Facile Synthesis of Surface-Modified Hollow-Silica (SiO2) Aerogel Particles via Oil–Water–Oil Double Emulsion Method
Abstract
1. Introduction
2. Results and Discussion
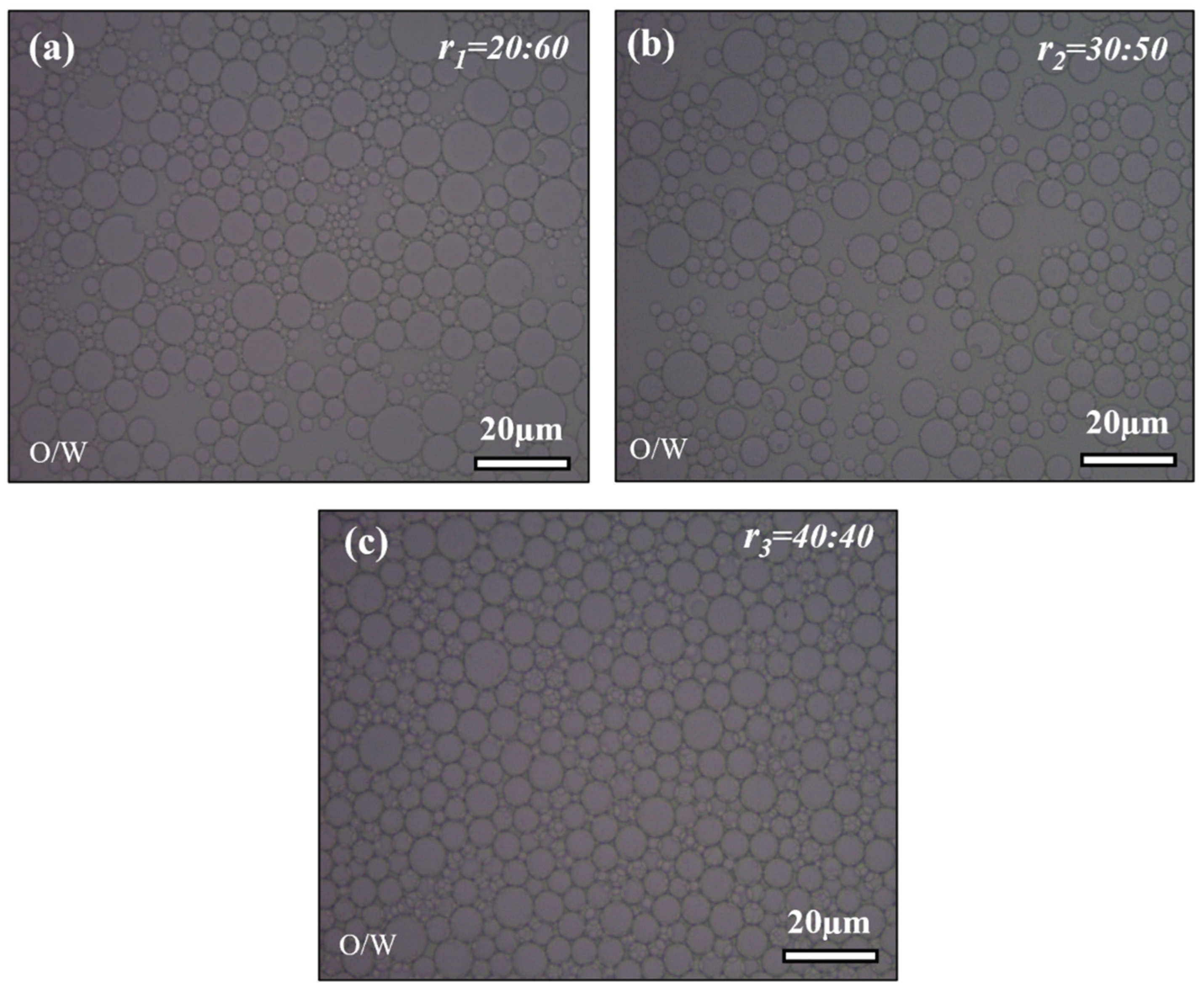
2.1. Effect of n-Hexane to Water Glass Ratio (r) in the Primary O/W Emulsion
2.1.1. Optical Microscope Analysis
2.1.2. SEM/TEM Analysis
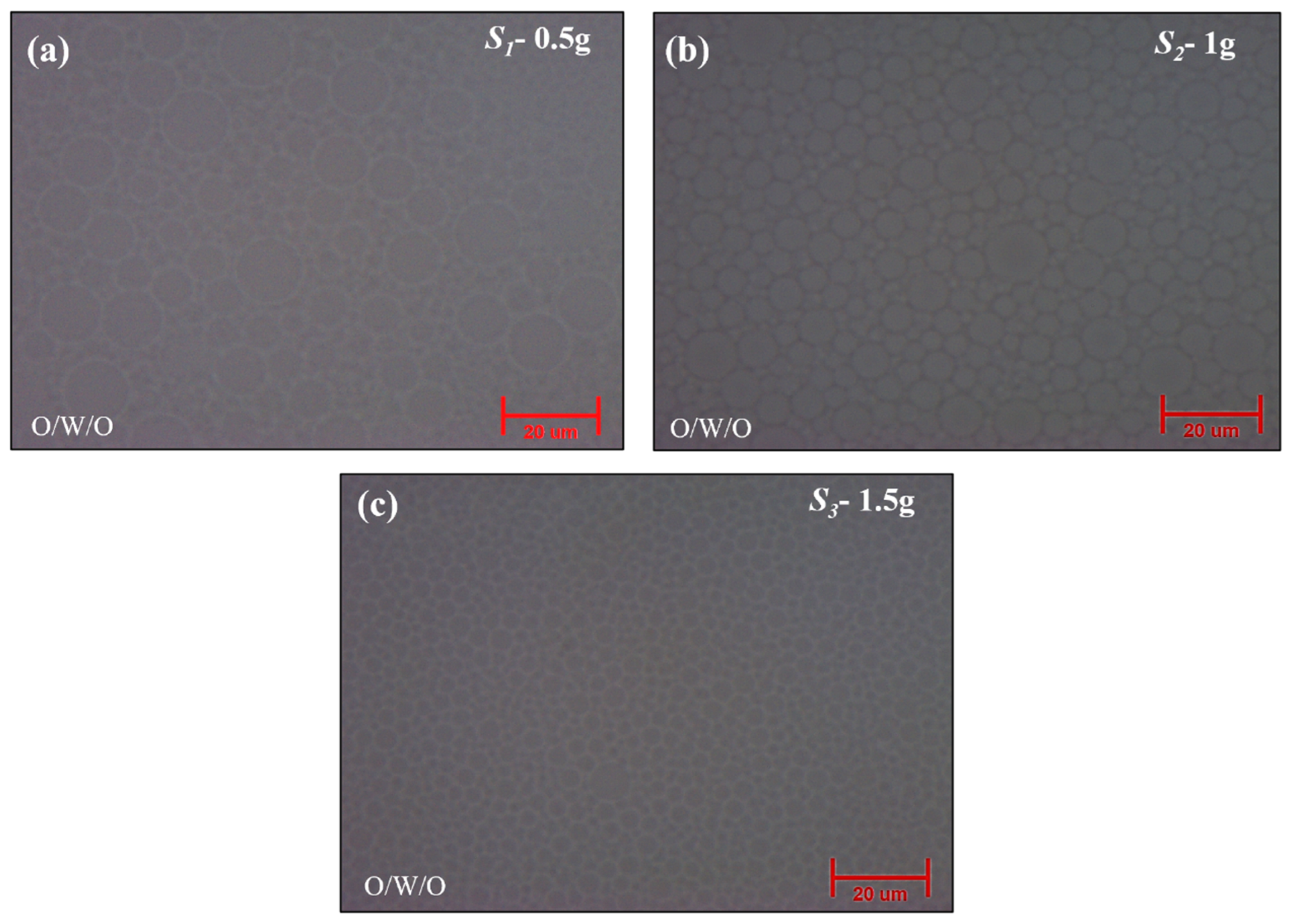
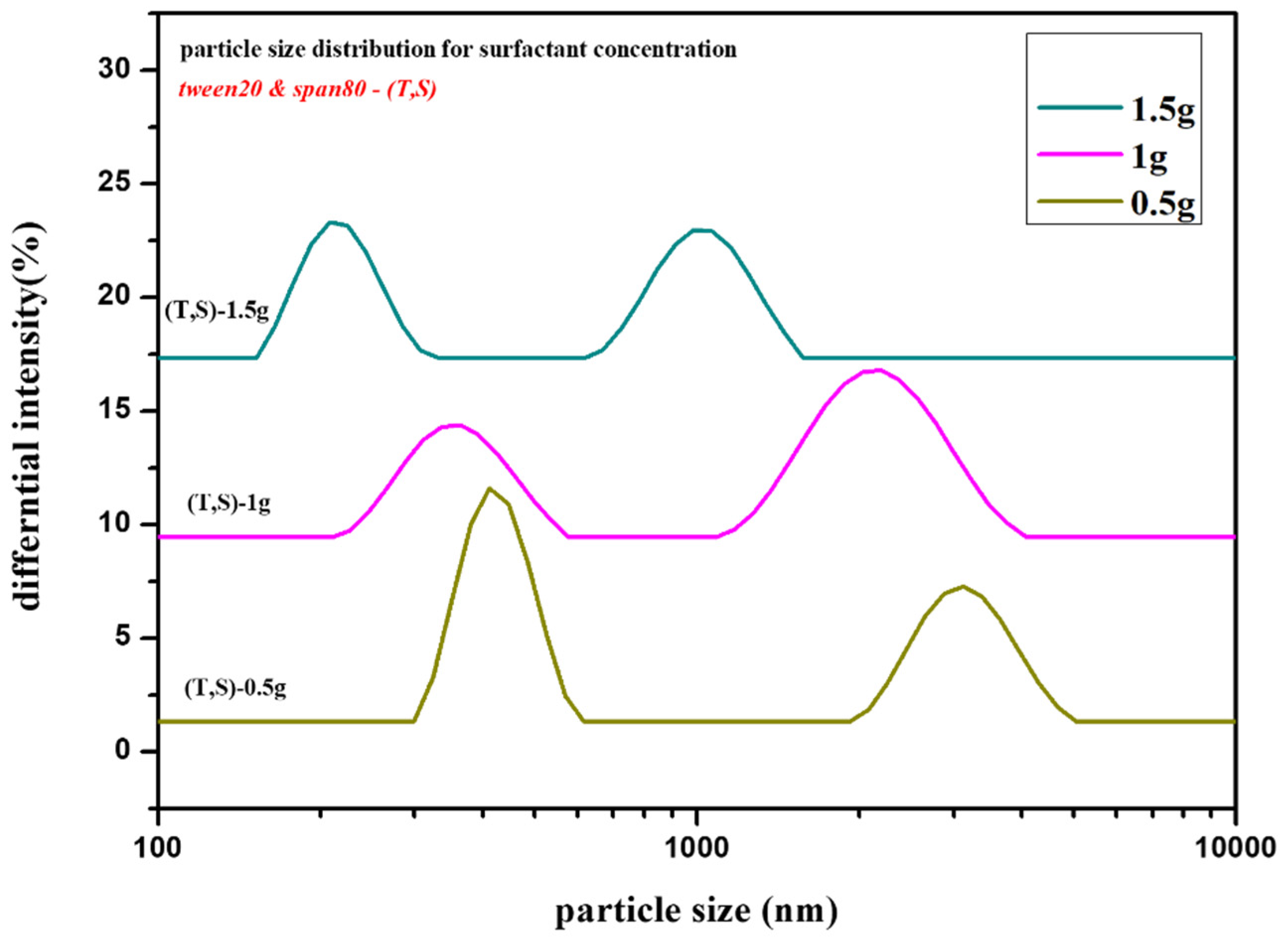
2.2. Effect of Surfactant [Tween20 and Span80] Concentration [s]
2.2.1. Optical Microscope Analysis
2.2.2. Zeta Potential
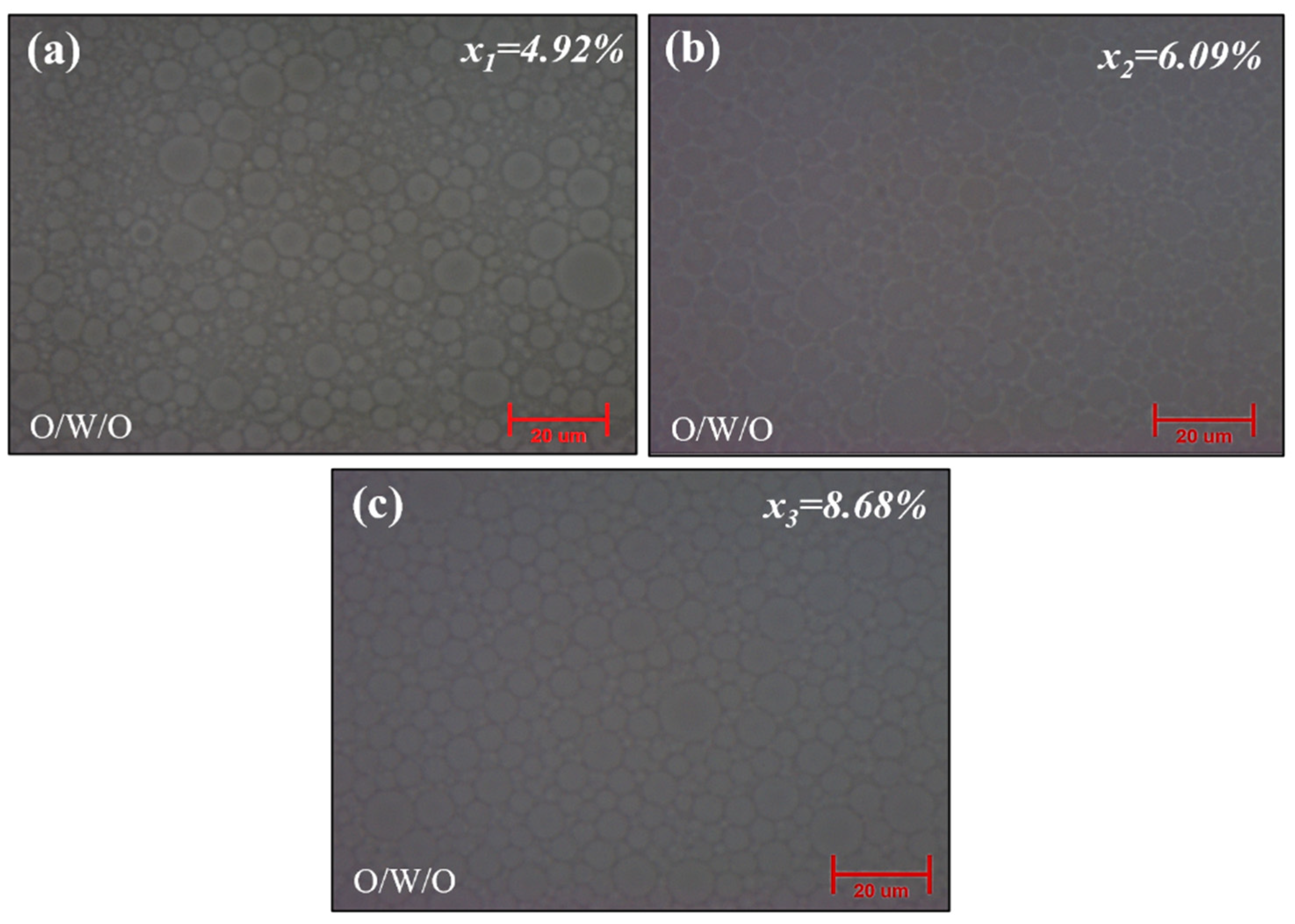
2.3. Effect of Silica (Water Glass) Content Concentration [x]
2.3.1. Formation Mechanism
- T is the thickness of the silica shell.
- C is the initial silica concentration.
- k is a proportionality constant.
- b is the intercept, representing the initial shell thickness when the silica concentration is minimal.
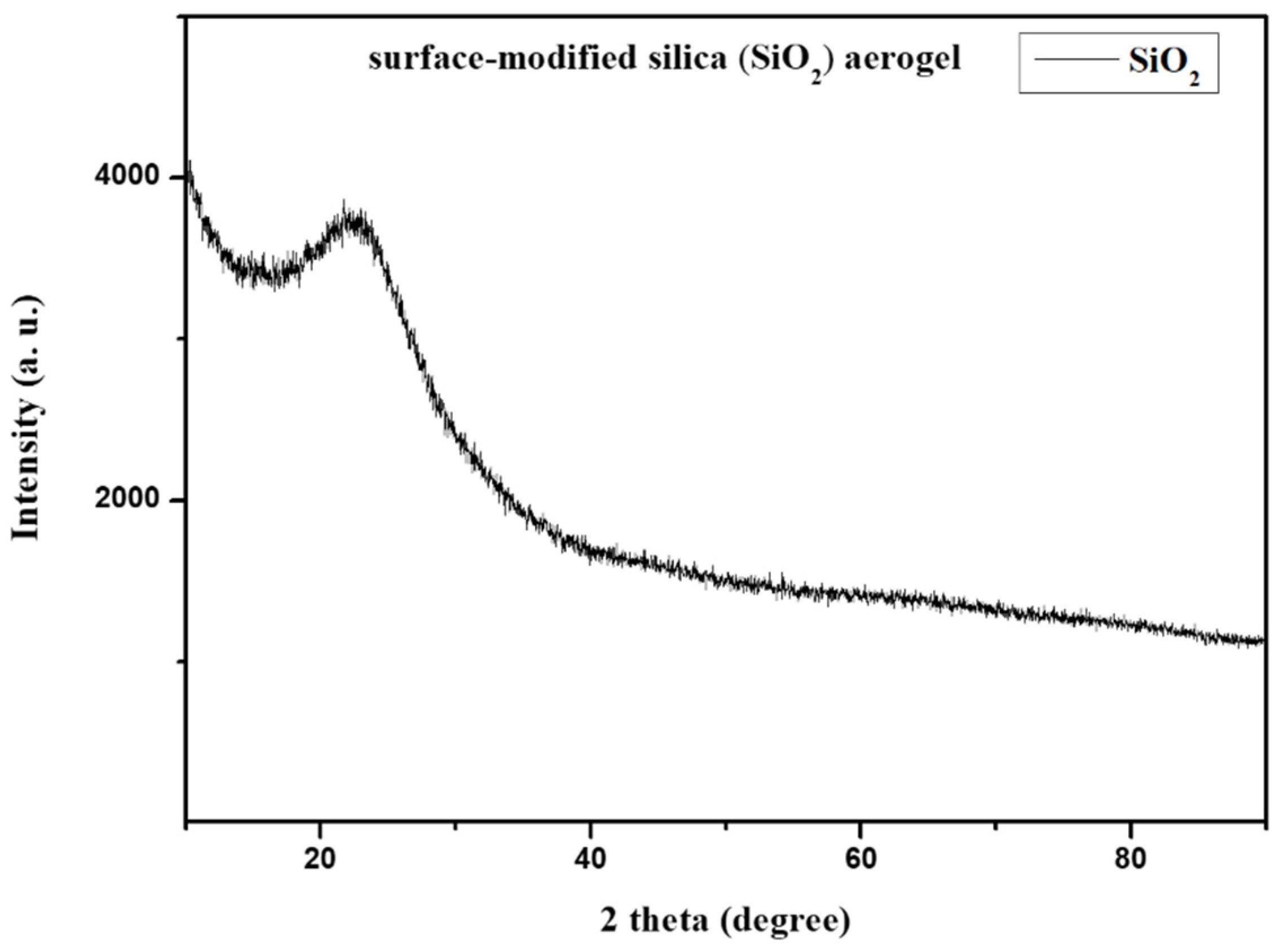
2.3.2. Morphological and Structural Study
3. Conclusions
4. Materials and Method
4.1. Materials
4.2. Method
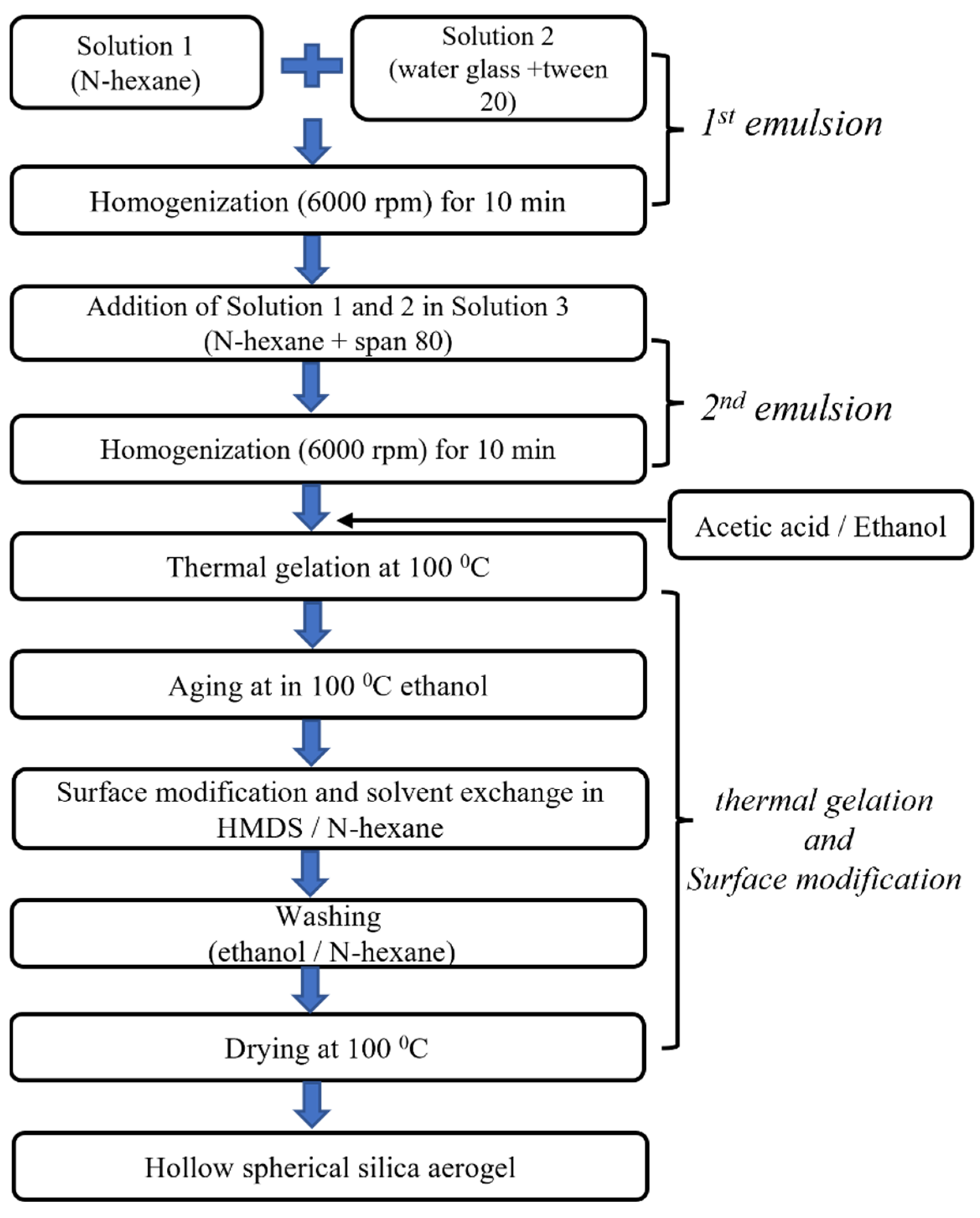
4.2.1. Preparation of Oil-in-Water-in-Oil (OWO) Double Emulsion
4.2.2. Preparation of Hollow Silica Aerogel Powder
