Short-Term Effects of Two COX-2 Selective Non-Steroidal Anti-Inflammatory Drugs on the Release of Growth Factors and Cytokines from Canine Platelet-Rich Gel Supernatants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Platelet and Leukocyte Concentrations in Whole Blood and Hemocomponents
2.2. Growth Factor Release from Hemocomponents
2.3. Cytokine Release from Hemocomponents
3. Conclusions
4. Materials and Methods
4.1. Animals
4.2. Study Design, Blood Procurement, and Hemocomponent Processing
4.3. Growth Factor and Cytokine Assessment in Hemocomponents
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apostolakis, S.; Kapetanakis, S. Platelet-Rich Plasma for Degenerative Spine Disease: A Brief Overview. Spine Surg. Relat. Res. 2024, 8, 10–21. [Google Scholar] [CrossRef]
- Giannotti, L.; Di Chiara Stanca, B.; Spedicato, F.; Nitti, P.; Damiano, F.; Demitri, C.; Calabriso, N.; Carluccio, M.A.; Palermo, A.; Siculella, L.; et al. Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications. Genes 2023, 14, 1669. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Bai, J.; Zhang, H.; Jiang, B.; Wang, J.; Fu, L.; Long, H.; Huang, X.; Zhao, J.; et al. Current advancements in therapeutic approaches in orthopedic surgery: A review of recent trends. Front. Bioeng. Biotechnol. 2024, 12, 1328997. [Google Scholar] [CrossRef]
- Pineda-Cortel, M.R.; Suarez, C.; Cabrera, J.T.; Daya, M.; Bernardo-Bueno, M.M.; Vergara, R.C.; Villavieja, A. Biotherapeutic Applications of Platelet-Rich Plasma in Regenerative Medicine. Tissue Eng. Regen. Med. 2023, 20, 811–828. [Google Scholar] [CrossRef]
- Pretorius, J.; Habash, M.; Ghobrial, B.; Alnajjar, R.; Ellanti, P. Current Status and Advancements in Platelet-Rich Plasma Therapy. Cureus 2023, 15, e47176. [Google Scholar] [CrossRef]
- Vladulescu, D.; Scurtu, L.G.; Simionescu, A.A.; Scurtu, F.; Popescu, M.I.; Simionescu, O. Platelet-Rich Plasma (PRP) in Dermatology: Cellular and Molecular Mechanisms of Action. Biomedicines 2023, 12, 7. [Google Scholar] [CrossRef]
- Zhu, L.; Li, P.; Qin, Y.; Xiao, B.; Li, J.; Xu, W.; Yu, B. Platelet-rich plasma in orthopedics: Bridging innovation and clinical applications for bone repair. J. Orthop. Surg. 2024, 32, 10225536231224952. [Google Scholar] [CrossRef]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef]
- Herber, A.; Covarrubias, O.; Daher, M.; Tung, W.S.; Gianakos, A.L. Platelet rich plasma therapy versus other modalities for treatment of plantar fasciitis: A systematic review and meta-analysis. Foot Ankle Surg. 2024, 30, 285–293. [Google Scholar] [CrossRef]
- Tao, X.; Aw, A.A.L.; Leeu, J.J.; Bin Abd Razak, H.R. Three Doses of Platelet-Rich Plasma Therapy Are More Effective Than One Dose of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Arthroscopy 2023, 39, 2568–2576.e2562. [Google Scholar] [CrossRef]
- Xu, Y.; Li, T.; Wang, L.; Yao, L.; Li, J.; Tang, X. Platelet-Rich Plasma Has Better Results for Long-term Functional Improvement and Pain Relief for Lateral Epicondylitis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2024, 2024, 3635465231213087. [Google Scholar] [CrossRef]
