape memory polymers. Polymer 2021, 212, 123174. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Zarrintaj, P.; Jafari, S.H.; Hosseini, S.M.; Aliakbari, S.; Pourbadie, H.G.; Naderi, N.; Zibaii, M.I.; Gholizadeh, S.S.; Ramsey, J.D.; et al. Fabricating an electroactive injectable hydrogel based on pluronic-chitosan/aniline-pentamer containing angiogenic factor for functional repair of the hippocampus ischemia rat model. Mater. Sci. Eng. C 2020, 117, 111328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, J.; Liu, W.; Liu, W.; Qiu, X. Synthesis of strong and highly stretchable, electrically conductive hydrogel with multiple stimuli responsive shape memory behavior. Polymer 2020, 188, 122147. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhang, W.; Deng, J.; Nie, Y.; Du, X.; Qin, L.; Lai, Y. 3D-printed NIR-responsive shape memory polyurethane/magnesium scaffolds with tight-contact for robust bone regeneration. Bioact. Mater. 2022, 16, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Jungmann, M.A.; Recalde Phillips, S.; Touchet, T.J.; Brinson, B.; Parish, K.; Petersen, C.; Hasan, S.M.; Nash, L.D.; Maitland, D.J.; Alge, D.L. Swellable and Thermally Responsive Hydrogel/Shape Memory Polymer Foam Composites for Sealing Lung Biopsy Tracts. ACS Biomater. Sci. Eng. 2023, 9, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater. 2020, 32, 1806538. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed]
- Ramaraju, H.; Akman, R.E.; Safranski, D.L.; Hollister, S.J. Designing biodegradable shape memory polymers for tissue repair. Adv. Funct. Mater. 2020, 30, 2002014. [Google Scholar] [CrossRef]
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. Biodegradable shape memory polymers in medicine. Adv. Healthc. Mater. 2017, 6, 1700694. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-Y.; Wang, C.-C.; Wu, T.-C.; Kuan, C.-H.; Liu, Y.-C.; Wang, T.-W. Peptide-functionalized double network hydrogel with compressible shape memory effect for intervertebral disc regeneration. Bioeng. Transl. Med. 2023, 8, e10447. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.W.; Lim, C.S.; Darai, A.; Lee, J.; Kim, W.; Han, I.; Kim, G.H. Shape-memory collagen scaffold combined with hyaluronic acid for repairing intervertebral disc. Biomater. Res. 2023, 27, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Zhu, S.; Wei, X.; Zhou, N.; Liao, X.; Peng, Y.; Tang, Y.; Zhang, L.; Yang, X.; et al. Cryoprinting of nanoparticle-enhanced injectable hydrogel with shape-memory properties. Mater. Des. 2022, 223, 111120. [Google Scholar] [CrossRef]
- Sokolowski, W.; Metcalfe, A.; Hayashi, S.; Raymond, J. Medical applications of shape memory polymers. Biomed. Mater. 2007, 2, S23. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrukh, A.; Nayab, S. Shape Memory Hydrogels for Biomedical Applications. Gels 2024, 10, 270. https://doi.org/10.3390/gels10040270
Farrukh A, Nayab S. Shape Memory Hydrogels for Biomedical Applications. Gels. 2024; 10(4):270. https://doi.org/10.3390/gels10040270
Chicago/Turabian StyleFarrukh, Aleeza, and Sana Nayab. 2024. "Shape Memory Hydrogels for Biomedical Applications" Gels 10, no. 4: 270. https://doi.org/10.3390/gels10040270
APA StyleFarrukh, A., & Nayab, S. (2024). Shape Memory Hydrogels for Biomedical Applications. Gels, 10(4), 270. https://doi.org/10.3390/gels10040270