The Injection of Gels Through an Intact Annulus Maintains Biomechanical Performance without Extrusion Risk
Abstract
1. Introduction
2. Results and Discussion
2.1. Injection Volume
2.2. Height Change
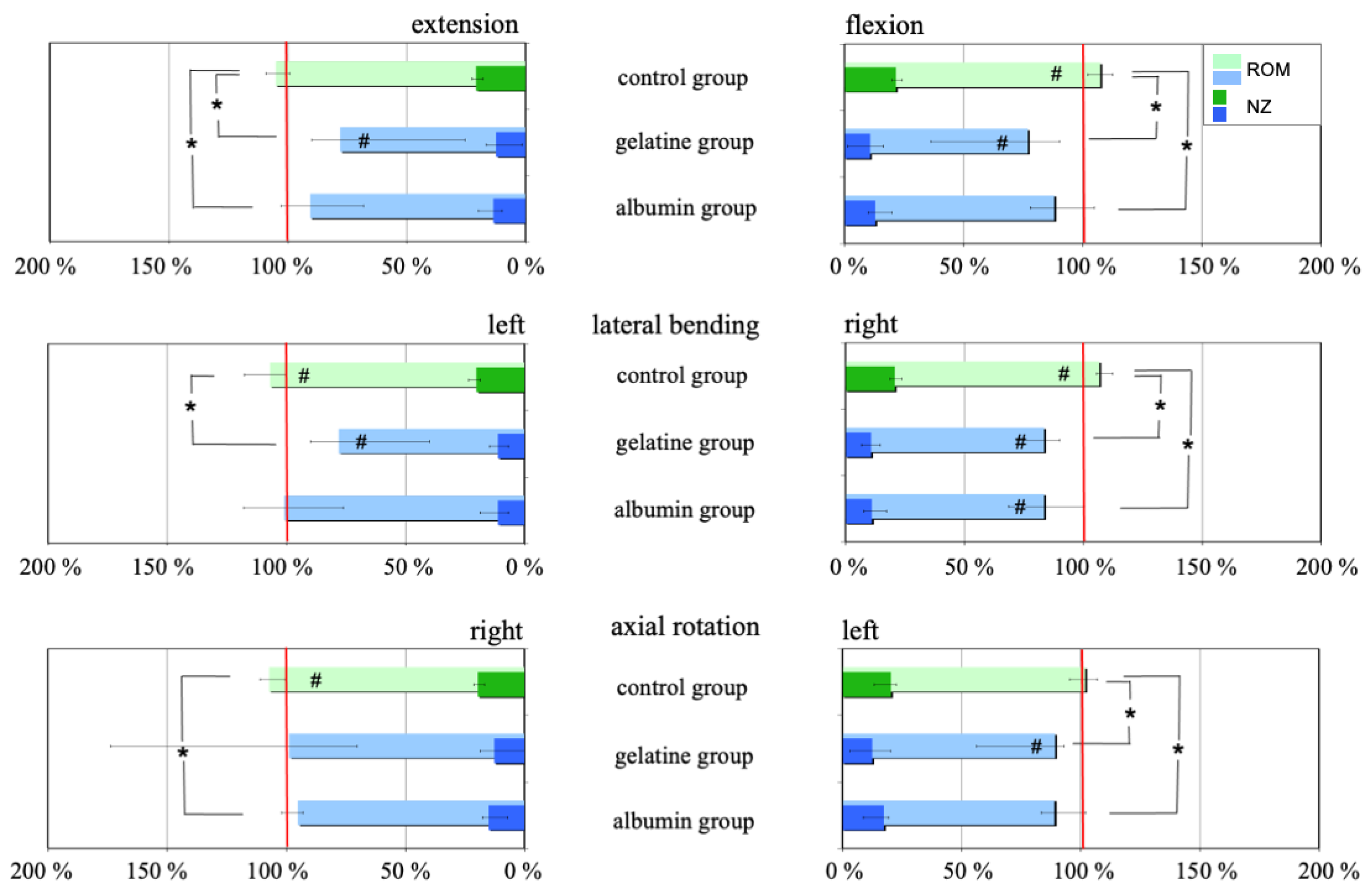
2.3. Range of Motion
2.4. Extrusion Risk
2.5. Macroscopic Examination
2.6. Discussion
3. Conclusions
4. Materials and Methods
4.1. Implants
4.2. Specimens and Preparation
4.3. Treatments
4.4. Height Measurements
4.5. Flexibility Tests
4.6. Dynamic Cyclic Loading
4.7. Macroscopic Inspection
4.8. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balkovec, C.; Vernengo, J.; McGill, S.M. The use of a novel injectable hydrogel nucleus pulposus replacement in restoring the mechanical properties of cyclically fatigued porcine intervertebral discs. J. Biomech. Eng. 2013, 135, 61004–61005. [Google Scholar] [CrossRef] [PubMed]
- Berlemann, U.; Schwarzenbach, O. An injectable nucleus replacement as an adjunct to microdiscectomy: 2 year follow-up in a pilot clinical study. Eur. Spine J. 2009, 18, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, A.A.; Knecht, S.; Baumgartner, D.; Gerber, H.; Endres, M.; Kaps, C.; Stussi, E.; Thome, C. Biomechanical testing of a polymer-based biomaterial for the restoration of spinal stability after nucleotomy. J. Orthop. Surg. Res. 2009, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.R.; Han, W.M.; Beckstein, J.; Cloyd, J.; Chen, W.; Elliott, D.M. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine (Phila Pa 1976) 2012, 37, E1099–E1105. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.J.; Ganey, T.; Hutton, W.C.; Libera, J.; Minkus, Y.; Alasevic, O. Clinical experience in cell-based therapeutics: Intervention and outcome. Eur. Spine J. 2006, 15 (Suppl. S3), S397–S405. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.J.; Siodla, V.; Ganey, T.; Minkus, Y.; Hutton, W.C.; Alasevic, O.J. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol. Eng. 2007, 24, 5–21. [Google Scholar] [CrossRef]
- Ruan, D.K.; Xin, H.; Zhang, C.; Wang, C.; Xu, C.; Li, C.; He, Q. Experimental intervertebral disc regeneration with tissue-engineered composite in a canine model. Tissue Eng. Part A 2010, 16, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.S.; Roberts, S.; Urban, J.P.; Menage, J.; Bramhill, J.; Campbell, D.; Franklin, V.J.; Lydon, F.; Merkher, Y.; Maroudas, A.; et al. Injectable hydrogels with high fixed charge density and swelling pressure for nucleus pulposus repair: Biomimetic glycosaminoglycan analogues. Acta Biomater. 2014, 10, 1124–1133. [Google Scholar] [CrossRef]
- Varma, D.M.; Lin, H.A.; Long, R.G.; Gold, G.T.; Hecht, A.C.; Iatridis, J.C.; Nicoll, S.B. Thermoresponsive, redox-polymerized cellulosic hydrogels undergo in situ gelation and restore intervertebral disc biomechanics post discectomy. Eur. Cell Mater. 2018, 35, 300–317. [Google Scholar] [CrossRef]