- Carr, B.J.; Miller, A.V.; Colbath, A.C.; Peralta, S.; Frye, C.W. Literature review details and supports the application of platelet-rich plasma products in canine medicine, particularly as an orthobiologic agent for osteoarthritis. J. Am. Vet. Med. Assoc. 2024, 262, 1–8. [Google Scholar] [CrossRef]
- Gines, J.A. Effect of Leukoreduced Platelet Rich Plasma on Intra-Articular Pro-Inflammatory Cytokines in a Canine Pilot Study. Animals 2022, 12, 2163. [Google Scholar] [CrossRef]
- Kaneps, A.J. A one-health perspective: Use of hemoderivative regenerative therapies in canine and equine patients. J. Am. Vet. Med. Assoc. 2023, 261, 301–308. [Google Scholar] [CrossRef]
- Peng, C.; Yang, L.; Labens, R.; Gao, Y.; Zhu, Y.; Li, J. A systematic review and meta-analysis of the efficacy of platelet-rich plasma products for treatment of equine joint disease. Equine Vet. J. 2024; early view. [Google Scholar] [CrossRef]
- Pérez Fraile, A.; González-Cubero, E.; Martínez-Flórez, S.; Olivera, E.R.; Villar-Suárez, V. Regenerative Medicine Applied to Musculoskeletal Diseases in Equines: A Systematic Review. Vet. Sci. 2023, 10, 666. [Google Scholar] [CrossRef]
- Jayaram, P.; Yeh, P.; Patel, S.J.; Cela, R.; Shybut, T.B.; Grol, M.W.; Lee, B.H. Effects of Aspirin on Growth Factor Release From Freshly Isolated Leukocyte-Rich Platelet-Rich Plasma in Healthy Men: A Prospective Fixed-Sequence Controlled Laboratory Study. Am. J. Sports Med. 2019, 47, 1223–1229. [Google Scholar] [CrossRef]
- Mannava, S.; Whitney, K.E.; Kennedy, M.I.; King, J.; Dornan, G.J.; Klett, K.; Chahla, J.; Evans, T.A.; Huard, J.; La Prade, R.F. The Influence of Naproxen on Biological Factors in Leukocyte-Rich Platelet-Rich Plasma: A Prospective Comparative Study. Arthroscopy 2019, 35, 201–210. [Google Scholar] [CrossRef]
- Brown, K.A.; Gregorio, E.N.; Barot, D.; Usimaki, A.; Linardi, R.L.; Missanelli, J.R.; You, Y.; Robinson, M.A.; Ortved, K.F. Single-dose nonsteroidal anti-inflammatory drugs in horses have no impact on concentrations of cytokines or growth factors in autologous protein solution and platelet-rich plasma. Am. J. Vet. Res. 2024, 85, 1–9. [Google Scholar] [CrossRef]
- Meeson, R.L.; Todhunter, R.J.; Blunn, G.; Nuki, G.; Pitsillides, A.A. Spontaneous dog osteoarthritis—A One Medicine vision. Nat. Rev. Rheumatol. 2019, 15, 273–287. [Google Scholar] [CrossRef]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, D.C.; Buchanan, B.; Dwyer, H.C.; Gabriele, J.P.; Kelly, S.; Araujo, J.A. Penetration and efficacy of transdermal NSAIDs in a model of acute joint inflammation. J. Pain Res. 2018, 11, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Arnold, D.; Strasser, E.; Ringwald, J.; Schlegel, A.; Wiltfang, J.; Eckstein, R. Sample preparation technique and white cell content influence the detectable levels of growth factors in platelet concentrates. Vox Sang. 2003, 85, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Tambella, A.M.; Martin, S.; Cantalamessa, A.; Serri, E.; Attili, A.R. Platelet-rich Plasma and Other Hemocomponents in Veterinary Regenerative Medicine. Wounds 2018, 30, 329–336. [Google Scholar] [PubMed]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.U.; López, C.; Ceballos-Márquez, A. Temporal Release and Denature of Several Mediators in Pure Platelet-Rich Plasma and Temperature-Induced Platelet Lysates Derived from a Similar Bovine Platelet Concentrate. Vet. Med. Int. 2022, 2022, 2609508. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Goszka, M.; Gliźniewicz, M.; Grygorcewicz, B.; Serwin, N.; Stodolak, P.; Słodzińska, W.; Birger, R.; Polikowska, A.; Budkowska, M.; et al. The Effect of a Rotating Magnetic Field on the Regenerative Potential of Platelets. Int. J. Mol. Sci. 2024, 25, 3644. [Google Scholar] [CrossRef]
- Warin, R.; Vongchan, P.; Suriyasathaporn, W.; Boripun, R. In Vitro Assessment of Lyophilized Advanced Platelet-Rich Fibrin from Dogs in Promotion of Growth Factor Release and Wound Healing. Vet. Sci. 2022, 9, 566. [Google Scholar] [CrossRef]
- Lai, F.; Dai, S.; Zhao, Y.; Sun, Y. Combination of PDGF-BB and adipose-derived stem cells accelerated wound healing through modulating PTEN/AKT pathway. Injury 2023, 54, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Gumede, D.B.; Abrahamse, H.; Houreld, N.N. Targeting Wnt/β-catenin signaling and its interplay with TGF-β and Notch signaling pathways for the treatment of chronic wounds. Cell Commun. Signal. 2024, 22, 244. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Qian, M.; Qin, T.; Liu, M.; Xu, H.; Xu, B. Increased Expression of Inflammatory Cytokines and Discogenic Neck Pain. Orthop. Surg. 2024, 16, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.J.; Beaumont, R.E.; McClellan, A.; Sze, C.; Palomino Lago, E.; Hazelgrove, L.; Dudhia, J.; Smith, R.K.W.; Guest, D.J. Tumour necrosis factor alpha, interleukin 1 beta and interferon gamma have detrimental effects on equine tenocytes that cannot be rescued by IL-1RA or mesenchymal stromal cell-derived factors. Cell Tissue Res. 2023, 391, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, K.; Qiu, X. Exploring causal correlations between inflammatory cytokines and knee osteoarthritis: A two-sample Mendelian randomization. Front. Immunol. 2024, 15, 1362012. [Google Scholar] [CrossRef] [PubMed]
- Hazewinkel, H.A.; van den Brom, W.E.; Theyse, L.F.; Pollmeier, M.; Hanson, P.D. Comparison of the effects of firocoxib, carprofen and vedaprofen in a sodium urate crystal induced synovitis model of arthritis in dogs. Res. Vet. Sci. 2008, 84, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Pollmeier, M.; Toulemonde, C.; Fleishman, C.; Hanson, P.D. Clinical evaluation of firocoxib and carprofen for the treatment of dogs with osteoarthritis. Vet. Rec. 2006, 159, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, M.L.; Giguère, S.; Pozor, M.A.; Burden, C.A.; Berghaus, L.J.; Berghaus, R.D.; Varner, J.C.; Hayna, J.T.; Benson, S.M.; Randell, S.A.; et al. Evidence for anti-inflammatory effects of firocoxib administered to mares with experimentally induced placentitis. Am. J. Reprod. Immunol. 2021, 86, e13396. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Gallego, M.; López, C.; Carmona, J.U. Evaluation of the Pro-, Anti-Inflammatory, and Anabolic Effects of Autologous Platelet-Rich Gel Supernatants in an in vitro Coculture System of Canine Osteoarthritis. Vet. Med. Int. 2022, 2022, 3377680. [Google Scholar] [CrossRef]
