Vancomycin-Loaded Silk Fibroin/Calcium Phosphate/Methylcellulose-Based In Situ Thermosensitive Hydrogel: A Potential Function for Bone Regeneration
Abstract
1. Introduction
2. Results
2.1. Cell Viability
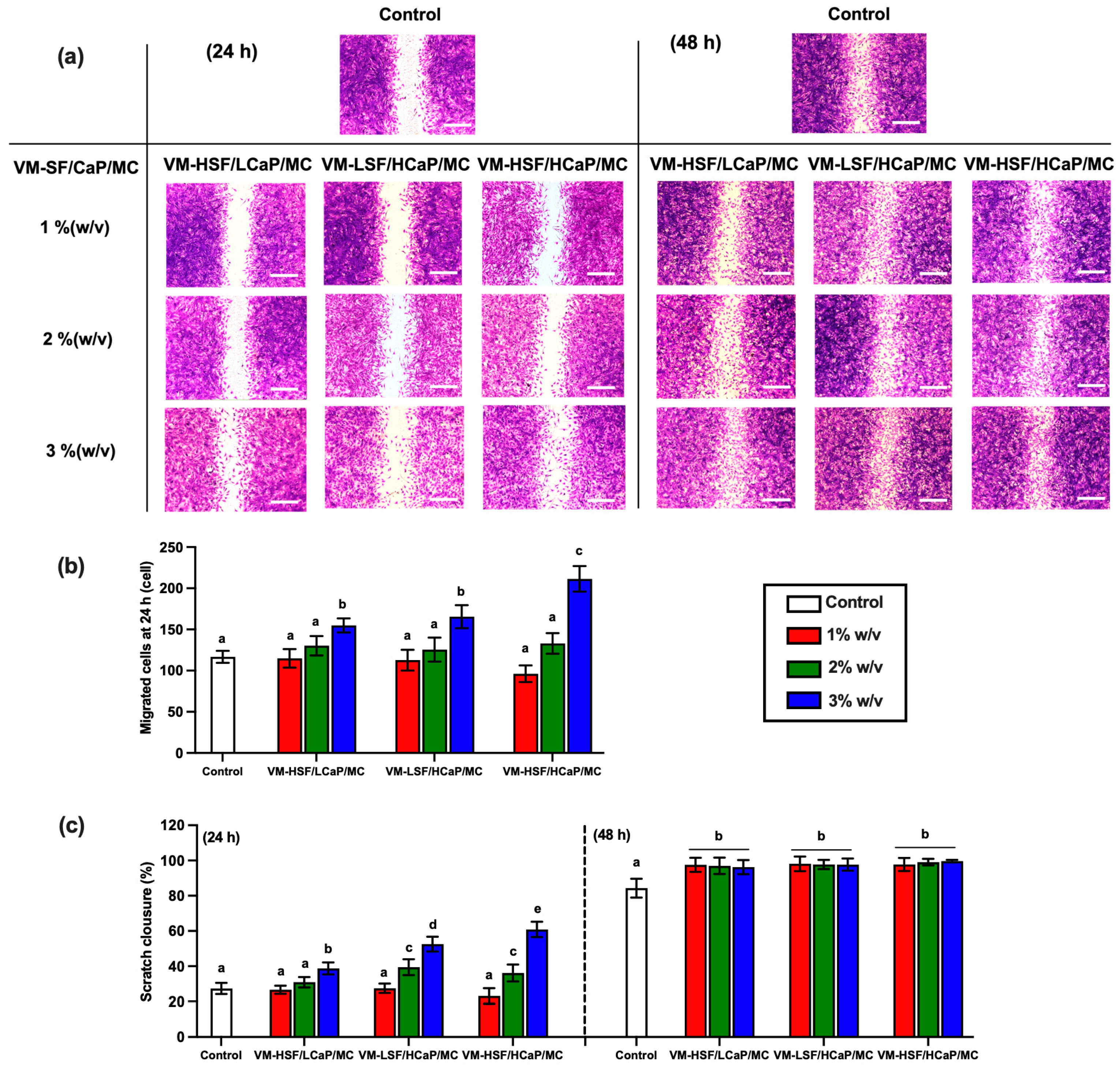
2.2. Cell Migration
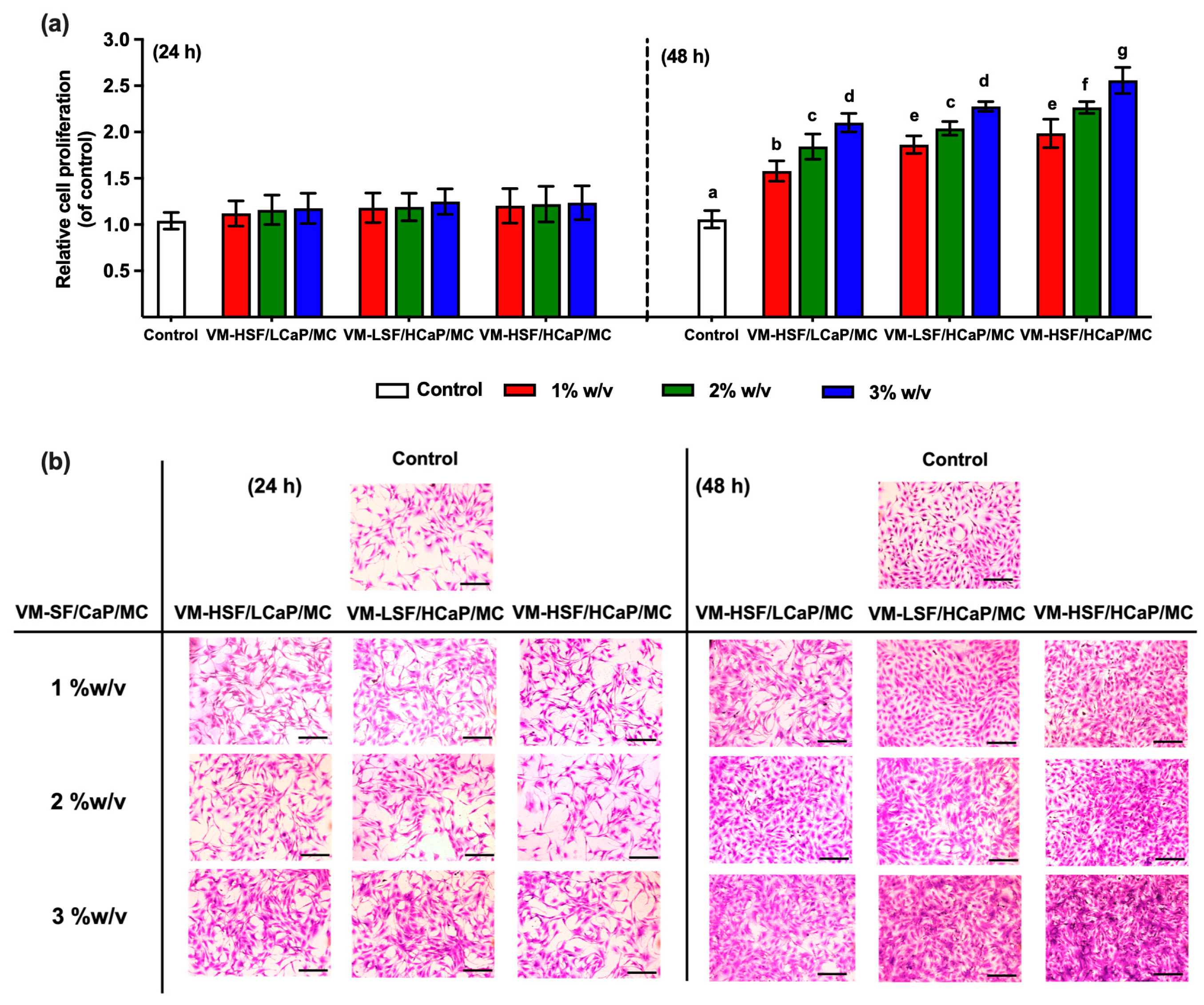
2.3. Cell Proliferation
2.4. Alkaline Phosphatase (ALP) Activity
2.5. Collagen Deposition
2.6. Extracellular Matrix Mineralization
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Extraction of Regenerated Silk Fibroin
5.3. Preparation of VM-SF/CaP/MC Hydrogels
5.4. Cell Culture and Specimen Preparation
5.5. Cell Viability Assay
5.6. Cell Migration Assay
5.7. Cell Proliferation Assay
5.8. Evaluation of Osteogenic Differentiation
5.8.1. Alkaline Phosphatase Activity
5.8.2. Collagen Deposition
5.8.3. Extracellular Matrix Mineralization
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel de Araujo, F.; Monaco, M.; Del Grosso, M.; Pirolo, M.; Visca, P.; Pantosti, A. Staphylococcus aureus clones causing osteomyelitis: A literature review (2000–2020). J. Glob. Antimicrob. Resist. 2021, 26, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Walter, G.; Kemmerer, M.; Kappler, C.; Hoffmann, R. Treatment Algorithms for Chronic Osteomyelitis. Dtsch. Ärztebl. Int. 2012, 109, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hatzenbuehler, J.; Pulling, T.J. Diagnosis and management of osteomyelitis. Am. Fam. Physician 2011, 84, 1027–1033. [Google Scholar] [CrossRef]
- Rao, S.; Kupfer, Y.; Pagala, M.; Chapnick, E.; Tessler, S. Systemic absorption of oral vancomycin in patients with Clostridium difficile infection. Scand. J. Infect. Dis. 2011, 43, 386–388. [Google Scholar] [CrossRef]
