Composite Hydrogel with Oleic Acid-Grafted Mesoporous Silica Nanoparticles for Enhanced Topical Delivery of Doxorubicin
Abstract
:1. Introduction
2. Results and Discussion
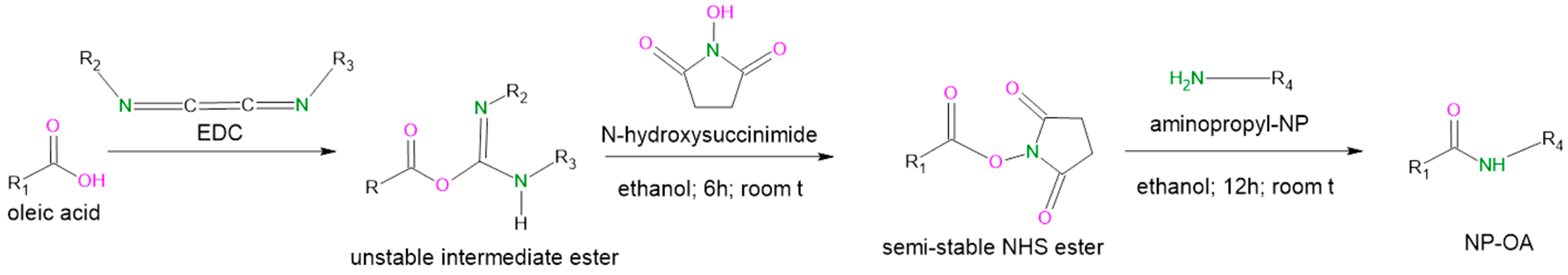
2.1. Nanoparticle Grafting with Oleic Acid and Physicochemical Characterization
2.2. FTIR
2.3. Nitrogen Physisorption
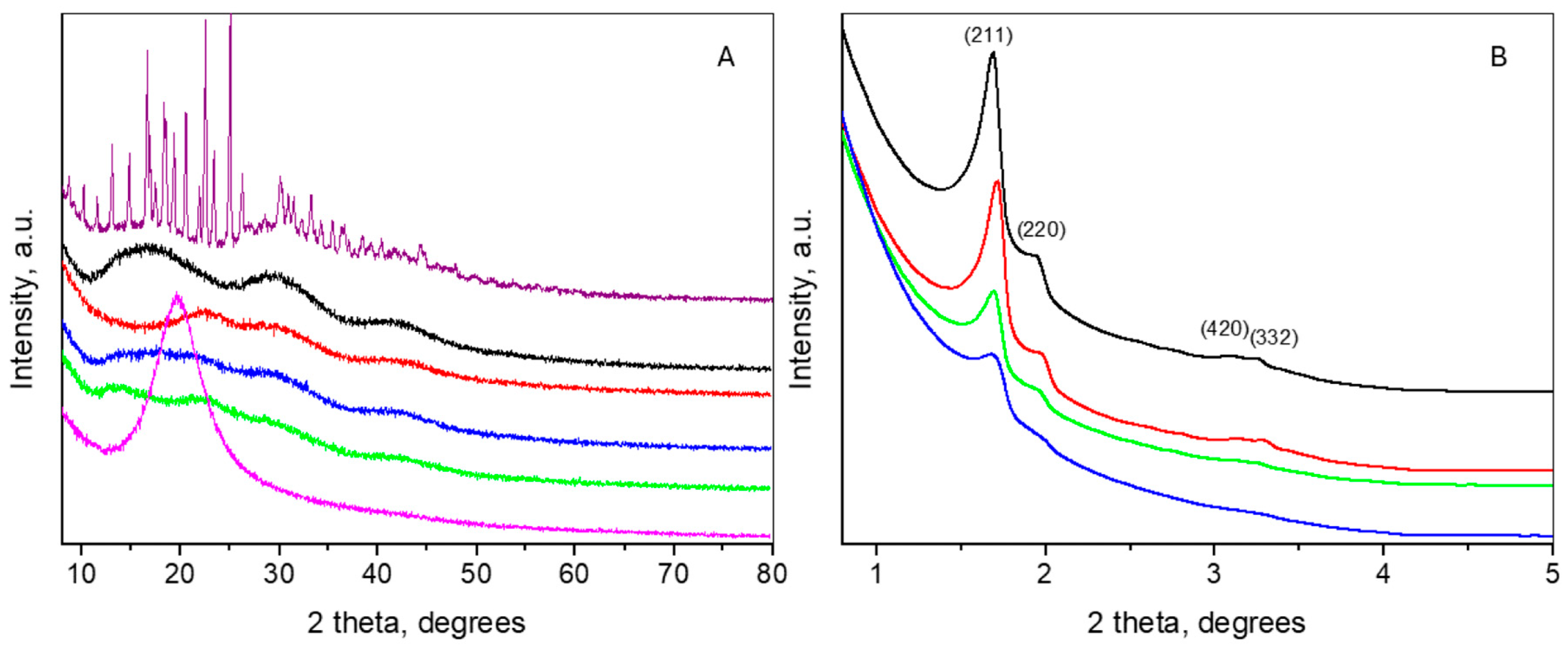
2.4. X-ray Diffraction
2.5. Drug Loading and Encapsulation Efficiency
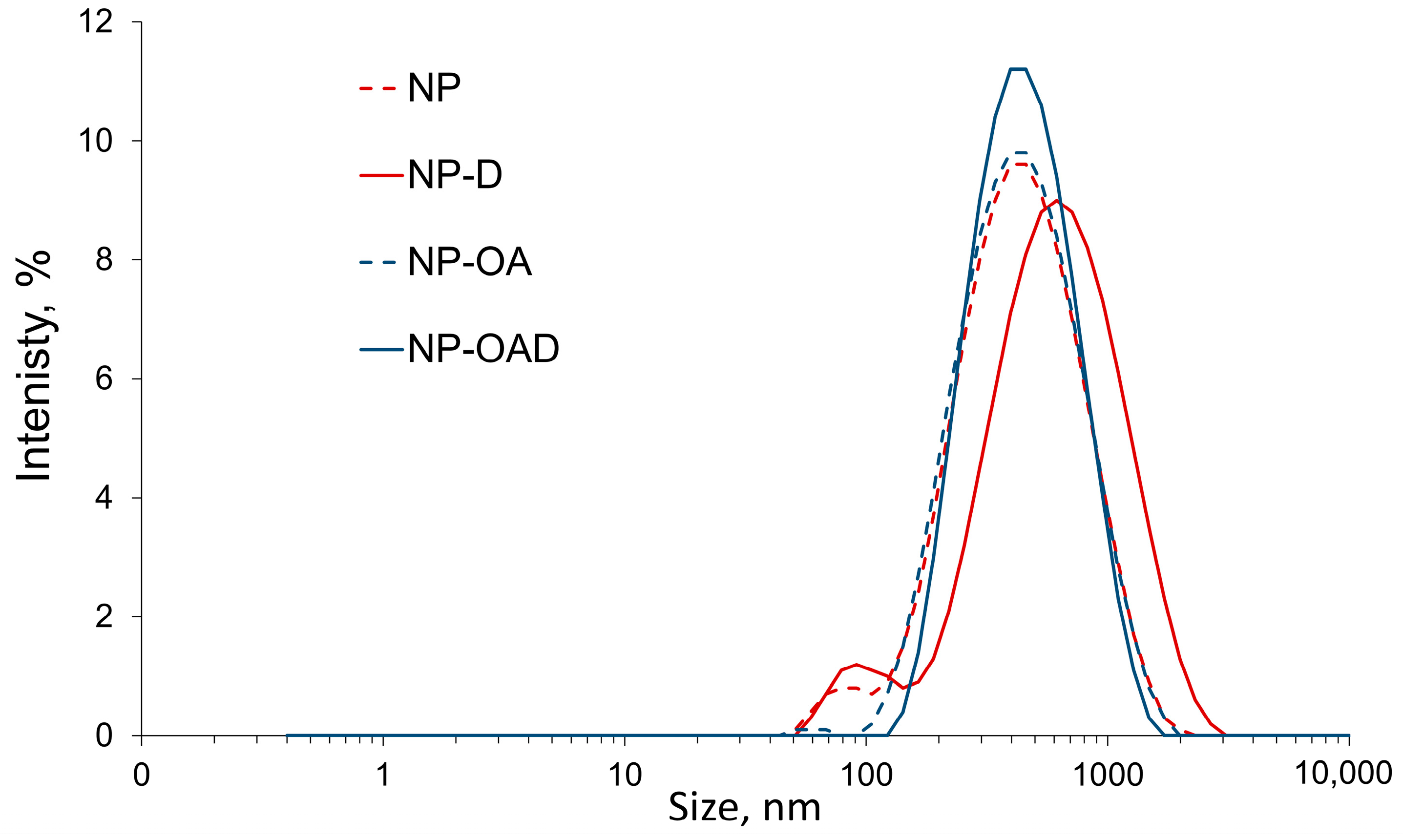
2.6. Particle Size, Polydisperisty and Zeta Potential
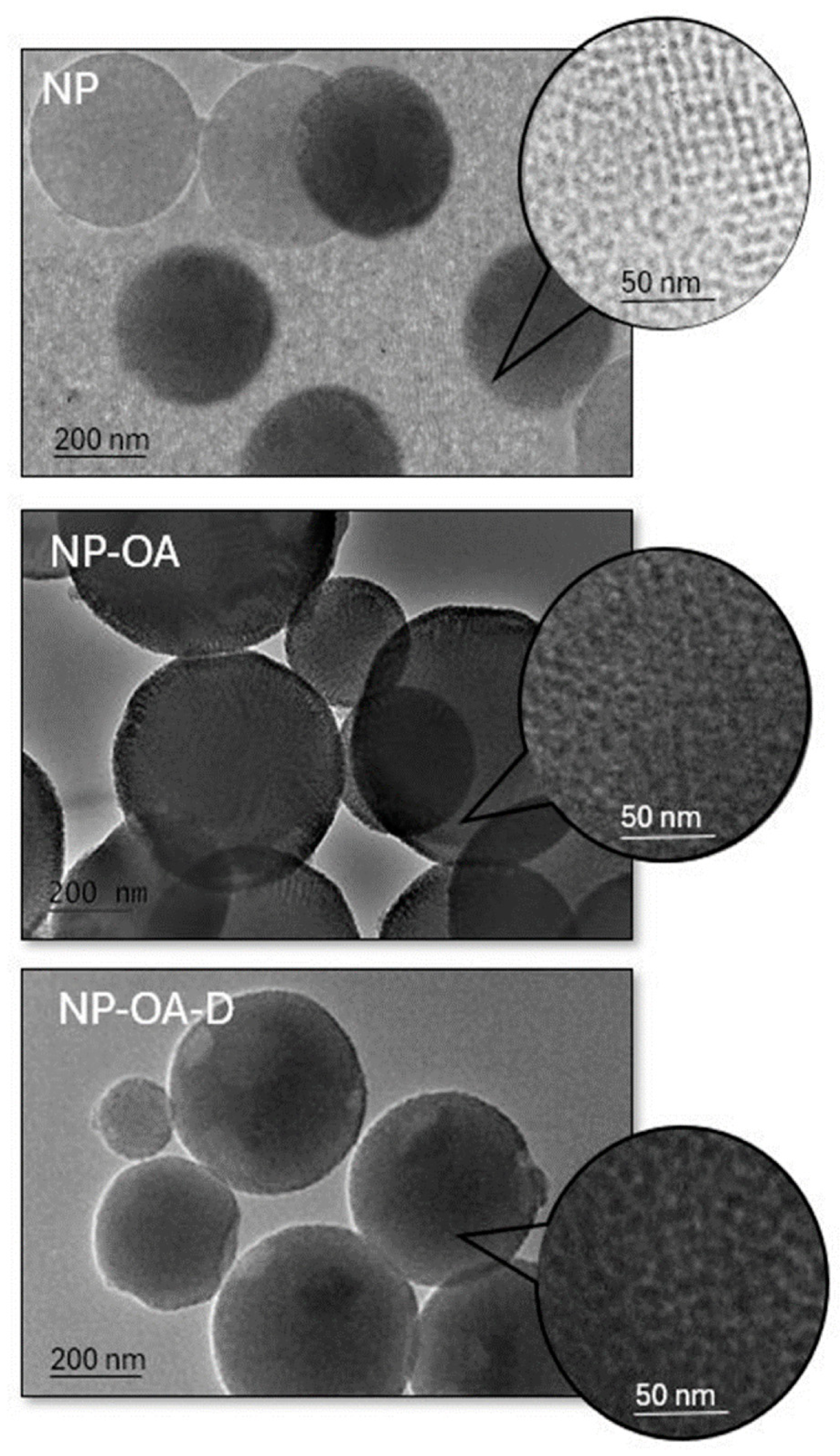
2.7. TEM Analysis
2.8. In Vitro Dissolution Test
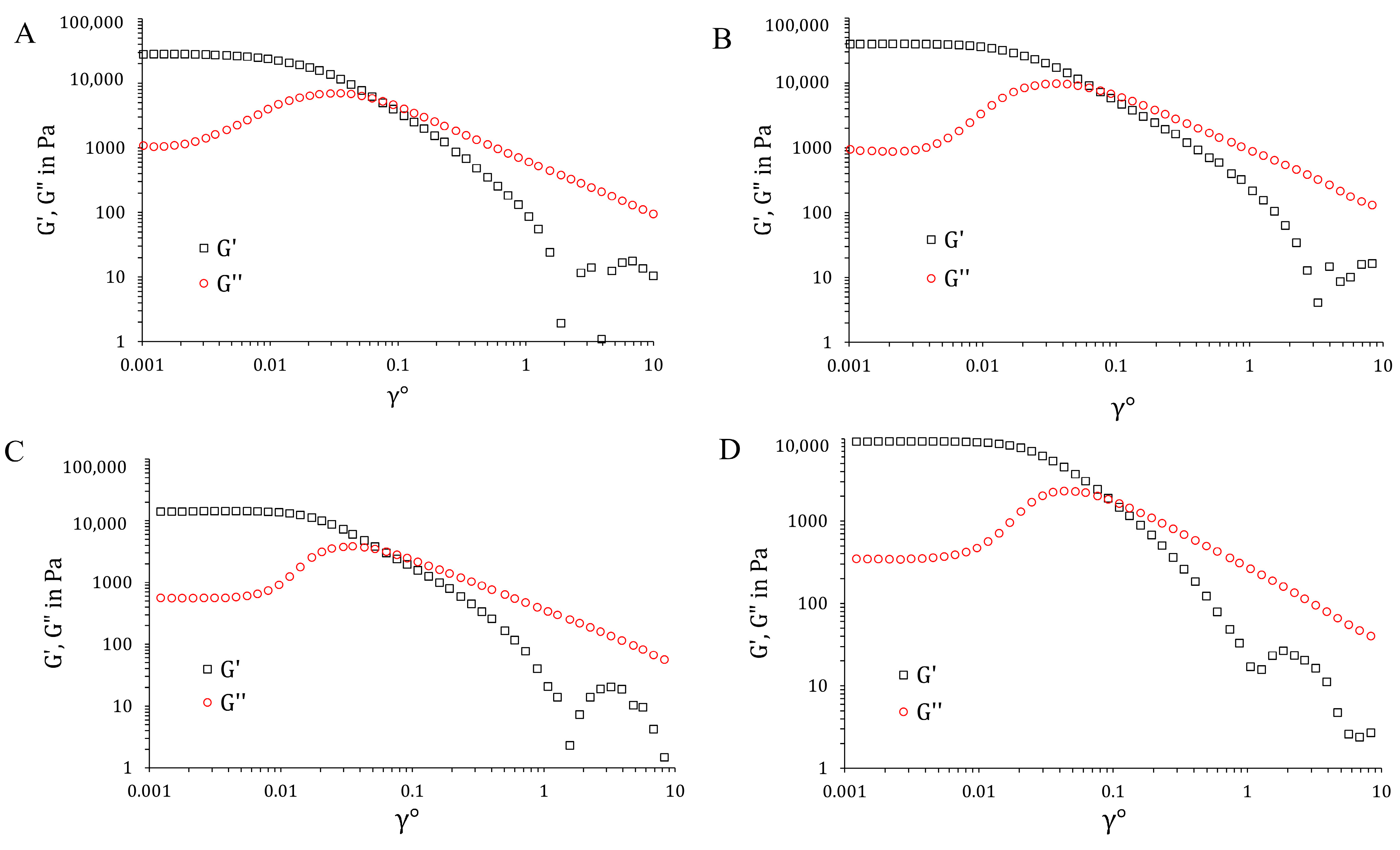
2.9. Physicochemical Characterization of the Hydrogels
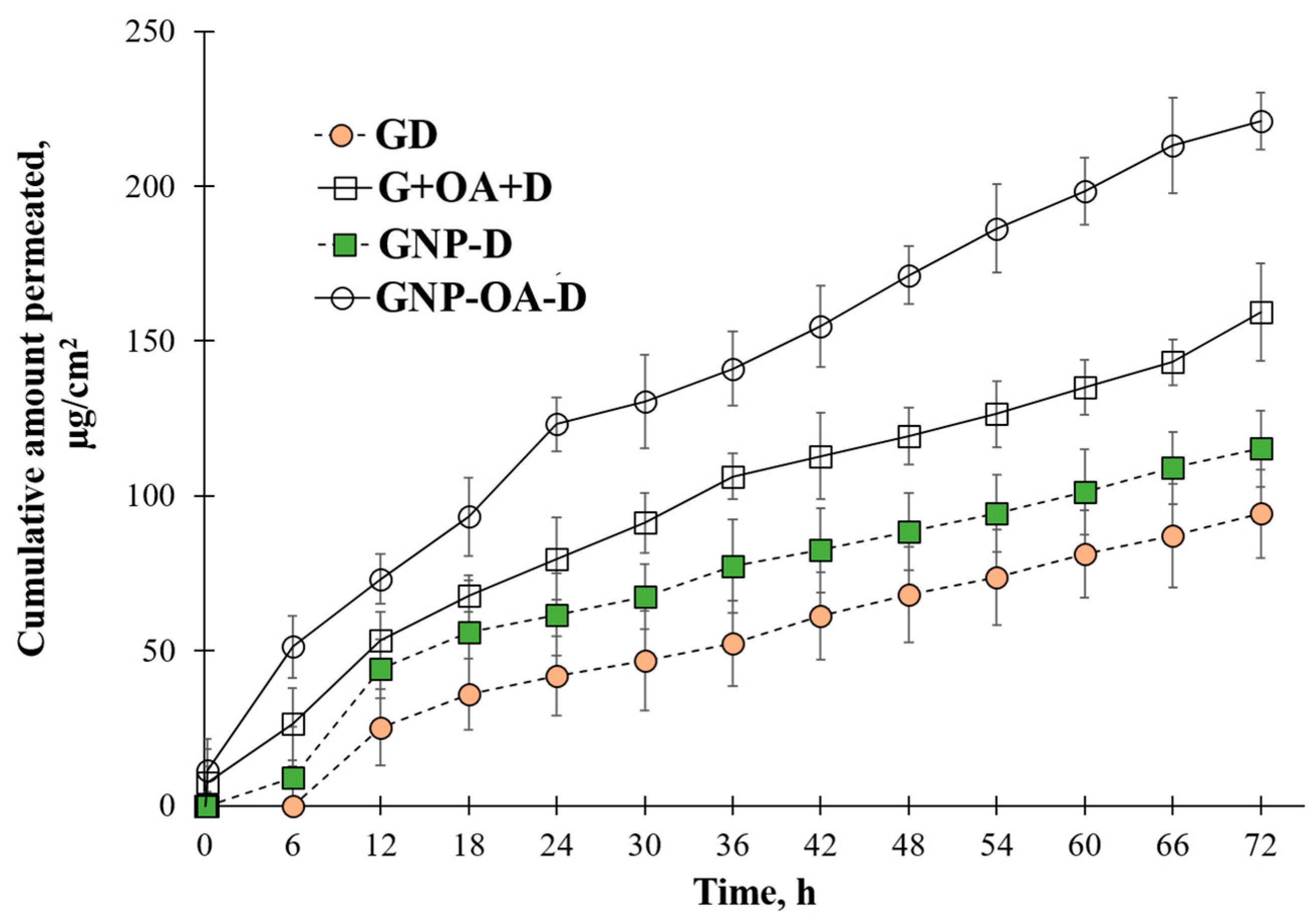
2.10. In Vitro Permeation Test
2.11. Cell Experiments
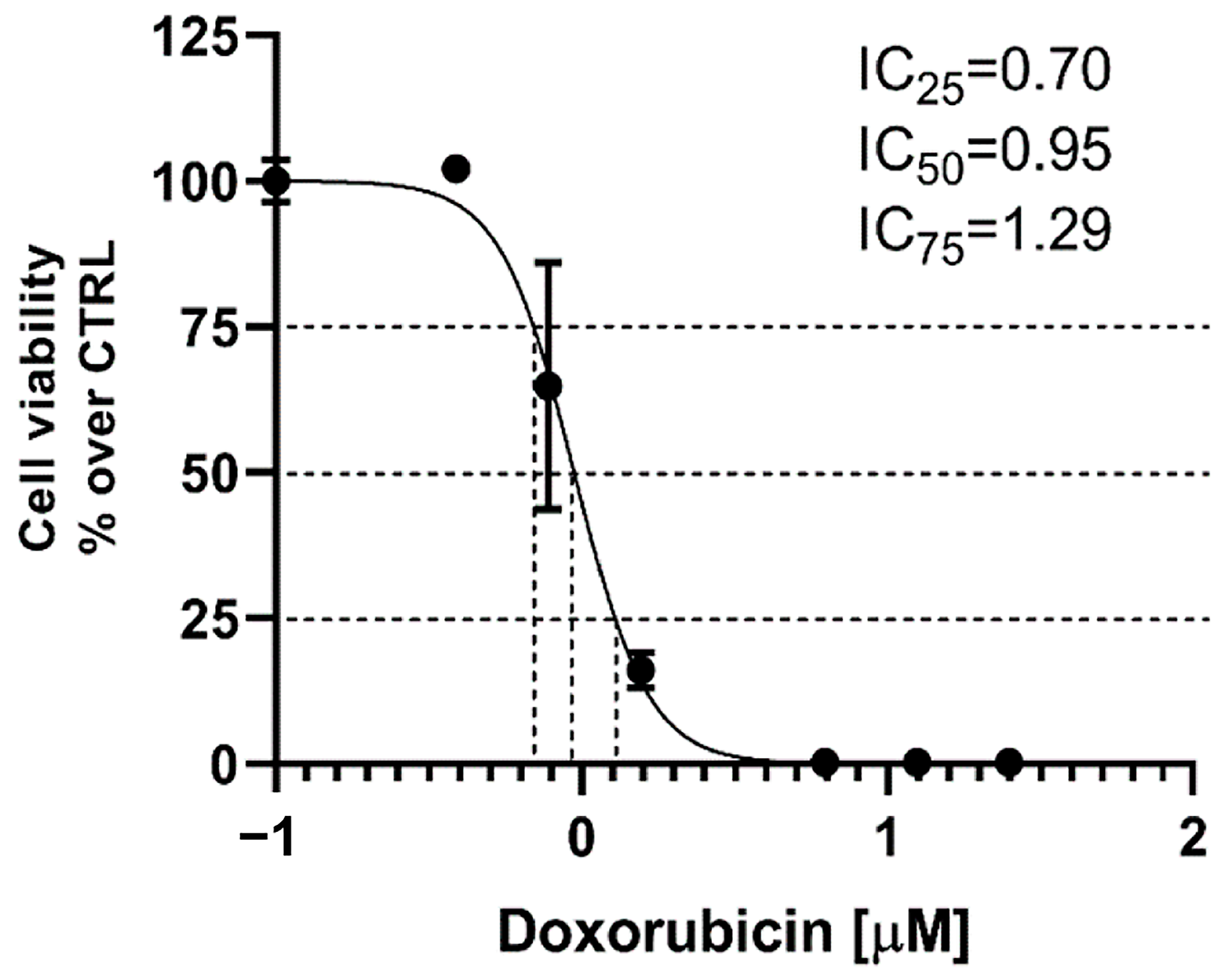
2.11.1. Cytotoxicity in A431 Cells
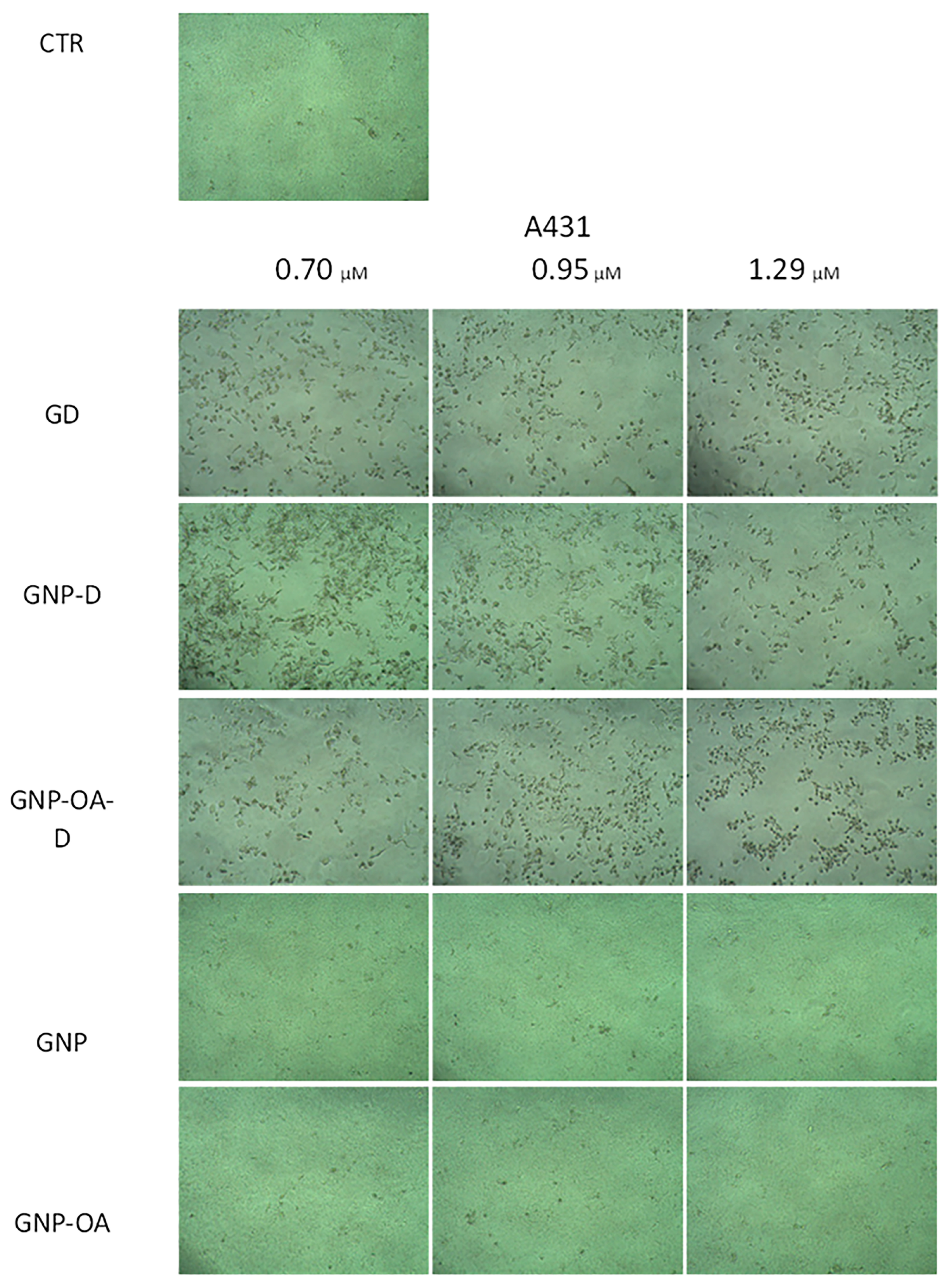
2.11.2. Phase-Contrast Micrography of A431 Cells
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Nanoparticle Grafting with Oleic Acid
4.3. Ninhydrin Reaction
4.4. Drug Loading and Encapsulation Efficiency
4.5. Fourier Transform Infrared Spectroscopy (FTIR)
4.6. Nitrogen Physisorption
4.7. X-ray Diffraction
4.8. Particle Size, Polydispersity Index and Zeta Potential
4.9. Transmission Electron Microscopy (TEM)
4.10. In Vitro Drug Dissolution and Kinetics Study
4.11. Hydrogel Preparation, Nanoparticles Incorporation, Appearance and pH
4.12. Hydrogel Rheology and Spreadability
4.13. In Vitro Occlusion Test
4.14. In Vitro Permeation Study
4.15. Cell Experiments
4.15.1. Cell Culture
4.15.2. Cytotoxicity Assay
4.15.3. Phase Contrast Micrography
4.15.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girdhar, V.; Patil, S.; Banerjee, S.; Singhvi, G. Nanocarriers For Drug Delivery: Mini Review. Curr. Nanomed. (Former. Recent Pat. Nanomed.) 2018, 8, 88–99. [Google Scholar] [CrossRef]
- Kumar, H.; Kumar, P.; Singh, V.; Pandey, S.S.; Pani, B. Synthesis and Surface Modification of Biocompatible Mesoporous Silica Nanoparticles (MSNs) and Its Biomedical Applications: A Review. Res. J. Chem. Environ. 2023, 27, 135–146. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Djayanti, K.; Maharjan, P.; Cho, K.H.; Jeong, S.; Kim, M.S.; Shin, M.C.; Min, K.A. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. Int. J. Mol. Sci. 2023, 24, 6349. [Google Scholar] [CrossRef]
- Morais, R.P.; Hochheim, S.; de Oliveira, C.C.; Riegel-Vidotti, I.C.; Marino, C.E.B. Skin Interaction, Permeation, and Toxicity of Silica Nanoparticles: Challenges and Recent Therapeutic and Cosmetic Advances. Int. J. Pharm. 2022, 614, 121439. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Shen, Y.-W.; Guan, Y.-Y.; Jiang, Y.-X.; Wu, Y.; Rahman, K.; Zhang, L.-J.; Liu, H.-J.; Luan, X. Mesoporous Silica Nanoparticles: Facile Surface Functionalization and Versatile Biomedical Applications in Oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef]
