PVA Hydrogels Supplemented with PLA Mesh for Tissue Regeneration Scaffold
Abstract
:1. Introduction
2. Results and Discussion
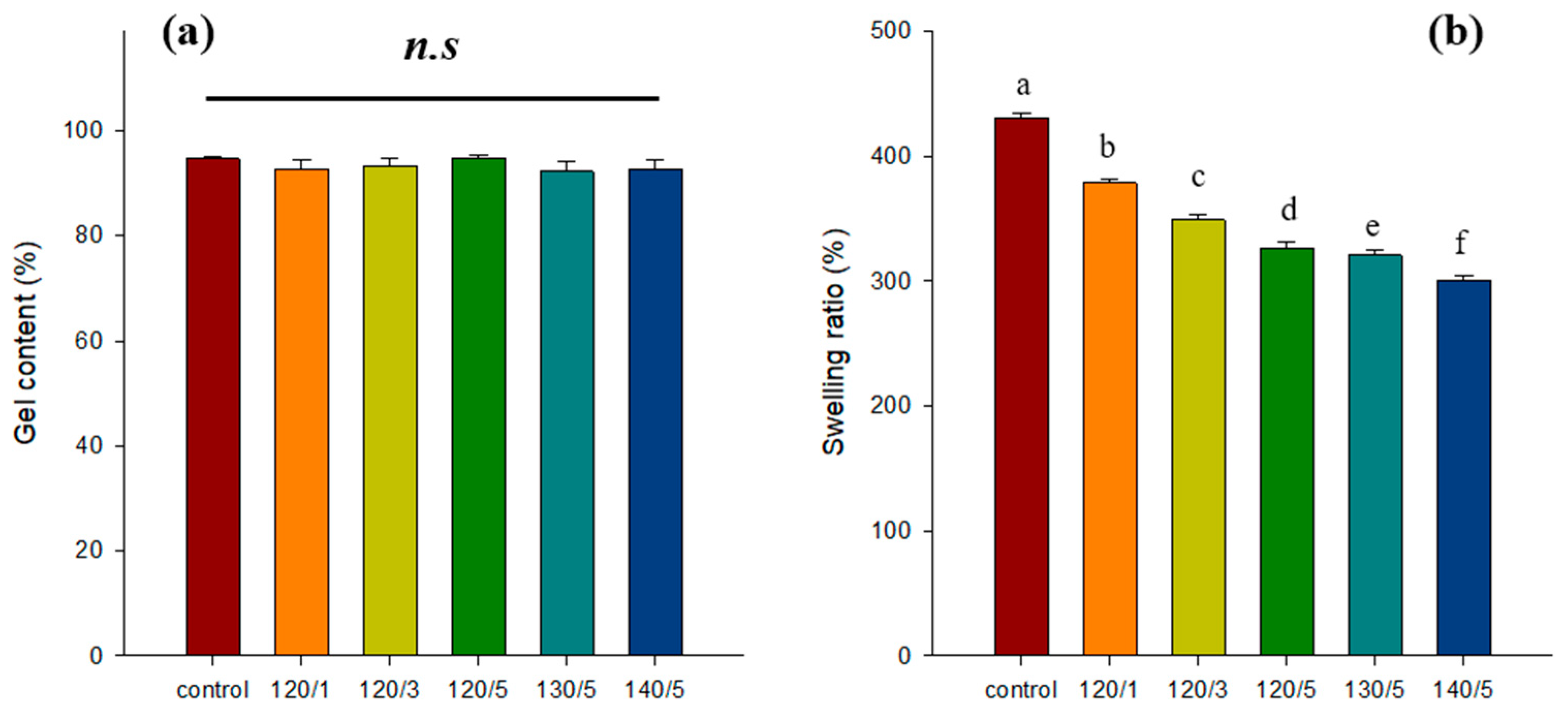
2.1. Gel Content and Swelling Ratio
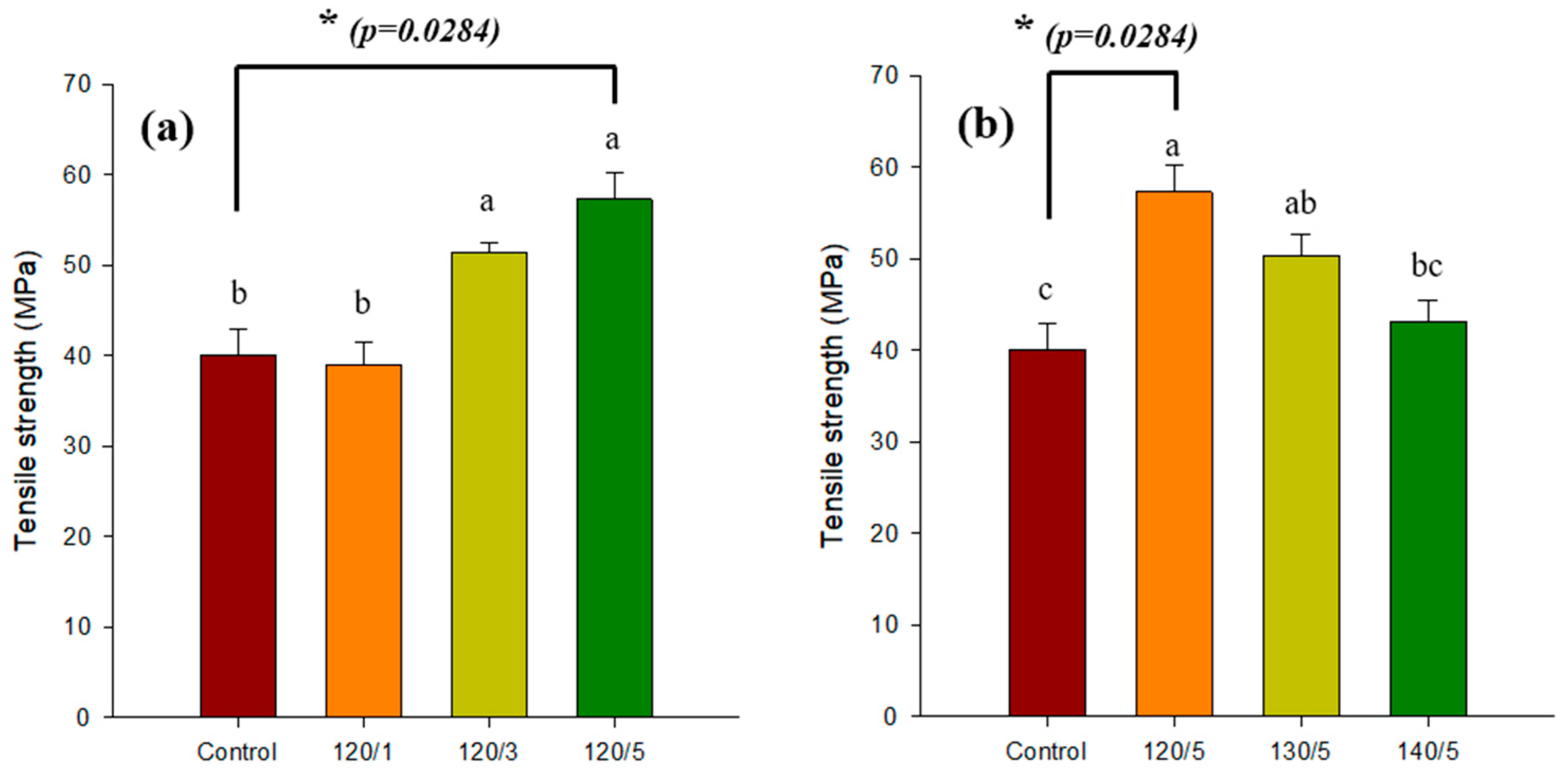
2.2. Gel Strength
2.3. FT-IR
2.4. DSC
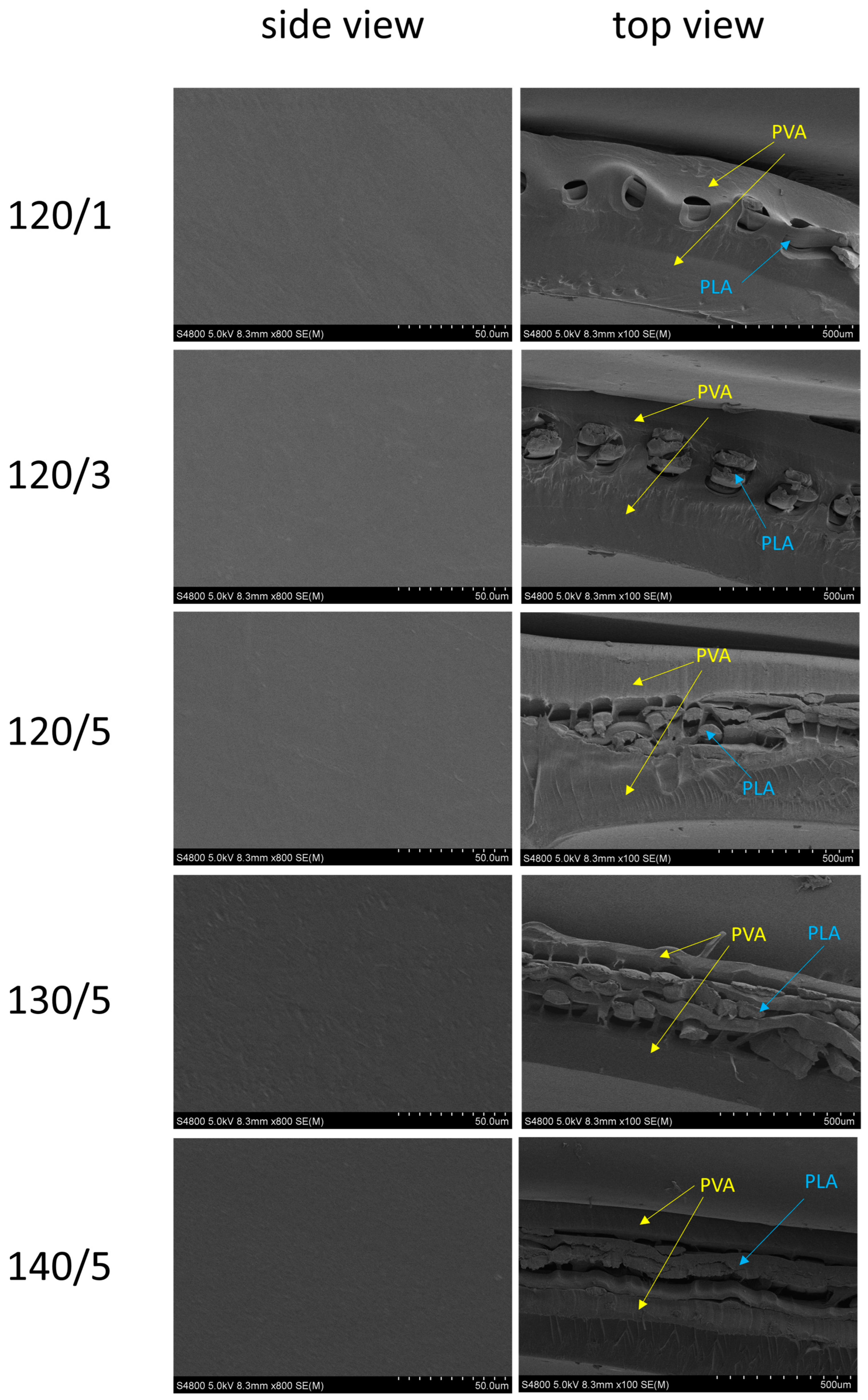
2.5. SEM
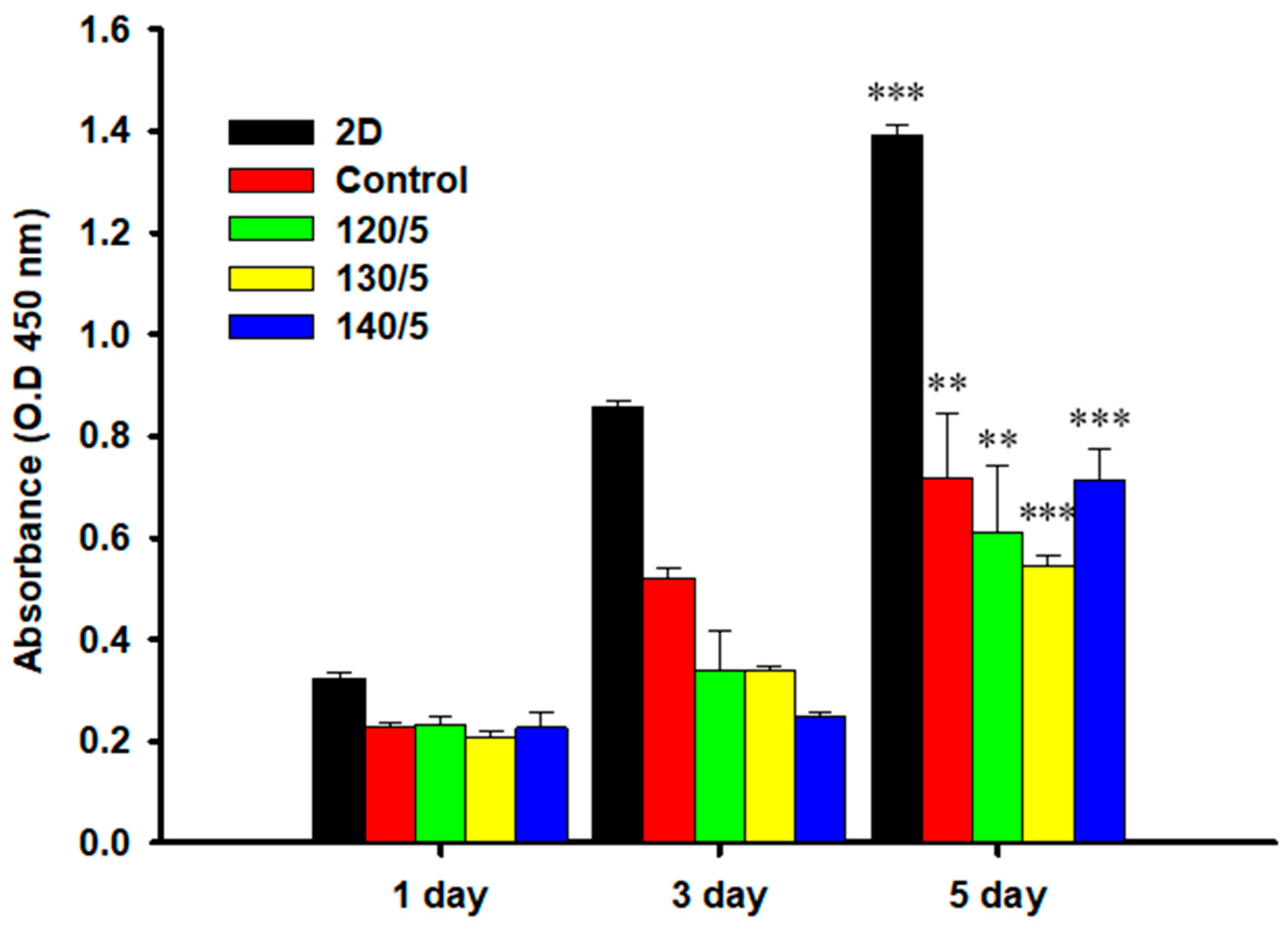
2.6. MTT Assay
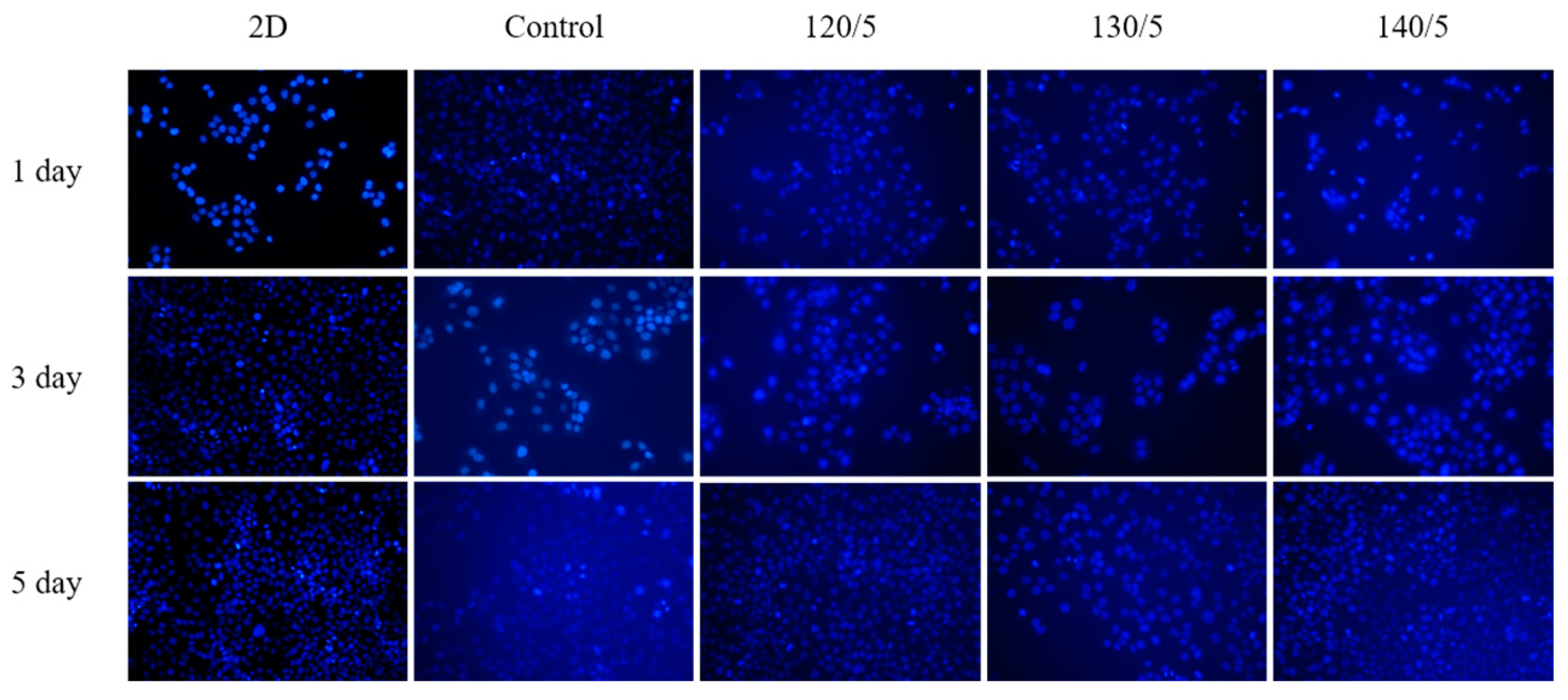
2.7. DAPI Staining
3. Conclusions
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of PVA/PLA Mesh Hydrogel Scaffold
4.3. Gel Content
4.4. Swelling Ratio
4.5. Gel Strength Analysis
4.6. FT-IR Analysis
4.7. DSC Analysis
4.8. SEM Analysis
4.9. MTT Assay
4.10. DAPI Staining
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capuana, E.; Lopresti, F.; Pavia, F.C.; Brucato, V.; La Carrubba, V. Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review. Polymers 2021, 13, 2041. [Google Scholar] [CrossRef] [PubMed]