- Humphrey, C.; Veve, M.P.; Walker, B.; Shorman, M.A. Long-term vancomycin use had low risk of ototoxicity. PLoS ONE 2019, 14, e0224561. [Google Scholar] [CrossRef]
- Heck, D.; Rosenberg, A.; Schink-Ascani, M.; Garbus, S.; Kiewitt, T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J. Arthroplast. 1995, 10, 470–475. [Google Scholar] [CrossRef]
- Walenkamp, G.H.; Kleijn, L.L.; de Leeuw, M. Osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 years. Acta Orthop. Scand. 1998, 69, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.T.; Chen, C.F.; Chu, I.M.; Li, Y.M.; Hsu, W.H.; Hsu, R.W.; Chang, P.J. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials 2010, 31, 5227–5236. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, C.; Peng, X.; Xiao, H.; Guo, C.; Wang, X.; Li, L.; Yu, X. A promising material for bone repair: PMMA bone cement modified by dopamine-coated strontium-doped calcium polyphosphate particles. R. Soc. Open Sci. 2019, 6, 191028. [Google Scholar] [CrossRef]
- Phewchan, P.; Laoruengthana, A.; Lamlertthon, S.; Tiyaboonchai, W. Injectable vancomycin-loaded silk fibroin/methylcellulose containing calcium phosphate-based in situ thermosensitive hydrogel for local treatment of osteomyelitis: Fabrication, characterization, and in vitro performance evaluation. J. Biomed. Mater. Res. Part A 2024, 112, 2210–2224. [Google Scholar] [CrossRef]
- Manissorn, J.; Wattanachai, P.; Tonsomboon, K.; Bumroongsakulsawat, P.; Damrongsakkul, S.; Thongnuek, P. Osteogenic enhancement of silk fibroin-based bone scaffolds by forming hybrid composites with bioactive glass through GPTMS during sol-gel process. Mater. Today Commun. 2021, 26, 101730. [Google Scholar] [CrossRef]