4.2.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sambucci, M.; Savoni, F.; Valente, M. Aerogel Technology for Thermal Insulation of Cryogenic Tanks—Numerical Analysis for Comparison with Traditional Insulating Materials. Gels 2023, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.K.; Kim, D.H.; Lim, H.S.; Lee, S.J. Fabrication of Transparent Amorphous Silica by Controlling Forming and Sintering Processes with Spherical Nano-Silica Powder. J. Korean Ceram. Soc. 2022, 59, 787–795. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Bao, Y.; Shi, C.; Wang, T.; Li, X.; Ma, J. Recent Progress in Hollow Silica: Template Synthesis, Morphologies and Applications. Microporous Mesoporous Mater. 2016, 227, 121–136. [Google Scholar] [CrossRef]
- Kandi, K.K.; Punugupati, G.; Madhukar, P.; Rao, C.S.P. Effect of Boron Nitride (BN) on Mechanical and Dielectric Properties of Fused Silica Ceramic Composites. J. Korean Ceram. Soc. 2022, 59, 565–577. [Google Scholar] [CrossRef]
- Sharma, J.; Polizos, G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials 2020, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Larismaa, J.; Keski-Honkola, A.; Vilonen, K.; Söderberg, O.; Hannula, S.P. Effect of Synthesis Time on Morphology of Hollow Porous Silica Microspheres. Medziagotyra 2012, 18, 66–71. [Google Scholar] [CrossRef][Green Version]