- Wilke, H.J.; Heuer, F.; Neidlinger-Wilke, C.; Claes, L. Is a collagen scaffold for a tissue engineered nucleus replacement capable of restoring disc height and stability in an animal model? Eur. Spine J. 2006, 15 (Suppl. S3), S433–S438. [Google Scholar] [CrossRef]
- Reitmaier, S.; Kreja, L.; Gruchenberg, K.; Kanter, B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Perugini, V.; Santin, M.; Ignatius, A.; et al. In vivo biofunctional evaluation of hydrogels for disc regeneration. Eur. Spine J. 2014, 23, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.C.; Salzer, E.; Crispim, J.F.; Fabra, G.T.; LeVisage, C.; Pandit, A.; Tryfonidou, M.; Maitre, C.L.; Ito, K. Characterization of biomaterials intended for use in the nucleus pulposus of degenerated intervertebral discs. Acta Biomater. 2020, 114, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mern, D.S.; Walsen, T.; Beierfuss, A.; Thome, C. Animal models of regenerative medicine for biological treatment approaches of degenerative disc diseases. Exp. Biol. Med. (Maywood) 2021, 246, 483–512. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.J.; Agarwal, N.; Hsieh, P.C.; Skelly, A.; Park, J.B.; Brodke, D.; Wang, J.C.; Yoon, S.T.; Buser, Z. Cell Therapy for Treatment of Intervertebral Disc Degeneration: A Systematic Review. Glob. Spine J. 2019, 9, 39S–52S. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.W.; Bertram, H.; Kleinschmidt, K.; Fischer, J.; Brohm, K.; Guehring, T.; Anton, M.; Richter, W. Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs. Eur. Spine J. 2010, 19, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.; Kroeber, M.; Wang, H.; Unglaub, F.; Guehring, T.; Carstens, C.; Richter, W. Matrix-assisted cell transfer for intervertebral disc cell therapy. Biochem. Biophys. Res. Commun. 2005, 331, 1185–1192. [Google Scholar] [CrossRef]
- Loibl, M.; Wuertz-Kozak, K.; Vadala, G.; Lang, S.; Fairbank, J.; Urban, J.P. Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine 2019, 2, e1043. [Google Scholar] [CrossRef]
- Buckley, C.T.; Hoyland, J.A.; Fujii, K.; Pandit, A.; Iatridis, J.C.; Grad, S. Critical aspects and challenges for intervertebral disc repair and regeneration-Harnessing advances in tissue engineering. JOR Spine 2018, 1, e1029. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J. Tissue Eng. Regen. Med. 2013, 7, 85–98. [Google Scholar] [CrossRef]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef]
- Reza, A.T.; Nicoll, S.B. Characterization of novel photocrosslinked carboxymethylcellulose hydrogels for encapsulation of nucleus pulposus cells. Acta Biomater. 2010, 6, 179–186. [Google Scholar] [CrossRef]
- Yan, C.; Wang, X.; Xiang, C.; Wang, Y.; Pu, C.; Chen, L.; Jiang, K.; Li, Y. Applications of Functionalized Hydrogels in the Regeneration of the Intervertebral Disc. Biomed. Res. Int. 2021, 2021, 2818624. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356. [Google Scholar] [CrossRef]