- He, Y.; Luo, L.; Liang, S.; Long, M.; Xu, H. Amino-Functionalized Mesoporous Silica Nanoparticles as Efficient Carriers for Anticancer Drug Delivery. J. Biomater. Appl. 2017, 32, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Mal, A.; Prabhuraj, R.S.; Malhotra, R.; Valvi, S.K.; Ingle, A.; Srivastava, R.; De, A.; Bandyopadhyaya, R. pH-Responsive Sustained Delivery of Doxorubicin Using Aminated and PEGylated Mesoporous Silica Nanoparticles Leads to Enhanced Antitumor Efficacy in Pre-Clinical Orthotopic Breast Cancer Model. J. Drug Deliv. Sci. Technol. 2022, 77, 103800. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Ho, Q.P.; Morand, J.; García, A.; Ortega, E.; Erthal, L.C.S.; Ruiz-Hernandez, E.; Santana, M.D.; Ruiz, J.; Vallet-Regí, M.; et al. Amino-Functionalized Mesoporous Silica Nanoparticle-Encapsulated Octahedral Organoruthenium Complex as an Efficient Platform for Combatting Cancer. Inorg. Chem. 2020, 59, 10275–10284. [Google Scholar] [CrossRef]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Khan, R.U.; Khalid, S.H.; Yousaf, A.M.; Hussain, T.; Asghar, S.; Khalid, I.; Asif, M.; et al. Amino-Decorated Mesoporous Silica Nanoparticles for Controlled Sofosbuvir Delivery. Eur. J. Pharm. Sci. 2020, 143, 105184. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Le, N.T.T.; Nguyen, N.H.; Ly, B.T.K.; Nguyen, T.D.; Nguyen, D.H. Aminated Hollow Mesoporous Silica Nanoparticles as an Enhanced Loading and Sustained Releasing Carrier for Doxorubicin Delivery. Microporous Mesoporous Mater. 2020, 309, 110543. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized Mesoporous Silica Nanoparticles and Biomedical Applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Rabasco Álvarez, A.M.; González Rodríguez, M.L. Lipids in Pharmaceutical and Cosmetic Preparations. Grasas Y Aceites 2000, 51, 74–96. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 01820. [Google Scholar] [CrossRef] [PubMed]
- Iannuccelli, V.; Bertelli, D.; Romagnoli, M.; Scalia, S.; Maretti, E.; Sacchetti, F.; Leo, E. In Vivo Penetration of Bare and Lipid-Coated Silica Nanoparticles across the Human Stratum Corneum. Colloids Surf. B Biointerfaces 2014, 122, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Cai, L.; Gong, X.; Zhang, L.; Zhang, J.; Zhu, P.; Jiang, H.; Wang, C.; Wang, S.; Chen, J. Drug-Loaded Oleic-Acid Grafted Mesoporous Silica Nanoparticles Conjugated with α-Lactalbumin Resembling BAMLET-like Anticancer Agent with Improved Biocompatibility and Therapeutic Efficacy. Mater. Today Bio 2022, 15, 100272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhou, L.; Song, Y.; Cai, L.; Ji, M.; Wang, J.; Ruan, G.; Chen, J. Encapsulating Insoluble Antifungal Drugs into Oleic Acid-Modified Silica Mesocomposites with Enhanced Fungicidal Activity. J. Mater. Chem. B 2020, 8, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Poyatos-Racionero, E.; Pérez-Esteve, É.; Dolores Marcos, M.; Barat, J.M.; Martínez-Máñez, R.; Aznar, E.; Bernardos, A. New Oleic Acid-Capped Mesoporous Silica Particles as Surfactant-Responsive Delivery Systems. ChemistryOpen 2019, 8, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef]