- Ciriminna, R.; Sciortino, M.; Alonzo, G.; De Schrijver, A.; Pagliaro, M. From Molecules to Systems: Sol-Gel Microencapsulation in Silica-Based Materials. Chem. Rev. 2011, 111, 765–789. [Google Scholar] [CrossRef]
- Niu, L.; An, Y.; Yang, X.; Bian, G.; Wu, Q.; Xia, Z.; Bai, G. Highly Dispersed Ni Nanoparticles Encapsulated in Hollow Mesoporous Silica Spheres as an Efficient Catalyst for Quinoline Hydrogenation. Mol. Catal. 2021, 514, 111855. [Google Scholar] [CrossRef]
- Kosari, M.; Borgna, A.; Zeng, H.C. Transformation of Stöber Silica Spheres to Hollow Nanocatalysts. ChemNanoMat 2020, 6, 889–906. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, P.; Han, R.; Luo, C.; Wei, Q. A Target-Triggered Signal Chemiluminescence Sensor for Prostate Specific Antigen Detection Based on Hollow Porous Silica Encapsulated Luminol by Aptamers. Sens. Actuators B Chem. 2021, 333, 129543. [Google Scholar] [CrossRef]
- Mofid, S.A.; Jelle, B.P.; Zhao, X.; Gao, T.; Grandcolas, M.; Cunningham, B.; Ng, S.; Yang, R. Utilization of Size-Tunable Hollow Silica Nanospheres for Building Thermal Insulation Applications. J. Build. Eng. 2020, 31, 101336. [Google Scholar] [CrossRef]
