Ionic Crosslinked Hydrogel Films for Immediate Decontamination of Chemical Warfare Agents
Abstract
1. Introduction
2. Results and Discussion
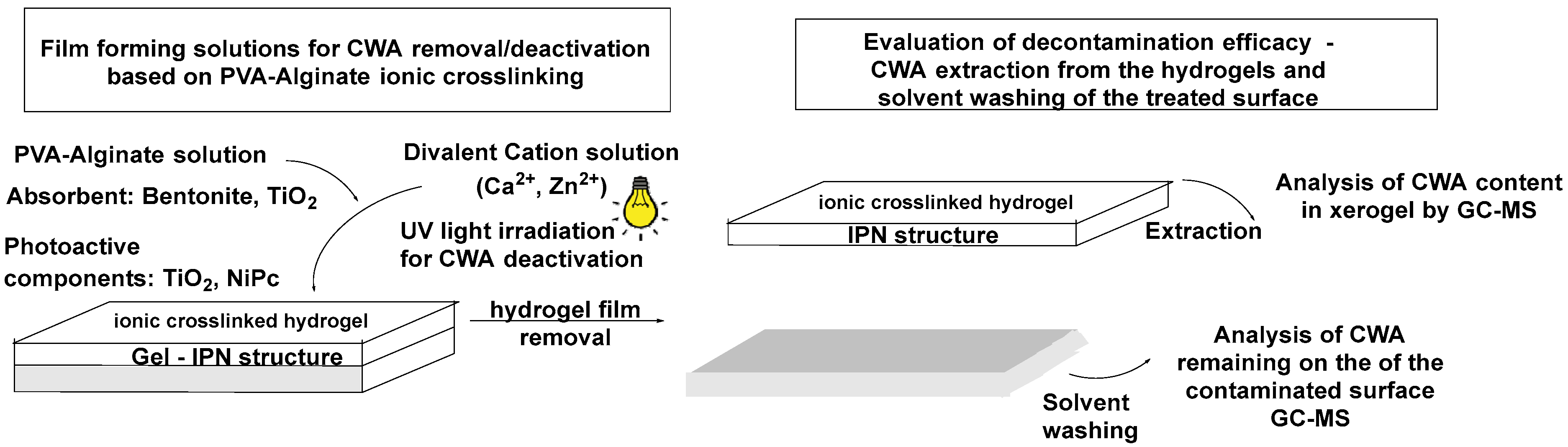