- Phewchan, P.; Laoruengthana, A.; Tiyaboonchai, W. Calcium phosphate incorporated in silk fibroin/methylcellulose based injectable hydrogel: Preparation, characterization, and in vitro biological evaluation for bone defect treatment. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1640–1652. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wu, F.; Xing, T.; Yadavalli, V.K.; Kundu, S.C.; Lu, S. A silk fibroin hydrogel with reversible sol–gel transition. RSC Adv. 2017, 7, 24085–24096. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; Magarinos, B.; Ferreira-Goncalves, T.; Starbird-Perez, R.; Alvarez-Lorenzo, C.; Reis, C.P.; Ardao, I.; Garcia-Gonzalez, C.A. Vancomycin-loaded methylcellulose aerogel scaffolds for advanced bone tissue engineering. Carbohydr. Polym. 2024, 324, 121536. [Google Scholar] [CrossRef]
- Thonglam, J.; Nuntanaranont, T.; Meesane, J. Silk fibroin incorporated with methylcellulose as mutually functional scaffolds: Bone nucleation and biological signal attachment. MRS Commun. 2021, 11, 596–602. [Google Scholar] [CrossRef]
- Narayanan, V.; Sumathi, S. Preparation, characterization and in vitro biological study of silk fiber/methylcellulose composite for bone tissue engineering applications. Polym. Bull. 2018, 76, 2777–2800. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, T.; Long, L.; Yang, Y.; Yang, X.; Luo, L.; Li, J.; Chen, Z.; Zou, C.; Luo, S. A dual PMMA/calcium sulfate carrier of vancomycin is more effective than PMMA-vancomycin at inhibiting Staphylococcus aureus growth in vitro. FEBS Open Bio 2020, 10, 552–560. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneve, Switzerland, 2009.