- Zhou, C.; Qi, Y.; Zhang, S.; Niu, W.; Wu, S.; Ma, W.; Tang, B. Bilayer Heterostructure Photonic Crystal Composed of Hollow Silica and Silica Sphere Arrays for Information Encryption. Langmuir 2020, 36, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.L.A.; Taniguchi, S.; Nguyen, T.T.; Arif, A.F.; Iskandar, F.; Ogi, T. Precisely Tailored Synthesis of Hexagonal Hollow Silica Plate Particles and Their Polymer Nanocomposite Films with Low Refractive Index. J. Colloid Interface Sci. 2020, 571, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Phattharachindanuwong, C.; Hansupalak, N.; Plank, J.; Chisti, Y. Template-Assisted Facile Synthesis and Characterization of Hollow Calcium Silicate Hydrate Particles for Use as Reflective Materials. Mater. Res. Bull. 2018, 97, 343–350. [Google Scholar] [CrossRef]
- Teng, Z.; Han, Y.; Li, J.; Yan, F.; Yang, W. Preparation of Hollow Mesoporous Silica Spheres by a Sol-Gel/Emulsion Approach. Microporous Mesoporous Mater. 2010, 127, 67–72. [Google Scholar] [CrossRef]
- Hu, W.; Gu, H.; Wang, J.; Li, Y.; Wang, Z. One-Step Synthesis of Silica Hollow Particles in a W/O Inverse Emulsion. Colloid Polym. Sci. 2013, 291, 2697–2704. [Google Scholar] [CrossRef]