- Lai, W.-F.; Tang, R.; Wong, W.-T. Ionically Crosslinked Complex Gels Loaded with Oleic Acid-Containing Vesicles for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef]
- Atef, B.; Ishak, R.A.H.; Badawy, S.S.; Osman, R. Exploring the Potential of Oleic Acid in Nanotechnology-Mediated Dermal Drug Delivery: An up-to-Date Review. J. Drug Deliv. Sci. Technol. 2022, 67, 103032. [Google Scholar] [CrossRef]
- Bruno, M.C.; Gagliardi, A.; Mancuso, A.; Barone, A.; Tarsitano, M.; Cosco, D.; Cristiano, M.C.; Fresta, M.; Paolino, D. Oleic Acid-Based Vesicular Nanocarriers for Topical Delivery of the Natural Drug Thymoquinone: Improvement of Anti-Inflammatory Activity. J. Control. Release 2022, 352, 74–86. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.R.; Faria de Freitas, Z.M.; de Brito Gitirana, L.; Ricci-Júnior, E. Improving the Topical Delivery of Zinc Phthalocyanine Using Oleic Acid as a Penetration Enhancer: In Vitro Permeation and Retention. Drug Dev. Ind. Pharm. 2011, 37, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.; Tzankov, B.; Popova, T.; Voycheva, C. Gel Formulations for Topical Treatment of Skin Cancer: A Review. Gels 2023, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K. Doxorubicin. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–5. ISBN 978-0-08-055232-3. [Google Scholar]
- Van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New Insights into the Activities and Toxicities of the Old Anticancer Drug Doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal Delivery of Doxorubicin-Loaded Solid Lipid Nanoparticles for the Treatment of Skin Cancer. J. Microencapsul. 2016, 33, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Carvalho, I.C.; Chagas, P.; de Oliveira, L.C.A.; Mansur, H.S. Bioengineered Carboxymethyl Cellulose-Doxorubicin Prodrug Hydrogels for Topical Chemotherapy of Melanoma Skin Cancer. Carbohydr. Polym. 2018, 195, 401–412. [Google Scholar] [CrossRef]
- Preet, S.; Pandey, S.K.; Kaur, K.; Chauhan, S.; Saini, A. Gold Nanoparticles Assisted Co-Delivery of Nisin and Doxorubicin against Murine Skin Cancer. J. Drug Deliv. Sci. Technol. 2019, 53, 101147. [Google Scholar] [CrossRef]
- He, Y.; Wu, S.; Rietveld, M.; Vermeer, M.; Cruz, L.J.; Eich, C.; El Ghalbzouri, A. Application of Doxorubicin-Loaded PLGA Nanoparticles Targeting Both Tumor Cells and Cancer-Associated Fibroblasts on 3D Human Skin Equivalents Mimicking Melanoma and Cutaneous Squamous Cell Carcinoma. Biomater. Adv. 2024, 160, 213831. [Google Scholar] [CrossRef]