- Okumo, T.; Sato, A.; Izukashi, K.; Ohta, M.; Oike, J.; Yagura, S.; Okuma, N.; Koya, T.; Sunagawa, M.; Kanzaki, K. Multifactorial Comparative Analysis of Platelet-Rich Plasma and Serum Prepared Using a Commercially Available Centrifugation Kit. Cureus 2023, 15, e48918. [Google Scholar] [CrossRef] [PubMed]
- Heiser, A.; McCarthy, A.; Wedlock, N.; Meier, S.; Kay, J.; Walker, C.; Crookenden, M.A.; Mitchell, M.D.; Morgan, S.; Watkins, K.; et al. Grazing dairy cows had decreased interferon-γ, tumor necrosis factor, and interleukin-17, and increased expression of interleukin-10 during the first week after calving. J. Dairy Sci. 2015, 98, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Carmona, J.U.; Rezende, C.M. Comparison of the effect of calcium gluconate and batroxobin on the release of transforming growth factor beta 1 in canine platelet concentrates. BMC Vet. Res. 2012, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.M.; Auchampach, J.A.; Drong, R.F.; Slightom, J.L. Cloning of a canine cDNA homologous to the human transforming growth factor-beta 1-encoding gene. Gene 1995, 155, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.L.; Dorneles, E.M.; Soares, R.P.; Magalhães, C.P.; Costa-Pereira, C.; Lage, A.P.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Araújo, M.S. Cross-reactivity of commercially available anti-human monoclonal antibodies with canine cytokines: Establishment of a reliable panel to detect the functional profile of peripheral blood lymphocytes by intracytoplasmic staining. Acta Vet. Scand. 2015, 57, 51. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, C.E.; Álvarez, M.E.; Carmona, J.U. Influence of calcium salts and bovine thrombin on growth factor release from equine platelet-rich gel supernatants. Vet. Comp. Orthop. Traumatol. 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Textor, J.A.; Willits, N.H.; Tablin, F. Synovial fluid growth factor and cytokine concentrations after intra-articular injection of a platelet-rich product in horses. Vet. J. 2013, 198, 217–223. [Google Scholar] [CrossRef]
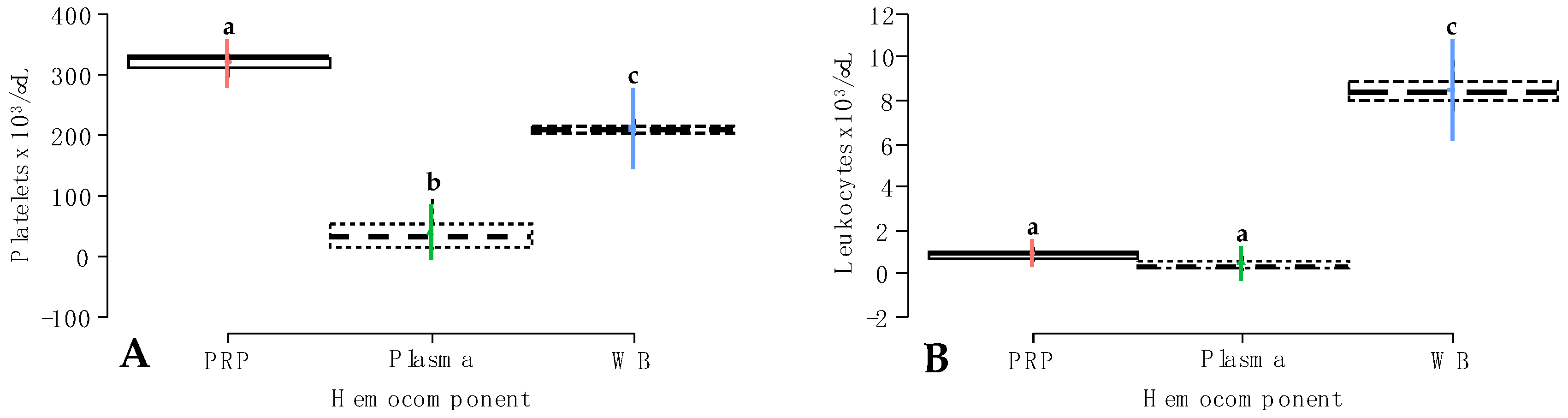
 Carprofen;
Carprofen;  Firocoxib. a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. TIPL = temperature-induced platelet lysate; PRGS = platelet-rich gel supernatant; CIPL = chemically induced platelet lysate. Other acronyms are the same as in Table 2.
Firocoxib. a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. TIPL = temperature-induced platelet lysate; PRGS = platelet-rich gel supernatant; CIPL = chemically induced platelet lysate. Other acronyms are the same as in Table 2.
 Carprofen;
Carprofen;  Firocoxib. a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. TIPL = temperature-induced platelet lysate; PRGS = platelet-rich gel supernatant; CIPL = chemically induced platelet lysate. Other acronyms are the same as in Table 2.