- Tan, M.N.; Park, Y.S. Synthesis of Stable Hollow Silica Nanospheres. J. Ind. Eng. Chem. 2009, 15, 365–369. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Xue, F.; Sun, G.; Luo, C.; Chen, J.; Xu, Q. A Simple and Efficient Route to Prepare Inorganic Hollow Microspheres Using Polymer Particles as Template in Supercritical Fluids. Colloids Surf. A Physicochem. Eng. Asp. 2010, 355, 45–52. [Google Scholar] [CrossRef]
- Fuji, M.; Shin, T.; Watanabe, H.; Takei, T. Shape-Controlled Hollow Silica Nanoparticles Synthesized by an Inorganic Particle Template Method. Adv. Powder Technol. 2012, 23, 562–565. [Google Scholar] [CrossRef]
- Song, J.C.; Xue, F.F.; Lu, Z.Y.; Sun, Z.Y. Controllable Synthesis of Hollow Mesoporous Silica Particles by a Facile One-Pot Sol-Gel Method. Chem. Commun. 2015, 51, 10517–10520. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990; 90, 33–72. [Google Scholar] [CrossRef]
- Palakurthy, S.; Azeem, P.A.; Reddy, K.V. Sol–Gel Synthesis of Soda Lime Silica-Based Bioceramics Using Biomass as Renewable Sources. J. Korean Ceram. Soc. 2022, 59, 76–85. [Google Scholar] [CrossRef]
- Fowler, C.E.; Khushalani, D.; Mann, S. Interfacial Synthesis of Hollow Microspheres of Mesostructured Silica. Chem. Commun. 2001, 1, 2028–2029. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Oh, C.; Shin, S.I.; Moon, S.K.; Oh, S.G. Preparation of Hollow Silica Microspheres in W/O Emulsions with Polymers. J. Colloid Interface Sci. 2003, 266, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gorsd, M.N.; Pizzio, L.R.; Blanco, M.N. Synthesis and Characterization of Hollow Silica Spheres. Procedia Mater. Sci. 2015, 8, 567–576. [Google Scholar] [CrossRef]
- Liu, M.; Gan, L.; Pang, Y.; Xu, Z.; Hao, Z.; Chen, L. Synthesis of Titania-Silica Aerogel-like Microspheres by a Water-in-Oil Emulsion Method via Ambient Pressure Drying and Their Photocatalytic Properties. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 490–495. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S.; Šubrt, J.; Szatmary, L. Titania Aerogel Prepared by Low Temperature Supercritical Drying. Microporous Mesoporous Mater. 2006, 91, 1–6. [Google Scholar] [CrossRef]
- Li, W.; Sha, X.; Dong, W.; Wang, Z. Synthesis of Stable Hollow Silica Microspheres with Mesoporous Shell in Nonionic W/O Emulsion. Chem. Commun. 2002, 2, 2434–2435. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Lee, J.M.; Nam, K.S.; Hwang, H. Thermal Gelation for Synthesis of Surface-Modified Silica Aerogel Powders. Gels 2021, 7, 242. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Rudžionis, Ž.; Tučkutė, S.; Ashish, D.K. Effects of Carbon Nanotubes on Expanded Glass and Silica Aerogel Based Lightweight Concrete. Sci. Rep. 2021, 11, 2104. [Google Scholar] [CrossRef]