- Methaneethorn, J.; Tengcharoen, K.; Leelakanok, N.; AlEjielat, R. Population Pharmacokinetics of Doxorubicin: A Systematic Review. Asia-Pac. J. Clin. Oncol. 2023, 19, 9–26. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to Different Experimental Organ Systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen; Iqubal, M.K.; Sartaj, A.; Khan, M.A.; Ali, J.; Baboota, S. Topical Delivery of Mannose Conjugated-Doxorubicin-Berberine Nanostructured Lipid Carrier Gel for Skin Cancer Amelioration: Formulation Optimization, In-Silico, In-Vitro, Ex-Vivo Assessment, and Dermatokinetic Analysis. J. Drug Deliv. Sci. Technol. 2024, 93, 105378. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Abou-Dahech, M.S.; Bachu, R.D.; Terrero, D.; Babu, R.J.; Tiwari, A.K. Transdermal Delivery of Chemotherapeutics: Strategies, Requirements, and Opportunities. Pharmaceutics 2021, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.Y.; Margaryan, A.; Huang, Y.; Schatton, T.; Waaga-Gasser, A.M.; Gasser, M.; Sayegh, M.H.; Sadee, W.; Frank, M.H. ABCB5-Mediated Doxorubicin Transport and Chemoresistance in Human Malignant Melanoma. Cancer Res. 2005, 65, 4320–4333. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Chapter 14—Microparticles and Nanoparticles. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 549–587. ISBN 978-0-12-382239-0. [Google Scholar]
- Guo, Y.; Wang, M.; Zhang, H.; Liu, G.; Zhang, L.; Qu, X. The Surface Modification of Nanosilica, Preparation of Nanosilica/Acrylic Core-shell Composite Latex, and Its Application in Toughening PVC Matrix. J. Appl. Polym. Sci. 2008, 107, 2671–2680. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Dash, S.; Sarkar, A. SBA-15 Functionalised with High Loading of Amino or Carboxylate Groups as Selective Adsorbent for Enhanced Removal of Toxic Dyes from Aqueous Solution. New J. Chem. 2016, 40, 3622–3634. [Google Scholar] [CrossRef]
- Parida, K.M.; Rath, D. Amine Functionalized MCM-41: An Active and Reusable Catalyst for Knoevenagel Condensation Reaction. J. Mol. Catal. A Chem. 2009, 310, 93–100. [Google Scholar] [CrossRef]
- Bootdee, K.; Nithitanakul, M.; Grady, B.P. Synthesis and Encapsulation of Magnetite Nanoparticles in PLGA: Effect of Amount of PLGA on Characteristics of Encapsulated Nanoparticles. Polym. Bull. 2012, 69, 795–806. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.-C. Oleic Acid Coating on the Monodisperse Magnetite Nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Harris, M.T. Surface Modification of Magnetic Nanoparticles Capped by Oleic Acids: Characterization and Colloidal Stability in Polar Solvents. J. Colloid. Interface Sci. 2006, 293, 401–408. [Google Scholar] [CrossRef]
- Okassa, L.N.; Marchais, H.; Douziech-Eyrolles, L.; Hervé, K.; Cohen-Jonathan, S.; Munnier, E.; Soucé, M.; Linassier, C.; Dubois, P.; Chourpa, I. Optimization of Iron Oxide Nanoparticles Encapsulation within Poly(d,l-Lactide-Co-Glycolide) Sub-Micron Particles. Eur. J. Pharm. Biopharm. 2007, 67, 31–38. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Fan, N.; Li, J.; He, Z.; Sun, J. Multimodal Nanoporous Silica Nanoparticles Functionalized with Aminopropyl Groups for Improving Loading and Controlled Release of Doxorubicin Hydrochloride. Mater. Sci. Eng. C 2017, 78, 370–375. [Google Scholar] [CrossRef]
- Li, J.; Shen, S.; Kong, F.; Jiang, T.; Tang, C.; Yin, C. Effects of Pore Size on in Vitro and in Vivo Anticancer Efficacies of Mesoporous Silica Nanoparticles. RSC Adv. 2018, 8, 24633–24640. [Google Scholar] [CrossRef]
