Assessment of Alginate Gel Films as the Orodispersible Dosage Form for Meloxicam
Abstract
:1. Introduction
2. Results and Discussion
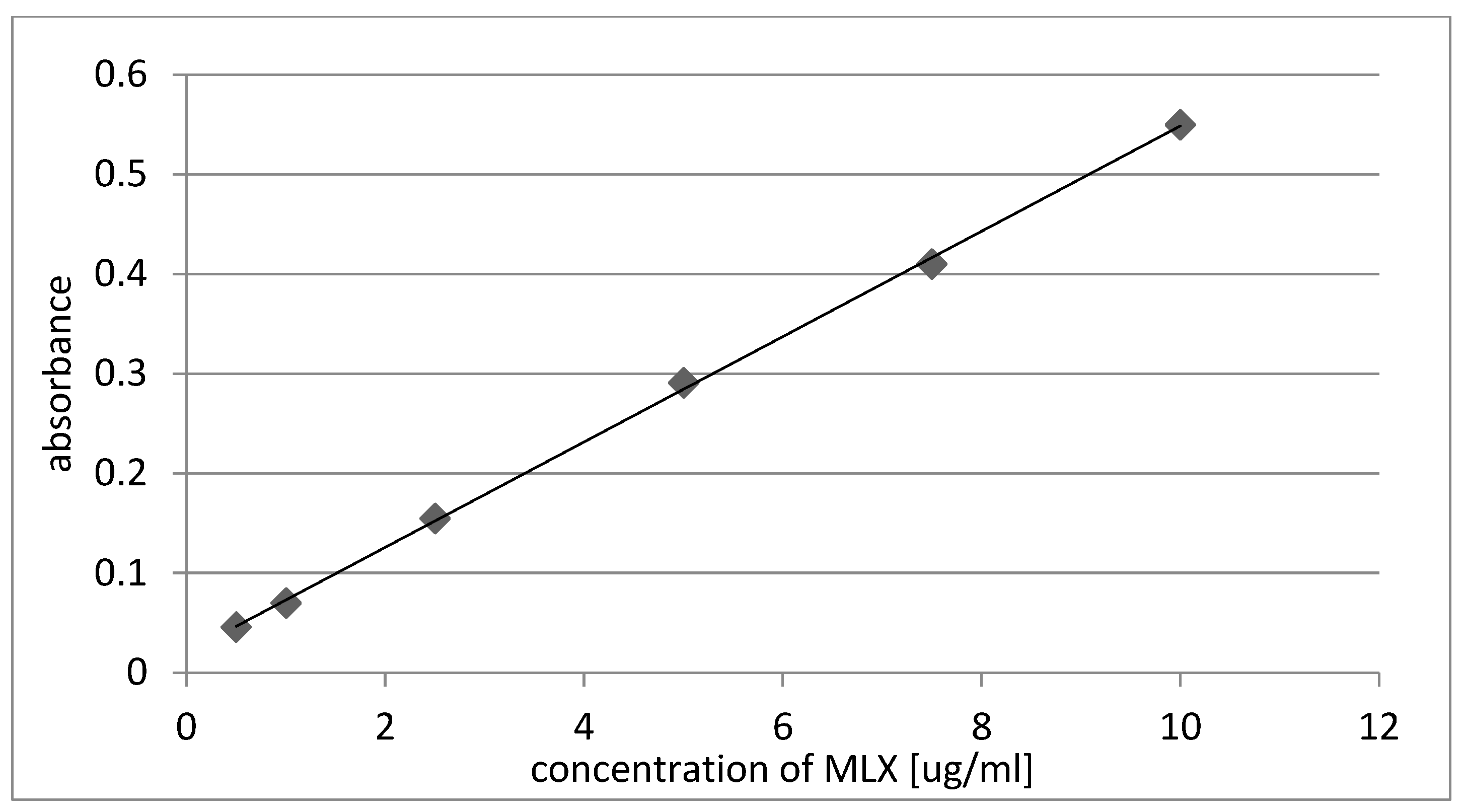
2.1. Amount of MLX
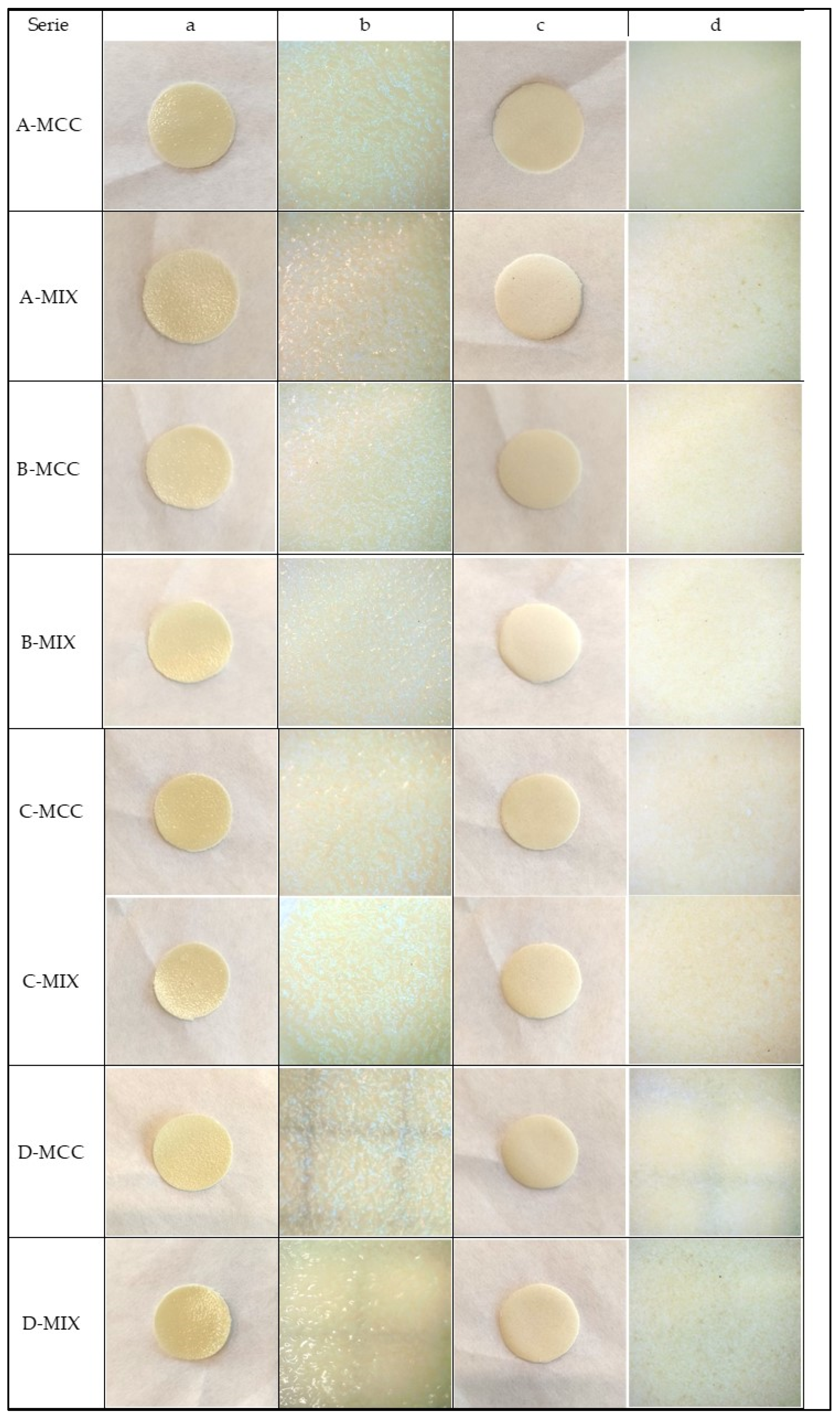
2.2. Appearance of Gel Films
2.3. Mass Uniformity of the Gel Films
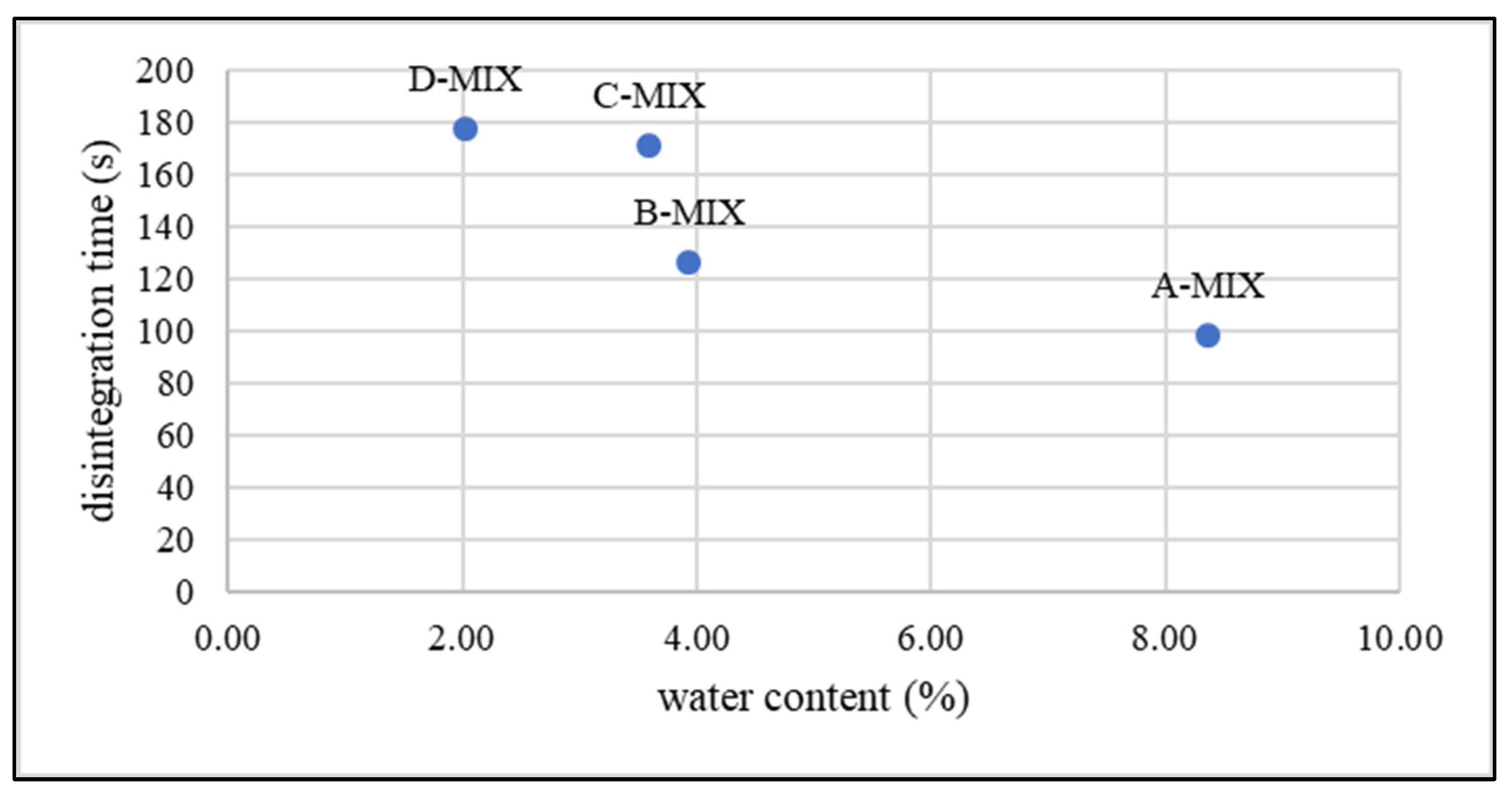
2.4. Water Content
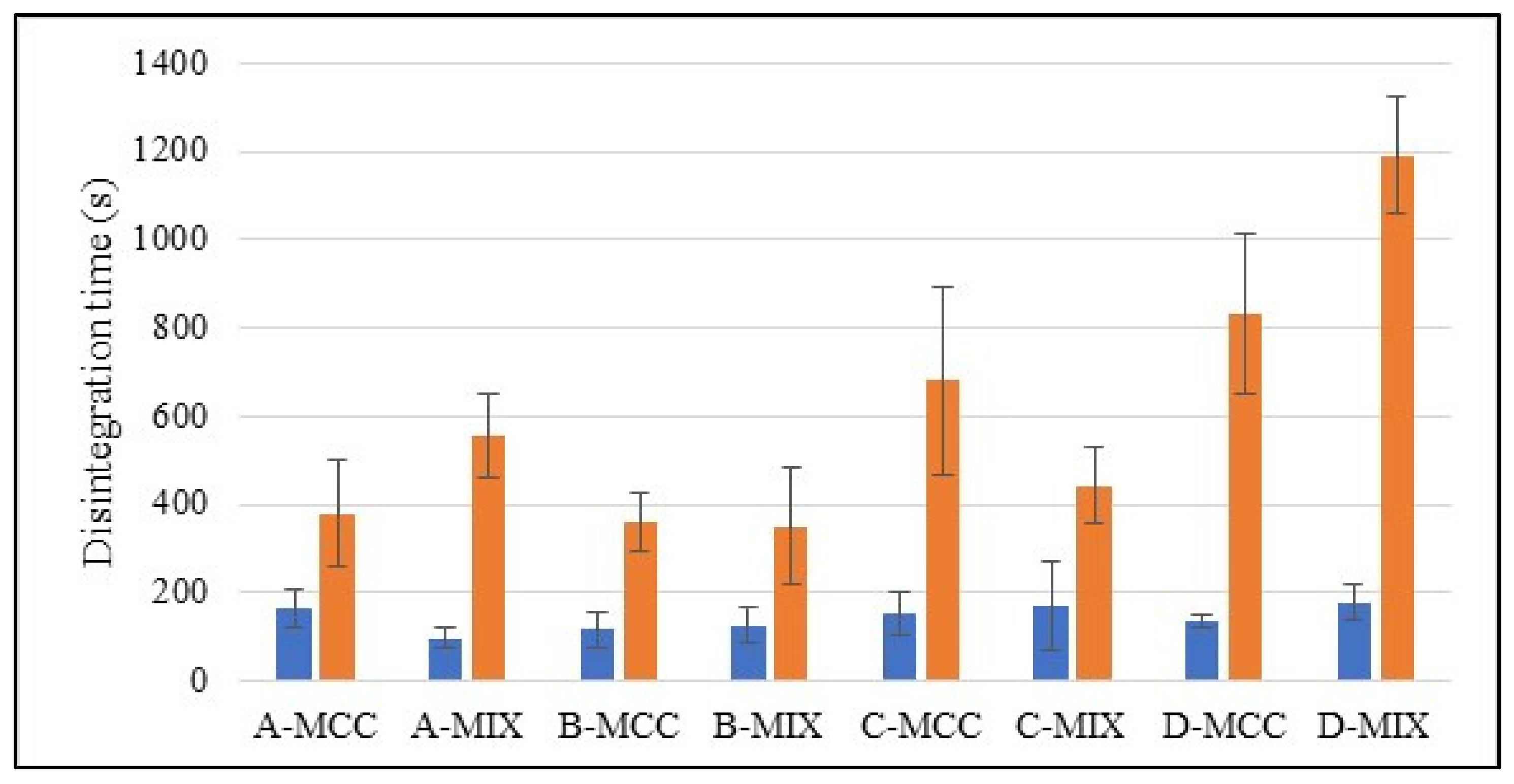
2.5. Disintegration Time
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Polymer Gel Films
4.3. Amount of MLX in Gel Films
4.4. Appearance, Size and Mass Uniformity of the Gel Films
4.5. Water Content
4.6. Disintegration Time of Gel Films
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Schiele, J.T.; Quinzler, R.; Klimm, H.-D.; Pruszydlo, M.G.; Haefeli, W.E. Difficulties swallowing solid oral dosage forms in a general practice population: Prevalence, causes, and relationship to dosage forms. Eur. J. Clin. Pharmacol. 2013, 69, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Khan, S.; Boateng, J.S.; Mitchell, J.; Trivedi, V. Formulation, Characterisation and Stabilisation of Buccal Films for Paediatric Drug Delivery of Omeprazole. AAPS PharmSciTech 2015, 16, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Krampe, R.; Sieber, D.; Pein-Hackelbusch, M.; Breitkreutz, J. A new biorelevant dissolution method for orodispersible films. Eur. J. Pharm. Biopharm. 2016, 98, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Khanna, S.; Pawar, P.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Olechno, K.; Basa, A.; Winnicka, K. ‘Success Depends on Your Backbone’—About the Use of Polymers as Essential Materials Forming Orodispersible Films. Materials 2021, 14, 4872. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Masen, M.; Cann, P.; Hanson, B.; Tuleu, C.; Orlu, M. Modernising Orodispersible Film Characterisation to Improve Palatability and Acceptability Using a Toolbox of Techniques. Pharmaceutics 2022, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Gupta, M.S.; Gowda, D.V.; Kumar, T.P.; Rosenholm, J.M. A Comprehensive Review of Patented Technologies to Fabricate Orodispersible Films: Proof of Patent Analysis (2000–2020). Pharmaceutics 2022, 14, 820. [Google Scholar] [CrossRef]
- Wojtyłko, M.; Froelich, A.; Jadach, B. Hypromellose-, Gelatin-and Gellan Gum-Based Gel Films with Chlorhexidine for Potential Application in Oral Inflammatory Diseases. Gels 2024, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Low, A.Q.J.; Parmentier, J.; Khong, Y.M.; Chai, C.C.E.; Tun, T.Y.; Berania, J.E.; Liu, X.; Gokhale, R.; Chan, S.Y. Effect of type and ratio of solubilising polymer on characteristics of hot-melt extruded orodispersible films. Int. J. Pharm. 2013, 455, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, A.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Development and evaluation of amorphous oral thin films using solvent-free processes: Comparison between 3d printing and hot-melt extrusion technologies. Pharmaceutics 2021, 13, 1613. [Google Scholar] [CrossRef] [PubMed]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.M.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The impact of the preparation method on the properties of orodispersible films with aripiprazole: Electrospinning vs. casting and 3D printing methods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Baek, S.H.; Lee, B.J.; Jin, H.E. Orodispersible polymer films with the poorly water-soluble drug, olanzapine: Hot-melt pneumatic extrusion for single-process 3D printing. Pharmaceutics 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Abraham, E.; Pothan, L.A.