- Dapčević Hadnadev, T.; Dokić, P.; Krstonošić, V.; Hadnadev, M. Influence of Oil Phase Concentration on Droplet Size Distribution and Stability of Oil-in-Water Emulsions. Eur. J. Lipid Sci. Technol. 2013, 115, 313–321. [Google Scholar] [CrossRef]
- Pouya, E.S.; Abolghasemi, H.; Fatoorehchi, H.; Rasem, B.; Hashemi, S.J. Effect of Dispersed Hydrophilic Silicon Dioxide Nanoparticles on Batch Adsorption of Benzoic Acid from Aqueous Solution Using Modified Natural Vermiculite: An Equilibrium Study. J. Appl. Res. Technol. 2016, 14, 325–337. [Google Scholar] [CrossRef]
- Cui, Z.G.; Yang, L.L.; Cui, Y.Z.; Binks, B.P. Effects of Surfactant Structure on the Phase Inversion of Emulsions Stabilized by Mixtures of Silica Nanoparticles and Cationic Surfactant. Langmuir 2010, 26, 4717–4724. [Google Scholar] [CrossRef] [PubMed]



| Sr. No | First Emulsion | Second Emulsion | Surfactant | Silica Content (%) | RPM | Time | ||
|---|---|---|---|---|---|---|---|---|
| Water Glass (mL) | N-Hexane (mL) | N-Hexane(ml) | Tween 20 (g) | Span 80 (g) | ||||
| 1 | 20 | 60 | 40 | 1 | 1 | 8.68 | 6000 | 20 min |
| 2 | 30 | 50 | ||||||