- Roik, N.V.; Belyakova, L.A. Mesoporous Silica Nanoparticles Equipped with Surface Nanovalves for pH-Controlled Liberation of Doxorubicin. Interface Focus 2016, 6, 20160041. [Google Scholar] [CrossRef]
- Dau, T.A.N.; Le, V.M.H.; Pham, T.K.H.; Le, V.H.; Cho, S.K.; Nguyen, T.N.U.; Ta, T.K.H.; Van Tran, T.T. Surface Functionalization of Doxorubicin Loaded MCM-41 Mesoporous Silica Nanoparticles by 3-Aminopropyltriethoxysilane for Selective Anticancer 9 Effect on A549 and A549/DOX Cells. J. Electron. Mater. 2021, 50, 2932–2939. [Google Scholar] [CrossRef]
- Bezzon, V.D.N.; Caturello, N.A.M.d.S.; Dalpian, G.M.; Ferreira, F.F. Crystal Structure Determination and DFT Analysis of Doxorubicin Hydrochloride for Controlled-Release Drug Formulations. J. Mol. Struct. 2023, 1294, 136412. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Refay, N.M.; El-Sherbeeny, A.M.; El-Meligy, M.A. Insight into the Loading and Release Properties of MCM-48/Biopolymer Composites as Carriers for 5-Fluorouracil: Equilibrium Modeling and Pharmacokinetic Studies. ACS Omega 2020, 5, 11745–11755. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Cui, Y.; Ma, S.; Suo, Y.; Zhang, W. Synthesis of Mo-MCM-48 and Their Isomerization Performances of n-Heptane. J. Porous Mater. 2019, 26, 1279–1286. [Google Scholar] [CrossRef]
- Yu, Q.; Zhuang, R.; Yi, H.; Gao, W.; Zhang, Y.; Tang, X. Application of MCM-48 with Large Specific Surface Area for VOCs Elimination: Synthesis and Hydrophobic Functionalization for Highly Efficient Adsorption. Environ. Sci. Pollut. Res. 2022, 29, 33595–33608. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; He, Q.; Gao, Y.; Shi, J.; Li, Y. Mesoporous Silica Nanoparticles Loading Doxorubicin Reverse Multidrug Resistance: Performance and Mechanism. Nanoscale 2011, 3, 4314–4322. [Google Scholar] [CrossRef]
- Rahmani, S.; Durand, J.-O.; Charnay, C.; Lichon, L.; Férid, M.; Garcia, M.; Gary-Bobo, M. Synthesis of Mesoporous Silica Nanoparticles and Nanorods: Application to Doxorubicin Delivery. Solid State Sci. 2017, 68, 25–31. [Google Scholar] [CrossRef]
- Ibraheem, L.M.; Khattabi, A.M. Studying the Effect of Functional Group and Size of Silica Nanoparticles Loaded with Quercetin on Their in Vitro Characteristics. Jordan J. Pharm. Sci. 2022, 15, 569–582. [Google Scholar] [CrossRef]
- Talavera-Pech, W.A.; Esparza-Ruiz, A.; Quintana-Owen, P.; Vilchis-Nestor, A.R.; Carrera-Figueiras, C.; Ávila-Ortega, A. Effects of Different Amounts of APTES on Physicochemical and Structural Properties of Amino-Functionalized MCM-41-MSNs. J. Sol-Gel Sci. Technol. 2016, 80, 697–708. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Nguyen, T.T.; Nghiem, T.H.L.; Nguyen, D.T.; Tran, T.T.H.; Vu, D.; Nguyen, T.B.N.; Nguyen, T.M.H.; Nguyen, V.T.; Nguyen, M.H. Optical Properties of Doxorubicin Hydrochloride Load and Release on Silica Nanoparticle Platform. Molecules 2021, 26, 3968. [Google Scholar] [CrossRef]