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable nanocomposite films based on sodium alginate and cellulose nanofibrils. Materials 2016, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Li, L. Enhanced stability and mechanical strength of sodium alginate composite films. Carbohydr. Polym. 2017, 160, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Janigová, N.; Elbl, J.; Pavloková, S.; Gajdziok, J. Effects of Various Drying Times on the Properties of 3D Printed Orodispersible Films. Pharmaceutics 2022, 14, 250. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef]
- Abedini, F.; Ebrahimi, M.; Roozbehani, A.H.; Domb, A.J.; Hosseinkhani, H. Overview on natural hydrophilic polysaccharide polymers in drug delivery. Polym. Adv. Technol. 2018, 29, 2564–2573. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhu, Y.; Men, Y.; Zeng, Y.; Sun, Y. Purification and characterization of a novel alginate lyase from the marine bacterium bacillus sp. Alg07. Mar. Drugs 2018, 16, 86. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K.; Kurakula, M.; Hoda, M.N. Alginate nanoparticles in drug delivery. In Alginates in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 129–152. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K. Alginate-inorganic composite particles as sustained drug delivery matrices. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–74. [Google Scholar] [CrossRef]
- Vicini, S.; Castellano, M.; Mauri, M.; Marsano, E. Gelling process for sodium alginate: New technical approach by using calcium rich micro-spheres. Carbohydr. Polym. 2015, 134, 767–774. [Google Scholar] [CrossRef]