- Peroglio, M.; Eglin, D.; Benneker, L.M.; Alini, M.; Grad, S. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J. 2013, 13, 1627–1639. [Google Scholar] [CrossRef]
- Peroglio, M.; Grad, S.; Mortisen, D.; Sprecher, C.M.; Illien-Junger, S.; Alini, M.; Eglin, D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur. Spine J. 2012, 21 (Suppl. S6), S839–S849. [Google Scholar] [CrossRef]
- Benz, K.; Stippich, C.; Osswald, C.; Gaissmaier, C.; Lembert, N.; Badke, A.; Steck, E.; Aicher, W.K.; Mollenhauer, J.A. Rheological and biological properties of a hydrogel support for cells intended for intervertebral disc repair. BMC Musculoskelet. Disord. 2012, 13, 54. [Google Scholar] [CrossRef]
- Omlor, G.W.; Fischer, J.; Kleinschmitt, K.; Benz, K.; Holschbach, J.; Brohm, K.; Anton, M.; Guehring, T.; Richter, W. Short-term follow-up of disc cell therapy in a porcine nucleotomy model with an albumin-hyaluronan hydrogel: In vivo and in vitro results of metabolic disc cell activity and implant distribution. Eur. Spine J. 2014, 23, 1837–1847. [Google Scholar] [CrossRef]
- Pimenta, L.; Marchi, L.; Coutinho, E.; Oliveira, L. Lessons Learned After 9 Years’ Clinical Experience with 3 Different Nucleus Replacement Devices. Semin. Spine Surg. 2012, 24, 43–47. [Google Scholar] [CrossRef]
- Klara, P.M.; Ray, C.D. Artificial nucleus replacement: Clinical experience. Spine (Phila Pa 1976) 2002, 27, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Vaccaro, A.R.; Lee, J.Y.; Denaro, V.; Lim, M.R. Nucleus pulposus replacement: Basic science and indications for clinical use. Spine (Phila Pa 1976) 2005, 30, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.B.; McCullen, G.M.; Higham, P.A.; Dumbleton, J.H.; Yuan, H.A. The artificial disc: Theory, design and materials. Biomaterials 1996, 17, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Zengerle, L.; Kohler, A.; Debout, E.; Hackenbroch, C.; Wilke, H.J. Nucleus replacement could get a new chance with annulus closure. Eur. Spine J. 2020, 29, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Coleman, P.J.; Mason, R.M.; Levick, J.R. Concentration dependence of interstitial flow buffering by hyaluronan in synovial joints. Microvasc. Res. 2000, 59, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.; Scott, J.E. Hyaluronan-mediated protective effect against cell damage caused by enzymatically produced hydroxyl (OH.) radicals is dependent on hyaluronan molecular mass. Cell Biochem. Funct. 1994, 12, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Hanus, M.; Belickas, J.; Laszlo, T.; Gudas, R.; Fiodorovas, M.; Cebatorius, A.; Pastucha, M.; Hoza, P.; Magos, K.; et al. Treatment of Large Cartilage Defects in the Knee by Hydrogel-Based Autologous Chondrocyte Implantation: Two-Year Results of a Prospective, Multicenter, Single-Arm Phase III Trial. Cartilage 2022, 13, 19476035221085146. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Mehnert, U.; Claes, L.E.; Bierschneider, M.M.; Jaksche, H.; Boszczyk, B.M. Biomechanical evaluation of vertebroplasty and kyphoplasty with polymethyl methacrylate or calcium phosphate cement under cyclic loading. Spine (Phila Pa 1976) 2006, 31, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Krischak, S.T.; Wenger, K.H.; Claes, L.E. Load-displacement properties of the thoracolumbar calf spine: Experimental results and comparison to known human data. Eur. Spine J. 1997, 6, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, P.C.; Kostuik, J.P.; D’Angelo, G.; Fernie, G.R.; Maki, B.E. An anatomical comparison of the human and bovine thoracolumbar spine. J. Orthop. Res. 1986, 4, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A. Mechanical testing of the spine. An appraisal of methodology, results, and conclusions. Spine (Phila Pa 1976) 1995, 20, 2151–2156. [Google Scholar] [CrossRef]
- Rohlmann, A.; Bergmann, G.; Graichen, F. Loads on an internal spinal fixation device during walking. J. Biomech. 1997, 30, 41–47. [Google Scholar] [CrossRef]
- Rohlmann, A.; Bergmann, G.; Graichen, F. Loads on internal spinal fixators measured in different body positions. Eur. Spine J. 1999, 8, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, A.; Graichen, F.; Weber, U.; Bergmann, G. 2000 Volvo Award winner in biomechanical studies: Monitoring in vivo implant loads with a telemeterized internal spinal fixation device. Spine (Phila Pa 1976) 2000, 25, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Ressel, L.; Heuer, F.; Graf, N.; Rath, S. Can prevention of a reherniation be investigated? Establishment of a herniation model and experiments with an anular closure device. Spine (Phila Pa 1976) 2013, 38, E587–E593. [Google Scholar] [CrossRef] [PubMed]
- Heuer, F.; Ulrich, S.; Claes, L.; Wilke, H.J. Biomechanical evaluation of conventional anulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J. Neurosurg. Spine 2008, 9, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Jungkunz, B.; Wenger, K.; Claes, L.E. Spinal segment range of motion as a function of in vitro test conditions: Effects of exposure period, accumulated cycles, angular-deformation rate, and moisture condition. Anat. Rec. 1998, 251, 15–19. [Google Scholar] [CrossRef]
- Comper, W.D.; Zamparo, O. Hydrodynamic properties of connective-tissue polysaccharides. Biochem. J. 1990, 269, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Benz, K.; Ahlers, M.; Gaissmaier, C.; Mollenhauer, J. Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transpl. 2011, 20, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Claes, L.; Schmitt, H.; Wolf, S. A universal spine tester for in vitro experiments with muscle force simulation. Eur. Spine J. 1994, 3, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Rohlmann, A.; Neller, S.; Schultheiss, M.; Bergmann, G.; Graichen, F.; Claes, L.E. Is it possible to simulate physiologic loading conditions by applying pure moments? A comparison of in vivo and in vitro load components in an internal fixator. Spine (Phila Pa 1976) 2001, 26, 636–642. [Google Scholar] [CrossRef]