Firocoxib. a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. TIPL = temperature-induced platelet lysate; PRGS = platelet-rich gel supernatant; CIPL = chemically induced platelet lysate. Other acronyms are the same as in Table 2.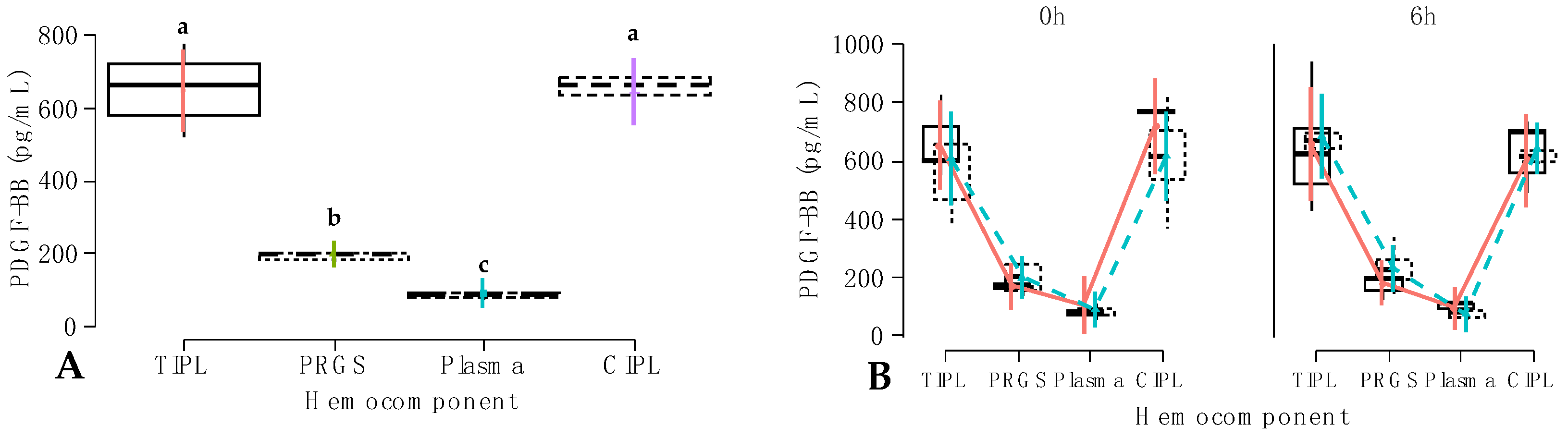
 Carprofen;
Carprofen;  Firocoxib. a–e = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 2.
Firocoxib. a–e = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 2.
 Carprofen;
Carprofen;  Firocoxib. a–e = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 2.
Firocoxib. a–e = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 2.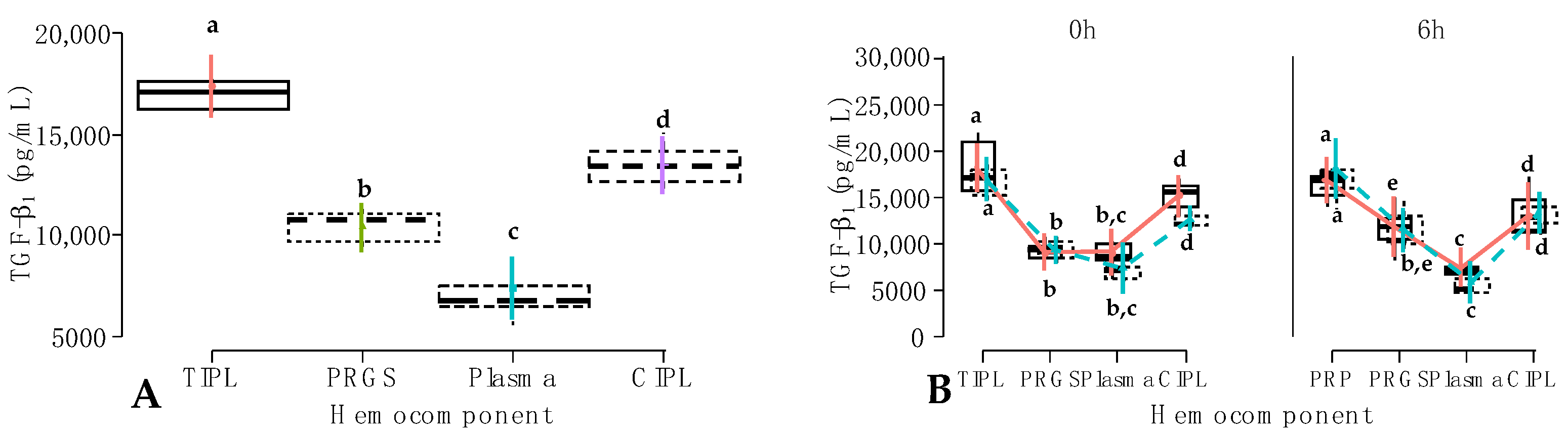
 One h;
One h;  6 h; (B) IL-1β concentrations (pg/mL) according to the interaction between hemocomponents, treatment, and time.