- Kostenko, A.; Swioklo, S.; Connon, C.J. Alginate in corneal tissue engineering. Biomed. Mater. 2022, 17, 022004. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. Alginate-PEGAc: A new mucoadhesive polymer. Acta Biomater. 2011, 7, 625–633. [Google Scholar] [CrossRef]
- Patil, S.; Murthy, R.; Mahajan, H.; Wagh, R.; Gattani, S. Mucoadhesive polymers: Means of improving drug delivery. Pharma Times 2006, 38, 25–28. [Google Scholar]
- Borbolla-Jiménez, F.V.; Peña-Corona, S.I.; Farah, S.J.; Jiménez-Valdés, M.T.; Pineda-Pérez, E.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chávez, S.A.; Magaña, J.J.; Leyva-Gómez, G. Films for Wound Healing Fabricated Using a Solvent Casting Technique. Pharmaceutics 2023, 15, 1914. [Google Scholar] [CrossRef] [PubMed]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-Based Materials Loaded with Nanoparticles in Wound Healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.G.; Lin, Z.T.; Deng, S.T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2016, 63, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Park, J.H.; Shin, U.S.; Kim, H.W. Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering. Acta Biomater. 2011, 7, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Luger, P.; Daneck, K.; Engel, W.; Trummlitz, G.; Wagner, K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 1996, 4, 175–187. [Google Scholar] [CrossRef]
- Peesa, J.P.; Yalavarthi, P.R.; Rasheed, A.; Mandava, V.B.R. A perspective review on role of novel NSAID prodrugs in the management of acute inflammation. J. Acute Dis. 2016, 5, 364–381. [Google Scholar] [CrossRef]