- Wilke, H.J.; Wenger, K.; Claes, L. Testing criteria for spinal implants: Recommendations for the standardization of in vitro stability testing of spinal implants. Eur. Spine J. 1998, 7, 148–154. [Google Scholar] [CrossRef]



| Flexion/ Extension | Intact | After Treatment | After 20,000 c. | After 40,000 c. | After 60,000 c. | After 100,000 c. |
|---|---|---|---|---|---|---|
| Control | 5.7 (4.7–11.2) | 6.2 (4.8–11.7) | 8.1 (6.9–13.6) | 8.7 (7.5–14.1) | 9.6 (7.8–14.5) | 10.0 (8.6–14.5) |
| Albumin | 6.4 (5.7–15.0) | 5.8 (5.1–11.0) | 8.1 (6.6–14.9) | 9.4 (7.5–16.2) | 9.8 (7.9–17.6) | 10.6 (8.6–18.5) |
| Gelatine | 6.1 (5.1–16.2) | 4.9 (3.9–7.4) | 6.9 (5.0–11.3) | 7.9 (6.3–12.5) | 8.4 (6.2–13.8) | 9.5 (6.4–14.8) |
| Lateral bending | Intact | After treatment | After 20,000 c. | After 40,000 c. | After 60,000 c. | After 100,000 c. |
| Control | 11.2 (6.8–12.1) | 12.0 (7.3–12.9) | 14.4 (9.3–17.4) | 15.2 (9.7–18.0) | 15.7 (9.8–18.6) | 16.0 (10.5–19.6) |
| Albumin | 9.8 (7.1–12.0) | 8.5 (6.2–12.5) | 10.8 (9.2–16.1) | 12.1 (10.1–17.7) | 12.9 (10.6–18.7) | 13.4 (10.9–20.5) |
| Gelatine | 10.0 (7.8–17.4) | 8.4 (6.3–10.4) | 11.4 (8.1–13.6) | 12.7 (9.4–14.8) | 13.3 (10.0–15.5) | 14.2 (10.6–17.2) |
| Axial rotation | Intact | After treatment | After 20,000 c. | After 40,000 c. | After 60,000 c. | After 100,000 c. |
| Control | 4.1 (3.4–5.3) | 4.2 (3.6–5.5) | 5.4 (4.1–6.3) | 5.5 (4.1–6.7) | 5.5 (4.2–7.1) | 5.9 (4.4–7.1) |
| Albumin | 4.3 (3.4–6.5) | 4.2 (3.0–5.8) | 5.2 (3.3–6.8) | 5.6 (3.9–7.1) | 5.8 (4.3–7.3) | 6.0 (4.0–7.8) |
| Gelatine | 4.0 (3.0–4.8) | 3.3 (2.9–4.4) | 4.3 (3.0–5.7) | 4.4 (3.5–6.0) | 4.6 (3.2–6.2) | 4.7 (3.4–6.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilke, H.-J.; Fuchs, H.; Benz, K.; Mollenhauer, J.; Gaissmaier, C.; Heuer, F.; Neidlinger-Wilke, C. The Injection of Gels Through an Intact Annulus Maintains Biomechanical Performance without Extrusion Risk. Gels 2024, 10, 269. https://doi.org/10.3390/gels10040269
Wilke H-J, Fuchs H, Benz K, Mollenhauer J, Gaissmaier C, Heuer F, Neidlinger-Wilke C. The Injection of Gels Through an Intact Annulus Maintains Biomechanical Performance without Extrusion Risk. Gels. 2024; 10(4):269. https://doi.org/10.3390/gels10040269
Chicago/Turabian StyleWilke, Hans-Joachim, Holger Fuchs, Karin Benz, Juergen Mollenhauer, Christoph Gaissmaier, Frank Heuer, and Cornelia Neidlinger-Wilke. 2024. "The Injection of Gels Through an Intact Annulus Maintains Biomechanical Performance without Extrusion Risk" Gels 10, no. 4: 269. https://doi.org/10.3390/gels10040269
APA StyleWilke, H.-J., Fuchs, H., Benz, K., Mollenhauer, J., Gaissmaier, C., Heuer, F., & Neidlinger-Wilke, C. (2024). The Injection of Gels Through an Intact Annulus Maintains Biomechanical Performance without Extrusion Risk. Gels, 10(4), 269. https://doi.org/10.3390/gels10040269