6 h; (B) IL-1β concentrations (pg/mL) according to the interaction between hemocomponents, treatment, and time.  Carprofen;
Carprofen;  Firocoxib. a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 3.
Firocoxib. a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 3.
 One h;
One h;  6 h; (B) IL-1β concentrations (pg/mL) according to the interaction between hemocomponents, treatment, and time.
6 h; (B) IL-1β concentrations (pg/mL) according to the interaction between hemocomponents, treatment, and time.  Carprofen;
Carprofen;  Firocoxib. a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 3.
Firocoxib. a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 3.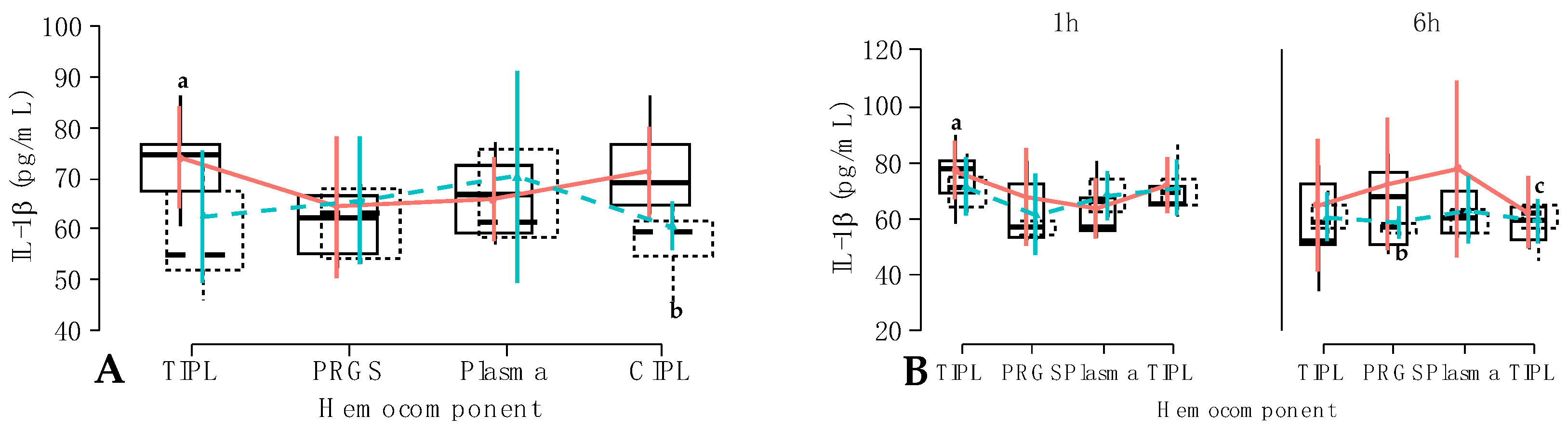
 Carprofen;
Carprofen;  Firocoxib; (B) IL-10 concentrations (pg/mL) according to the hemocomponent factor. a,b = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 3 and Table 4.
Firocoxib; (B) IL-10 concentrations (pg/mL) according to the hemocomponent factor. a,b = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 3 and Table 4.
 Carprofen;
Carprofen;  Firocoxib; (B) IL-10 concentrations (pg/mL) according to the hemocomponent factor. a,b = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 3 and Table 4.
Firocoxib; (B) IL-10 concentrations (pg/mL) according to the hemocomponent factor. a,b = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test. Acronyms are the same as in Figure 2 and Table 3 and Table 4.
 Carprofen;
Carprofen;  Firocoxib a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test.
Firocoxib a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test.
 Carprofen;
Carprofen;  Firocoxib a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test.