- Davies, N.M.; Skjodt, N.M. Clinical pharmacokinetics of meloxicam: A cyclo-oxygenase-2 preferential nonsteroidal anti-inflammatory drug. Clin. Pharmacokinet. 1999, 36, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Euller-Ziegler, L.; Vélicitat, P.; Bluhmki, E.; Türck, D.; Scheuerer, S.; Combe, B. Meloxicam: A review of its pharmacokinetics, efficacy and tolerability following intramuscular administration. Inflamm. Res. 2001, 50 (Suppl. 1), S5–S9. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11339521 (accessed on 10 February 2024). [CrossRef] [PubMed]
- Meyer-Kirchrath, J.; Schror, K. Cyclooxygenase-2 Inhibition and Side-effects of Non-steroidal Antiinflammatory Drugs in the Gastrointestinal Tract. Curr. Med. Chem. 2000, 7, 1121–1129. [Google Scholar] [CrossRef]
- Del Tacca, M.; Colucci, R.; Fornai, M.; Blandizzi, C. Efficacy and Tolerability of Meloxicam, a COX-2 Preferential Nonsteroidal Anti-Inflammatory Drug. Clin. Drug Investig. 2002, 22, 799–818. [Google Scholar] [CrossRef]
- Scarpa, M.; Paudel, A.; Kloprogge, F.; Hsiao, W.K.; Bresciani, M.; Gaisford, S.; Orlu, M. Key acceptability attributes of orodispersible films. Eur. J. Pharm. Biopharm. 2018, 125, 131–140. [Google Scholar] [CrossRef]
- Jadach, B.; Misek, M.; Ferlak, J. Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery. Gels 2023, 9, 687. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Speer, I.; Steiner, D.; Thabet, Y.; Breitkreutz, J.; Kwade, A. Comparative study on disintegration methods for oral film preparations. Eur. J. Pharm. Biopharm. 2018, 132, 50–61. [Google Scholar] [CrossRef]



| Series | Mass Uniformity (g) | Amount of MLX (mg) |
|---|---|---|
| A-MCC | 0.124 ± 0.014 | 5.8 ± 0.4 |
| A-MIX | 0.129 ± 0.015 | 4.7 ± 0.4 |
| B-MCC | 0.119 ± 0.013 | 4.9 ± 0.3 |
| B-MIX | 0.114 ± 0.013 | 4.7 ± 0.4 |
| C-MCC | 0.134 ± 0.015 | 6.3 ± 0.3 |
| C-MIX | 0.128 ± 0.010 | 5.3 ± 0.1 |
| D-MCC | 0.123 ± 0.011 | 5.7 ± 0.5 |
| D-MIX | 0.124 ± 0.017 | 6.0 ± 1.0 |