- Manzini, B.M.; Machado, L.M.R.; Noritomi, P.Y.; da Silva, J.V.L. Advances in Bone tissue engineering: A fundamental review. J. Biosci. 2021, 46, 1–18. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, K.M.; Lee, K.S.; You, C.K.; Lee, Y.K. Enhanced Strength of the Tissue Engineering Scaffold. J. Korean Res. Soc. Dent. Mater. 2011, 38, 321–326. [Google Scholar]
- Amanda, J.R.W.; Hilldore, J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. J. Biomater. 2007, 28, 45–54. [Google Scholar]
- Harley, B.A.; Leung, J.H.; Silva, E.C.C.M.; Gibson, L.J. Mechanical characterization of collagen–glycosami-noglycan scaffolds. J. Acta Biomater. 2007, 3, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Paralikar, S.A.; Simonsen, J.; Lombardi, J. Poly(vinyl alcohol)/Cellulose Nanocrystal Barrier Membrane. J. Membr. Sci. 2008, 320, 248–258. [Google Scholar] [CrossRef]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly(vinyl alcohol) Films Crosslinked by Glutaraldehyde Under Mild Conditions. J. Appl. Polym. Sci. 2009, 111, 3074–3080. [Google Scholar] [CrossRef]
- Campos, E.; Coimbra, P.; Gill, M.H. An Improved Method for Preparing Glutaraldehyde Cross-linked Chitosan-poly(vinyl alcohol) Microparticles. J. Polym. Bull. 2013, 70, 549–561. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Badiei, A. Cross-linked Poly(vinyl alcohol)/Sulfonated Nanoporous Silica Hybrid Membranes for Proton Exchange Membrane Fuel Cell. J. Nanostruct. Chem. 2014, 97, 2–9. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly(vinyl alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. J. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking Reaction of Poly(vinyl alcohol) with Glyoxal. J. Polym. Res. 2010, 17, 725–730. [Google Scholar] [CrossRef]
- Krumova, M.; López, D.; Benavente, R.; Mijangos, C.; Perena, J.M. Effect of Crosslinking on the Mechanical and Thermal Properties of Poly(vinyl alcohol). J. Polym. 2000, 41, 9265–9272. [Google Scholar] [CrossRef]
- Miyazaki, T.; Takeda, Y.; Akane, S.; Itou, T.; Hoshiko, A.; En, K. Role of Boric Acid for a Poly(vinyl alcohol) Film as a Crosslinking Agent: Melting Behaviors of the Films with Boric Acid. J. Polym. 2010, 51, 5539–5549. [Google Scholar] [CrossRef]
- Lim, M.; Kwon, H.; Kim, D.; Seo, J.; Han, H.; Khan, S.B. Highly-enhanced Water Resistant and Oxygen Barrier Properties of Cross-linked Poly(vinyl alcohol) Hybrid Films for Packaging Application. J. Prog. Org. Coat. 2015, 85, 68–75. [Google Scholar] [CrossRef]
- Yin, Y.; Li, J.; Liu, Y.; Li, Z. Starch Crosslinked with Poly(vinyl alcohol) by Boric Acid. J. Appl. Polym. Sci. 2005, 96, 1394–1397. [Google Scholar] [CrossRef]
- Kim, G.; Kim, H.; Kang, H.J. Crosslinking Characteristic of Poly(vinyl alcohol) by Natural Dye. J. Polym. 2011, 35, 72–76. [Google Scholar]
- Jo, S.Y.; Lim, Y.M.; Youn, M.H.; Gwon, H.J.; Park, J.S.; Nho, Y.C.; Shin, H.S. Fabrication and Characterization of PVA/CMC Hydrogels by Freezing-Thawing Technique and Gamma-Ray Irradiation. J. Polym. 2009, 33, 551–554. [Google Scholar]
- Sahoo, S.K.; Panda, A.K.; Labhasetwar, V. Characterization of porous PLGA/PLA microparticles as a scaffold for three dimensional growth of breast cancer cells. J. Biomacromol. 2005, 6, 1132–1139. [Google Scholar] [CrossRef]