Firocoxib a–c = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test.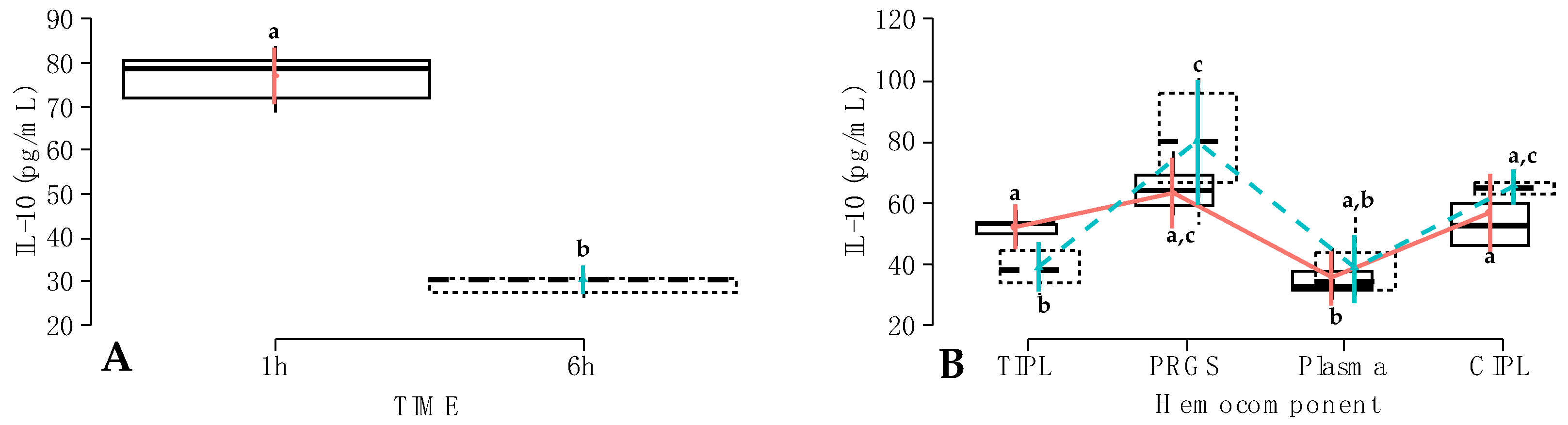
 Carprofen;
Carprofen;  Firocoxib. a–f = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test.
Firocoxib. a–f = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test.
 Carprofen;
Carprofen;  Firocoxib. a–f = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test.
Firocoxib. a–f = different lowercase letters denote significant differences (p < 0.001) for the evaluated variables by the Tukey test.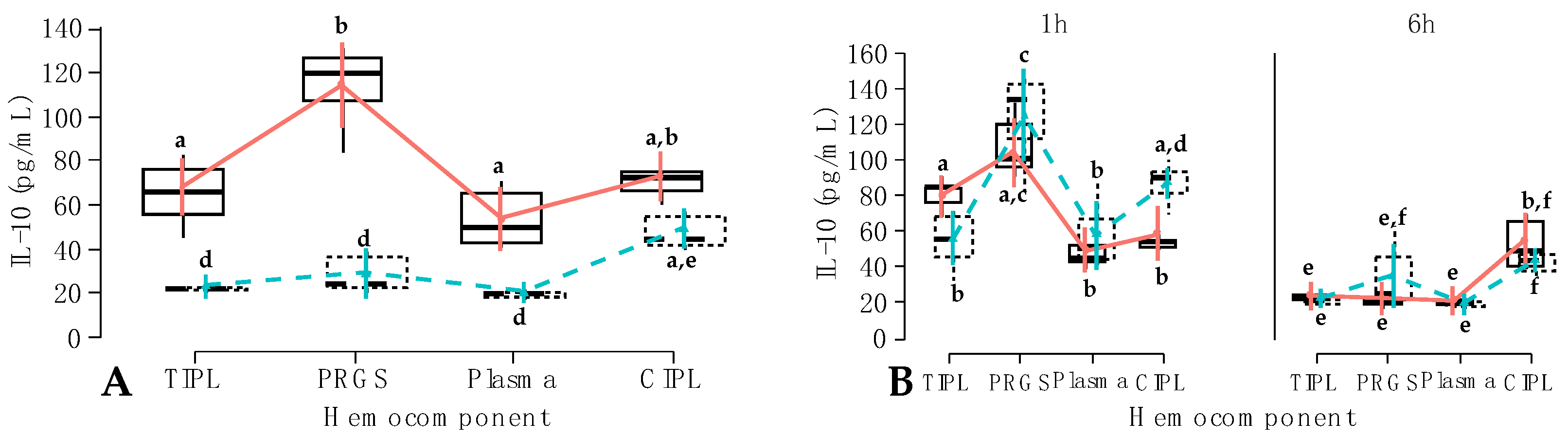
| GLMM Number | Fixed Effect | p-Value |
|---|---|---|
| Intercept | <0.001 | |
| Hemocomponent (HC) | <0.001 | |
| Treatment | 0.432 | |
| 1 | Time | 0.552 |
| HC × Treatment | 0.961 | |
| HC × Time | 0.816 | |
| Treatment × Time | 0.826 | |
| HC × Treatment × Time | 0.736 | |
| Intercept | <0.001 | |
| HC | <0.001 | |
| Treatment | 0.198 | |
| 2 | Time | 0.269 |
| HC × Treatment | 0.242 | |
| HC × Time | 0.156 | |
| Treatment × Time | 0.201 | |
| HC × Treatment × Time | 0.549 |
| GLMM Number | Fixed Effect | p-Value |
|---|---|---|
| Intercept | <0.001 | |
| Hemocomponent (HC) | <0.001 | |
| Treatment | 0.891 | |
| 3 | Time | 0.994 |
| HC × Treatment | 0.596 | |
| HC × Time | 0.215 | |
| Treatment × Time | 0.257 | |
| HC × Treatment × Time | 0.083 | |
| Intercept | <0.001 | |
| HC | <0.001 | |
| Treatment | 0.136 | |
| 4 | Time | 0.917 |
| HC × Treatment | 0.546 | |
| HC × Time | 0.058 | |
| Treatment × Time | 0.350 | |
| HC × Treatment × Time | 0.013 |
| GLMM Number | Fixed Effect | p-Value |
|---|---|---|