| Serie | Cracking | Bending | Homogenity |
|---|---|---|---|
| A-MCC | did not crack | very slight breakage or non-existent | yes |
| A-MIX | cracked and curled slightly, but remained very flexible | no fracture visible and the film does not constantly deform | on the bottom side slight heterogeneity |
| B-MCC | only slightly cracked | slight break and deformation occurs | yes |
| B-MIX | cracked more than the B-MCC series | slight deformation when bent without any fractures | yes |
| C-MCC | cracked slightly | slight break when bending, but in general the films are flexible | yes |
| C-MIX | clearly cracked | no breakage but there is a slight deformation | heterogeneity on the bottom surface |
| D-MCC | visibly cracked and curled | fracture and deformation remain on the bent film | yes |
| D-MIX | visibly cracked and curled | fracture and deformation remain on the bent film | yes |
| Ingredient * | ALG-MCC | ALG-MIX |
|---|---|---|
| Weight (g) | ||
| Sodium alginate | 3.0 | 3.0 |
| PROSOLVE EASYtab SP | - | 2.0 |
| VIVAPUR 112 (microcrystaline cellulose) | 2.0 | 2.0 |
| Meloxicam | 0.5 | 0.5 |
| Glycerol | 5.0 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadach, B.; Kowalczyk, M.; Froelich, A. Assessment of Alginate Gel Films as the Orodispersible Dosage Form for Meloxicam. Gels 2024, 10, 379. https://doi.org/10.3390/gels10060379
Jadach B, Kowalczyk M, Froelich A. Assessment of Alginate Gel Films as the Orodispersible Dosage Form for Meloxicam. Gels. 2024; 10(6):379. https://doi.org/10.3390/gels10060379
Chicago/Turabian StyleJadach, Barbara, Martyna Kowalczyk, and Anna Froelich. 2024. "Assessment of Alginate Gel Films as the Orodispersible Dosage Form for Meloxicam" Gels 10, no. 6: 379. https://doi.org/10.3390/gels10060379