- Lindfors, N.C.; Hyvonen, P.; Nyyssonen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Zhang, J.; Sakisaka, Y.; Ishihata, H.; Maruyama, K.; Nemoto, E.; Chiba, S.; Nagamine, M.; Hasegawa, H.; Yamada, S. Evaluation of Preosteoblast MC3T3-E1 Cells Cultured on a Microporous Titanium Membrane Fabricated Using a Precise Mechanical Punching Process. Materials 2020, 13, 5288. [Google Scholar] [CrossRef]
- Yan, X.Z.; Yang, W.; Yang, F.; Kersten-Niessen, M.; Jansen, J.A.; Both, S.K. Effects of continuous passaging on mineralization of MC3T3-E1 cells with improved osteogenic culture protocol. Tissue Eng. Part C Methods 2014, 20, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Pneumaticos, S.G.; Triantafyllopoulos, G.K.; Basdra, E.K.; Papavassiliou, A.G. Segmental bone defects: From cellular and molecular pathways to the development of novel biological treatments. J. Cell. Mol. Med. 2010, 14, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, K.; Huang, F.; Wang, C. Interaction of hydroxyapatite nanoparticles with endothelial cells: Internalization and inhibition of angiogenesis in vitro through the PI3K/Akt pathway. Int. J. Nanomed. 2017, 12, 5781–5795. [Google Scholar] [CrossRef] [PubMed]
- Lishchynskyi, O.; Stetsyshyn, Y.; Raczkowska, J.; Awsiuk, K.; Orzechowska, B.; Abalymov, A.; Skirtach, A.G.; Bernasik, A.; Nastyshyn, S.; Budkowski, A. Fabrication and Impact of Fouling-Reducing Temperature-Responsive POEGMA Coatings with Embedded CaCO3 Nanoparticles on Different Cell Lines. Materials 2021, 14, 1417. [Google Scholar] [CrossRef]
- Huang, W.S.; Chu, I.M. Injectable polypeptide hydrogel/inorganic nanoparticle composites for bone tissue engineering. PLoS ONE 2019, 14, 0210285. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, H.; Tian, Y.; Fan, Y.; Li, S.; Wang, G.; Wang, Y.; Peng, C.; Wu, D. Dual-functional composite scaffolds for inhibiting infection and promoting bone regeneration. Mater. Today Bio 2022, 16, 100409. [Google Scholar] [CrossRef]
- Thiel, A.; Reumann, M.K.; Boskey, A.; Wischmann, J.; von Eisenhart-Rothe, R.; Mayer-Kuckuk, P. Osteoblast migration in vertebrate bone. Biol. Rev. Camb. Philos. Soc. 2018, 93, 350–363. [Google Scholar] [CrossRef]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Kwon, K.J.; Seok, H. Silk protein-based membrane for guided bone regeneration. Appl. Sci. 2018, 8, 1214. [Google Scholar] [CrossRef]
- Cheng, G.; Chen, J.; Wang, Q.; Yang, X.; Cheng, Y.; Li, Z.; Tu, H.; Deng, H.; Li, Z. Promoting osteogenic differentiation in pre-osteoblasts and reducing tibial fracture healing time using functional nanofibers. Nano Res. 2018, 11, 3658–3677. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, G.; Xie, C.; Yin, C.; Dong, X.; Li, Z.; Zhou, X.; Wang, Q.; Deng, H.; Li, Z. Biomimetic Silk Fibroin Hydrogels Strengthened by Silica Nanoparticles Distributed Nanofibers Facilitate Bone Repair. Adv. Healthc. Mater. 2021, 10, 2001646. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wei, L.; Xu, Z.; Qin, L.; Yang, J.; Zou, Y.; Zhao, C.; Chen, L.; Hu, N. Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy. Biomedicines 2023, 11, 2244. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F. Bioinspired Materials for Wound Healing Application: The Potential of Silk Fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef]