| Intercept | <0.001 | |
| Hemocomponent (HC) | 0.105 | |
| Treatment | 0.188 | |
| 5 | Time | 0.576 |
| HC × Treatment | 0.596 | |
| HC × Time | 0.019 | |
| Treatment × Time | 0.248 | |
| HC × Treatment × Time | <0.001 | |
| Intercept | <0.001 | |
| HC | 0.840 | |
| Treatment | 0.144 | |
| 6 | Time | 0.884 |
| HC × Treatment | 0.367 | |
| HC × Time | 0.553 | |
| Treatment × Time | 0.990 | |
| HC × Treatment × Time | <0.001 |
| GLMM Number | Fixed Effect | p-Value |
|---|---|---|
| Intercept | <0.001 | |
| Hemocomponent (HC) | 0.001 | |
| Treatment | 0.263 | |
| 7 | Time | <0.001 |
| HC × Treatment | 0.046 | |
| HC × Time | 0.001 | |
| Treatment × Time | 0.066 | |
| HC × Treatment × Time | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ospina, J.; Carmona, J.U.; López, C. Short-Term Effects of Two COX-2 Selective Non-Steroidal Anti-Inflammatory Drugs on the Release of Growth Factors and Cytokines from Canine Platelet-Rich Gel Supernatants. Gels 2024, 10, 396. https://doi.org/10.3390/gels10060396
Ospina J, Carmona JU, López C. Short-Term Effects of Two COX-2 Selective Non-Steroidal Anti-Inflammatory Drugs on the Release of Growth Factors and Cytokines from Canine Platelet-Rich Gel Supernatants. Gels. 2024; 10(6):396. https://doi.org/10.3390/gels10060396
Chicago/Turabian StyleOspina, Julián, Jorge U. Carmona, and Catalina López. 2024. "Short-Term Effects of Two COX-2 Selective Non-Steroidal Anti-Inflammatory Drugs on the Release of Growth Factors and Cytokines from Canine Platelet-Rich Gel Supernatants" Gels 10, no. 6: 396. https://doi.org/10.3390/gels10060396