- Liu, X.; Gao, J.; Cui, X.; Nie, S.; Wu, X.; Zhang, L.; Tang, P.; Liu, J.; Li, M. Functionalized 3D-Printed PLA Biomimetic Scaffold for Repairing Critical-Size Bone Defects. J. Bioeng. 2023, 10, 1019. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, G.; Li, Z.; Lou, Z.; Gong, Z.; Wang, X.; Li, C.; Wang, B. A PLA-tPU based magnesium ion incorporated CSH/nHA bioactive porous composite scaffold for critical bone defect repair. J. Mater. Adv. 2023, 4, 3583. [Google Scholar] [CrossRef]
- Lee, G.; Lee, H.M.; Kim, Y.H. Thermal and Mechanical Properties of Poly(L-lactic Acid) Films Plasticized with Propylene Carbonate. J. Polym. 2019, 43, 113–122. [Google Scholar]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. J. Chem. Eng. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.; Jin, Y.Z.; Park, J.W.; Lee, K.M.; Han, S.H.; Choi, B.S.; Lee, J.H. Three-dimensional printed polylactic acid scaffold integrated with BMP-2 laden hydrogel for precise bone regeneration. J. Biomater. Res. 2021, 25, 35. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. J. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Nasr, M.; Awad, G.A.; Mansour, S.; Al Shamy, A.; Mortada, N.D. Hydrophilic versus hydrophobic porogens for engineering of poly(lactide-co-glycolide) microparticles containing risedronate sodium. J. Pharm. Dev. Technol. 2013, 18, 1078–1088. [Google Scholar] [CrossRef]
- Seo, Y.H.; Zo, S.M.; Han, S.S.; Oh, T.H. Study on Mechanical Properties and Biocompatibility of Scaffold Made of using PLA Mesh Fabric. J. Text. Sci. Eng. 2023, 60, 345–352. [Google Scholar]

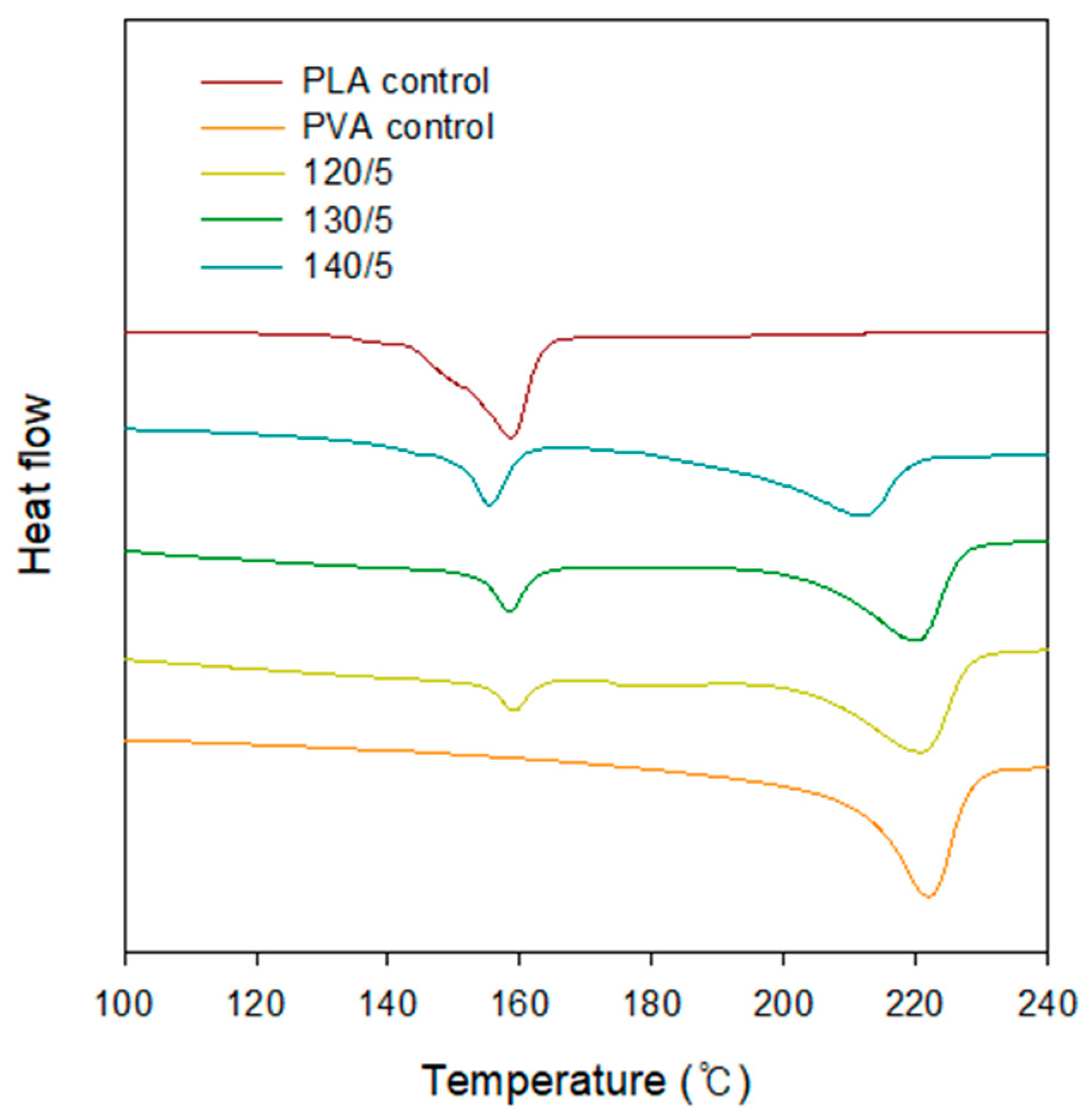

| Hot Press Temperature (°C)/Number of PLA Layer | Melting Temperature (°C) | |
|---|---|---|
| T1 | T2 | |
| Control | - | 222.01 |
| 120/5 | 158.88 | 221.30 |
| 130/5 | 158.28 | 220.59 |
| 140/5 | 155.39 | 211.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.-H.; Lee, J.-M.; Park, S.-Y.; Kim, M.-H.; Kim, S.-B.; Oh, T.-H. PVA Hydrogels Supplemented with PLA Mesh for Tissue Regeneration Scaffold. Gels 2024, 10, 364. https://doi.org/10.3390/gels10060364
Seo Y-H, Lee J-M, Park S-Y, Kim M-H, Kim S-B, Oh T-H. PVA Hydrogels Supplemented with PLA Mesh for Tissue Regeneration Scaffold. Gels. 2024; 10(6):364. https://doi.org/10.3390/gels10060364
Chicago/Turabian StyleSeo, Young-Ho, Jae-Man Lee, Sun-Young Park, Myung-Hoo Kim, Seon-Beom Kim, and Tae-Hwan Oh. 2024. "PVA Hydrogels Supplemented with PLA Mesh for Tissue Regeneration Scaffold" Gels 10, no. 6: 364. https://doi.org/10.3390/gels10060364