| 3 | 40 | 40 | ||||||
| 4 | 40 | 40 | 40 | 0.5 | 0.5 | 8.68 | 6000 | 20 min |
| 5 | 1.5 | 1.5 | ||||||
| 6 | 40 | 40 | 40 | 1 | 1 | 4.92 | 6000 | 20 min |
| 7 | 6.09 | |||||||
| 8 | 8.68 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapadnis, P.S.; Nam, K.-S.; Kim, H.-Y.; Park, H.-H.; Hwang, H. Facile Synthesis of Surface-Modified Hollow-Silica (SiO2) Aerogel Particles via Oil–Water–Oil Double Emulsion Method. Gels 2024, 10, 380. https://doi.org/10.3390/gels10060380
Kapadnis PS, Nam K-S, Kim H-Y, Park H-H, Hwang H. Facile Synthesis of Surface-Modified Hollow-Silica (SiO2) Aerogel Particles via Oil–Water–Oil Double Emulsion Method. Gels. 2024; 10(6):380. https://doi.org/10.3390/gels10060380
Chicago/Turabian StyleKapadnis, Pratik S., Ki-Sun Nam, Hyun-Young Kim, Hyung-Ho Park, and Haejin Hwang. 2024. "Facile Synthesis of Surface-Modified Hollow-Silica (SiO2) Aerogel Particles via Oil–Water–Oil Double Emulsion Method" Gels 10, no. 6: 380. https://doi.org/10.3390/gels10060380
APA StyleKapadnis, P. S., Nam, K.-S., Kim, H.-Y., Park, H.-H., & Hwang, H. (2024). Facile Synthesis of Surface-Modified Hollow-Silica (SiO2) Aerogel Particles via Oil–Water–Oil Double Emulsion Method. Gels, 10(6), 380. https://doi.org/10.3390/gels10060380