- Xie, M.; Shi, H.; Li, Z.; Shen, H.; Ma, K.; Li, B.; Shen, S.; Jin, Y. A Multifunctional Mesoporous Silica Nanocomposite for Targeted Delivery, Controlled Release of Doxorubicin and Bioimaging. Colloids Surf. B Biointerfaces 2013, 110, 138–147. [Google Scholar] [CrossRef]
- Peng, S.; Huang, B.; Lin, Y.; Pei, G.; Zhang, L. Effect of Surface Functionalization and Pore Structure Type on the Release Performance of Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2022, 336, 111862. [Google Scholar] [CrossRef]
- Beňová, E.; Bergé-Lefranc, D.; Zeleňák, V.; Almáši, M.; Huntošová, V.; Hornebecq, V. Adsorption Properties, the pH-Sensitive Release of 5-Fluorouracil and Cytotoxicity Studies of Mesoporous Silica Drug Delivery Matrix. Appl. Surf. Sci. 2020, 504, 144028. [Google Scholar] [CrossRef]
- Doadrio, J.C.; Sousa, E.M.B.; Izquierdo-Barba, I.; Doadrio, A.L.; Perez-Pariente, J.; Vallet-Regí, M. Functionalization of Mesoporous Materials with Long Alkyl Chains as a Strategy for Controlling Drug Delivery Pattern. J. Mater. Chem. 2006, 16, 462–466. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Inal, O.; Yapar, E.A. Effect of Mechanical Properties on the Release of Meloxicam from Poloxamer Gel Bases. Indian J. Pharm. Sci. 2013, 75, 700–706. [Google Scholar]
- Iqubal, M.K.; Iqubal, A.; Anjum, H.; Gupta, M.M.; Ali, J.; Baboota, S. Determination of in Vivo Virtue of Dermal Targeted Combinatorial Lipid Nanocolloidal Based Formulation of 5-Fluorouracil and Resveratrol against Skin Cancer. Int. J. Pharm. 2021, 610, 121179. [Google Scholar] [CrossRef]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef]
- Stamatas, G.N.; de Sterke, J.; Hauser, M.; von Stetten, O.; van der Pol, A. Lipid Uptake and Skin Occlusion Following Topical Application of Oils on Adult and Infant Skin. J. Dermatol. Sci. 2008, 50, 135–142. [Google Scholar] [CrossRef]
- Moreira, T.S.; de Sousa, V.P.; Pierre, M.B.R. Influence of Oleic Acid on the Rheology and in Vitro Release of Lumiracoxib from Poloxamer Gels. J. Pharm. Pharm. Sci. 2010, 13, 286–302. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef]
- Hyun, K.; Nam, J.G.; Wilhellm, M.; Ahn, K.H.; Lee, S.J. Large Amplitude Oscillatory Shear Behavior of PEO-PPO-PEO Triblock Copolymer Solutions. Rheol. Acta 2006, 45, 239–249. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; de Araújo, D.R.; Seabra, A.B. S-Nitrosoglutathione-Containing Chitosan Nanoparticles Dispersed in Pluronic F-127 Hydrogel: Potential Uses in Topical Applications. J. Drug Deliv. Sci. Technol. 2018, 43, 211–220. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; da Costa, T.G.; de Lima, R.; Hedtrich, S.; Fraceto, L.F.; de Araujo, D.R. Hydrogels Containing Budesonide-Loaded Nanoparticles to Facilitate Percutaneous Absorption for Atopic Dermatitis Treatment Applications. ACS Appl. Polym. Mater. 2021, 3, 4436–4449. [Google Scholar] [CrossRef]