- Martinez-Mora, C.; Mrowiec, A.; Garcia-Vizcaino, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolas, F.J. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Gabor, R.; Doubkova, M.; Gorosova, S.; Malanik, K.; Vandrovcova, M.; Cvrcek, L.; Drobikova, K.; Mamulova Kutlakova, K.; Bacakova, L. Preparation of highly wettable coatings on Ti-6Al-4V ELI alloy for traumatological implants using micro-arc oxidation in an alkaline electrolyte. Sci. Rep. 2020, 10, 19780. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, M.; Kostina, A.; Zihlavnikova Enayati, K.; Zabirnyk, A.; Malashicheva, A.; Stenslokken, K.O.; Sullivan, G.J.; Kaljusto, M.L.; Kvitting, J.P.; Kostareva, A.; et al. Inflammation and Mechanical Stress Stimulate Osteogenic Differentiation of Human Aortic Valve Interstitial Cells. Front. Physiol. 2018, 9, 1635. [Google Scholar] [CrossRef]
- Jung, S.R.; Song, N.J.; Yang, D.K.; Cho, Y.J.; Kim, B.J.; Hong, J.W.; Yun, U.J.; Jo, D.G.; Lee, Y.M.; Choi, S.Y.; et al. Silk proteins stimulate osteoblast differentiation by suppressing the Notch signaling pathway in mesenchymal stem cells. Nutr. Res. 2013, 33, 162–170. [Google Scholar] [CrossRef]
- Gao, X.; Di, X.; Li, J.; Kang, Y.; Xie, W.; Sun, L.; Zhang, J. Extracellular Calcium-Induced Calcium Transient Regulating the Proliferation of Osteoblasts through Glycolysis Metabolism Pathways. Int. J. Mol. Sci. 2023, 24, 4991. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Tan, Y.; Fan, H.; Zhang, X. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen. Biomater. 2018, 5, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.Y.; Park, Y.J.; Han, J.S. Effects of HA released calcium ion on osteoblast differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Tullberg-Reinert, H.; Jundt, G. In situ measurement of collagen synthesis by human bone cells with a Sirius Red-based colorimetric microassay: Effects of transforming growth factor β2 and ascorbic acid 2-phosphate. Histochem. Cell Biol. 1999, 112, 271–276. [Google Scholar] [CrossRef] [PubMed]




| Compositions | VM-SF/CaP/MC | ||
|---|---|---|---|
| VM-HSF/LCaP/MC | VM-LSF/HCaP/MC | VM-HSF/HCaP/MC | |
| SF content (% w/v) | 2.0 | 1.5 | 2.0 |
| CaCl2:Na2HPO4 molar ratio | 0.3:0.2 | 0.5:0.3 | 0.5:0.3 |
| MC content (% w/v) | 2.0 | 2.0 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phewchan, P.; Laoruengthana, A.; Chomchalao, P.; Lamlertthon, S.; Tiyaboonchai, W. Vancomycin-Loaded Silk Fibroin/Calcium Phosphate/Methylcellulose-Based In Situ Thermosensitive Hydrogel: A Potential Function for Bone Regeneration. Gels 2024, 10, 695. https://doi.org/10.3390/gels10110695
Phewchan P, Laoruengthana A, Chomchalao P, Lamlertthon S, Tiyaboonchai W. Vancomycin-Loaded Silk Fibroin/Calcium Phosphate/Methylcellulose-Based In Situ Thermosensitive Hydrogel: A Potential Function for Bone Regeneration. Gels. 2024; 10(11):695. https://doi.org/10.3390/gels10110695
Chicago/Turabian StylePhewchan, Premchirakorn, Artit Laoruengthana, Pratthana Chomchalao, Supaporn Lamlertthon, and Waree Tiyaboonchai. 2024. "Vancomycin-Loaded Silk Fibroin/Calcium Phosphate/Methylcellulose-Based In Situ Thermosensitive Hydrogel: A Potential Function for Bone Regeneration" Gels 10, no. 11: 695. https://doi.org/10.3390/gels10110695
APA StylePhewchan, P., Laoruengthana, A., Chomchalao, P., Lamlertthon, S., & Tiyaboonchai, W. (2024). Vancomycin-Loaded Silk Fibroin/Calcium Phosphate/Methylcellulose-Based In Situ Thermosensitive Hydrogel: A Potential Function for Bone Regeneration. Gels, 10(11), 695. https://doi.org/10.3390/gels10110695