- Liu, X.; Shen, B.; Shen, C.; Zhong, R.; Wang, X.; Yuan, H. Nanoparticle-Loaded Gels for Topical Delivery of Nitrofurazone: Effect of Particle Size on Skin Permeation and Retention. J. Drug Deliv. Sci. Technol. 2018, 45, 367–372. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Herai, H.; Gratieri, T.; Thomazine, J.A.; Bentley, M.V.L.B.; Lopez, R.F.V. Doxorubicin Skin Penetration from Monoolein-Containing Propylene Glycol Formulations. Int. J. Pharm. 2007, 329, 88–93. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993–5; 2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Tixeira, R.; Caruso, S.; Paone, S.; Baxter, A.A.; Atkin-Smith, G.K.; Hulett, M.D.; Poon, I.K.H. Defining the Morphologic Features and Products of Cell Disassembly during Apoptosis. Apoptosis 2017, 22, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Ulfo, L.; Turrini, E.; Marconi, A.; Costantini, P.E.; Marforio, T.D.; Mattioli, E.J.; Di Giosia, M.; Danielli, A.; Fimognari, C.; et al. Light-Enhanced Cytotoxicity of Doxorubicin by Photoactivation. Cells 2023, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Giulitti, F.; Petrungaro, S.; Mandatori, S.; Tomaipitinca, L.; de Franchis, V.; D’Amore, A.; Filippini, A.; Gaudio, E.; Ziparo, E.; Giampietri, C. Anti-Tumor Effect of Oleic Acid in Hepatocellular Carcinoma Cell Lines via Autophagy Reduction. Front. Cell Dev. Biol. 2021, 9, 629182. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, T.; Li, C.; Dai, Z.; Che, D.; Yao, Y.; Li, L.; Ma, J.; Yang, X.; Gao, G. High Metastaticgastric and Breast Cancer Cells Consume Oleic Acid in an AMPK Dependent Manner. PLoS ONE 2014, 9, e97330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, X.; Huang, C.; Liu, Z.; Ye, Y. Oleic Acid Copolymer as A Novel Upconversion Nanomaterial to Make Doxorubicin-Loaded Nanomicelles with Dual Responsiveness to pH and NIR. Pharmaceutics 2020, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Carrillo Pérez, C.; Cavia Camarero, M.D.M.; Alonso de la Torre, S. Antitumor Effect of Oleic Acid; Mechanisms of Action. A Review. Nutr. Hosp. 2012, 27, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic Acid Induces Apoptosis and Autophagy in the Treatment of Tongue Squamous Cell Carcinomas. Sci. Rep. 2017, 7, 11277. [Google Scholar] [CrossRef]
- Bachelard, C.M.; Coquan, E.; du Rusquec, P.; Paoletti, X.; Tourneau, C.L. Risks and Benefits of Anticancer Drugs in Advanced Cancer Patients: A Systematic Review and Meta-Analysis. eClinicalMedicine 2021, 40, 101130. [Google Scholar] [CrossRef]
- Westheim, A.J.F.; Stoffels, L.M.; Dubois, L.J.; van Bergenhenegouwen, J.; van Helvoort, A.; Langen, R.C.J.; Shiri-Sverdlov, R.; Theys, J. The Modulatory Effects of Fatty Acids on Cancer Progression. Biomedicines 2023, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-T. Synthesis and Characterization of Amino-Functionalized Silica Nanoparticles. Colloid. J. 2013, 75, 311–318. [Google Scholar] [CrossRef]
- Sakeye, M.; Smått, J.-H. Comparison of Different Amino-Functionalization Procedures on a Selection of Metal Oxide Microparticles: Degree of Modification and Hydrolytic Stability. Available online: https://pubs.acs.org/doi/pdf/10.1021/la303925x (accessed on 19 April 2023).
- Ferreira, L.M.; Sari, M.H.M.; Azambuja, J.H.; da Silveira, E.F.; Cervi, V.F.; Marchiori, M.C.L.; Maria-Engler, S.S.; Wink, M.R.; Azevedo, J.G.; Nogueira, C.W.; et al. Xanthan Gum-Based Hydrogel Containing Nanocapsules for Cutaneous Diphenyl Diselenide Delivery in Melanoma Therapy. Investig. New Drugs 2020, 38, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Caldas, A.R.; Catita, J.; Machado, R.; Ribeiro, A.; Cerqueira, F.; Horta, B.; Medeiros, R.; Lúcio, M.; Lopes, C.M. Omega-3- and Resveratrol-Loaded Lipid Nanosystems for Potential Use as Topical Formulations in Autoimmune, Inflammatory, and Cancerous Skin Diseases. Pharmaceutics 2021, 13, 1202. [Google Scholar] [CrossRef]
- Hasler-Nguyen, N. EMA’s Proposed Guideline on Quality and Equivalence of Topical Products and Nanotechnology: Future Advances and Challenges. Acta Pharm. Hung. 2021, 91, 232–233. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Nadeem, M.S.; Altayb, H.N.; Alshehri, S.; Imam, S.S. Formulation, Optimization and Evaluation of Luteolin-Loaded Topical Nanoparticulate Delivery System for the Skin Cancer. Pharmaceutics 2021, 13, 1749. [Google Scholar] [CrossRef]
- Saleem, M.N.; Idris, M. Formulation Design and Development of a Unani Transdermal Patch for Antiemetic Therapy and Its Pharmaceutical Evaluation. Scientifica 2016, 2016, e7602347. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Raval, S.; Peer, S.; Upadhyay, U.M. A Comparative Evaluation of Different Membranes for Their Diffusion Efficiency: An in Vitro Study. Pharma Sci. Monit. 2010, 1, 41–49. [Google Scholar]
- Zheng, J.; Shen, C.-Y.; Pang, J.-Y.; Xu, F.-C.; Liao, W.-B.; Hu, C.-X.; Xu, P.-H.; Han, J.; Yuan, H.-L. Preparation of tanshinone ⅡA loaded nanostructured lipid carrier and its in vitro transdermal permeation characteristics. Zhongguo Zhong Yao Za Zhi 2016, 41, 3232–3238. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]




| Sample | SBET (m2·g−1) | Vt (m2·g−1) | Dav (nm) |
|---|---|---|---|
| NP | 586 | 0.76 | 5.3 |
| NP-OA | 576 | 0.74 | 5.2 |
| NP-D | 387 | 0.48 | 5.0 |
| NP-OA-D | 250 | 0.33 | 5.1 |
| Sample Coding | Full Name | Size (nm) (Mean ± SD) | PDI | Zeta Potential (mV) (Mean ± SD) | EE (%) (Mean ± SD) | LC (%) (Mean ± SD) |
|---|---|---|---|---|---|---|
| NP | Aminopropyl-functionalized silica | 352.7 ± 2.6 | 0.251 | −23.9 ± 0.3 | - | - |
| NP-D | NP loaded with D | 449.3 ± 3.1 | 0.359 | −40.7 ± 0.2 | 99.14 ± 0.83 | 19.63 ± 0.09 |
| NP-OA | OA grafted NP | 372.4 ± 2.9 | 0.219 | −50.2 ± 0.4 | - | - |
| NP-OA-D | NP-OA loaded with D | 402.8 ± 3.3 | 0.142 | −14.5 ± 0.4 | 97.66 ± 1.81 | 18.96 ± 0.23 |
| Formulation | Zero Order | First Order | Higuchi | Korsmeyer–Peppas |
|---|---|---|---|---|
| NP-D | R2 = 0.803 | R2 = 0.9249 | R2 = 0.9435 | R2 = 0.983 |
| k = 2.366 | k = −0.024 | k = 14.421 | n = 0.312 | |
| NP-OA-D | R2 = 0.970 | R2 = 0.989 | R2 = 0.984 | R2 = 0.908 |
| k = 1.785 | k = −0.011 | k = 10.103 | n = 0.762 |
| Gel Formulations with | Physicochemical Property | Mean | SD |
|---|---|---|---|
| Free D (GD) | pH | 5.75 | 0.21 |
| F, (%) (after 48 h) | 57.19 | 0.52 | |
| SF (mm2/g) | 3.20 | 0.74 | |
| ŋ (Pa·s) | 4521 | 121 | |
| G′ (Pa) | 28,390 | 149 | |
| G″ (Pa) | 1032 | 67 | |
| Jss (µg/cm2/h) | 1.12 | 0.35 | |
| Qt (µg/cm2) | 94.23 | 11.56 | |
| NP-D (GNP-D) | pH | 6.01 | 0.23 |
| F (%) (after 48 h) | 53.12 | 0.73 | |
| SF (mm2/g) | 2.70 | 0.74 | |
| ŋ (Pa·s) | 2161 | 98 | |
| G′ (Pa) | 13,570 | 203 | |
| G″ (Pa) | 388.4 | 97 | |
| Jss (µg/cm2/h) | 1.34 | 0.31 | |
| Qt (µg/cm2) | 115.33 | 15.36 | |
| Free D and OA (G+OA+D) | pH | 5.15 | 0.09 |
| F (%) (after 48 h) | 62.81 | 0.76 | |
| SF (mm2/g) | 2.87 | 0.42 | |
| ŋ (Pa·s) | 4573 | 103 | |
| G′ (Pa) | 28,720 | 91 | |
| G″ (Pa) | 819.1 | 86 | |
| Jss (µg/cm2/h) | 1.93 | 0.27 | |
| Qt (µg/cm2) | 159.33 | 17.25 | |
| NP-OA-D (GNP-OA-D) | pH | 6.91 | 0.17 |
| F (%) (after 48 h) | 53.91 | 0.47 | |
| SF (mm2/g) | 2.87 | 0.42 | |
| ŋ (Pa·s) | 1486 | 162 | |
| G′ (Pa) | 9327 | 174 | |
| G″ (Pa) | 363.2 | 73 | |
| Jss (µg/cm2/h) | 2.72 | 0.12 | |
| Qt (µg/cm2) | 221.01 | 16.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavkova, M.; Dimitrova, D.; Voycheva, C.; Popova, T.; Spassova, I.; Kovacheva, D.; Yordanov, Y.; Tzankova, V.; Tzankov, B. Composite Hydrogel with Oleic Acid-Grafted Mesoporous Silica Nanoparticles for Enhanced Topical Delivery of Doxorubicin. Gels 2024, 10, 356. https://doi.org/10.3390/gels10060356
Slavkova M, Dimitrova D, Voycheva C, Popova T, Spassova I, Kovacheva D, Yordanov Y, Tzankova V, Tzankov B. Composite Hydrogel with Oleic Acid-Grafted Mesoporous Silica Nanoparticles for Enhanced Topical Delivery of Doxorubicin. Gels. 2024; 10(6):356. https://doi.org/10.3390/gels10060356
Chicago/Turabian StyleSlavkova, Marta, Diana Dimitrova, Christina Voycheva, Teodora Popova, Ivanka Spassova, Daniela Kovacheva, Yordan Yordanov, Virginia Tzankova, and Borislav Tzankov. 2024. "Composite Hydrogel with Oleic Acid-Grafted Mesoporous Silica Nanoparticles for Enhanced Topical Delivery of Doxorubicin" Gels 10, no. 6: 356. https://doi.org/10.3390/gels10060356