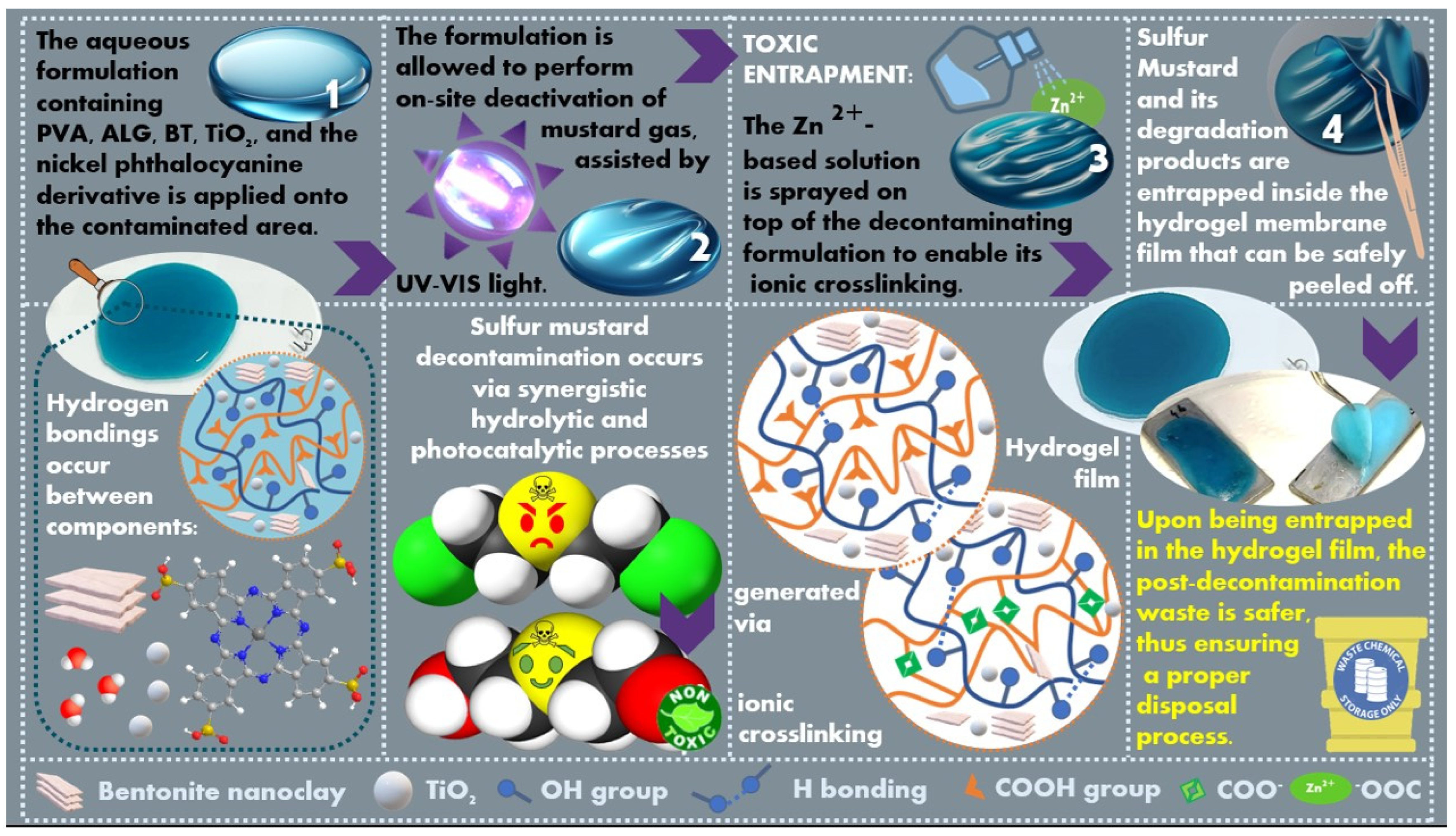
2.1. Method Principles
2.2. Morphological Characterization of the Hydrogels
2.3. Swelling Degree
2.4. FTIR Analysis
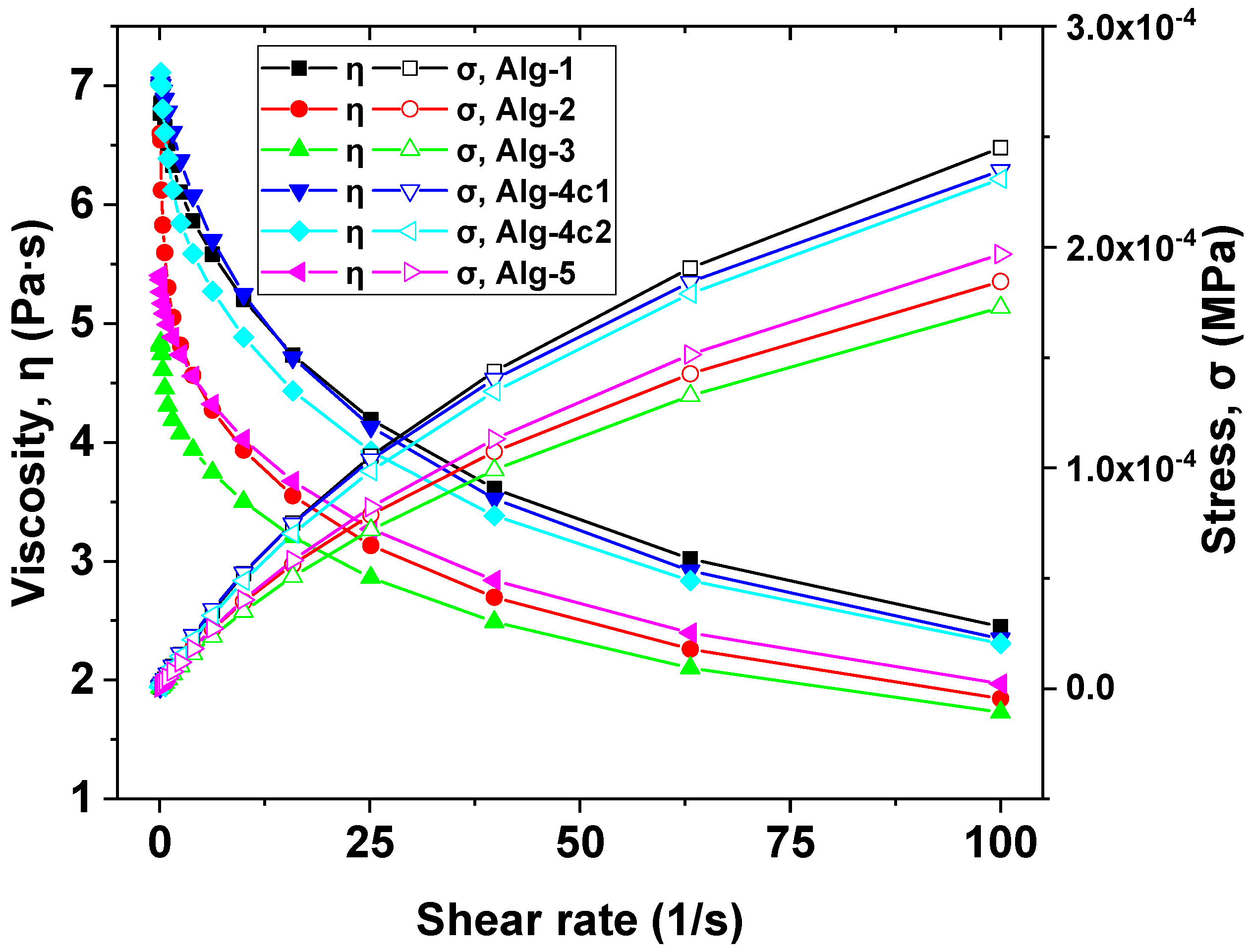
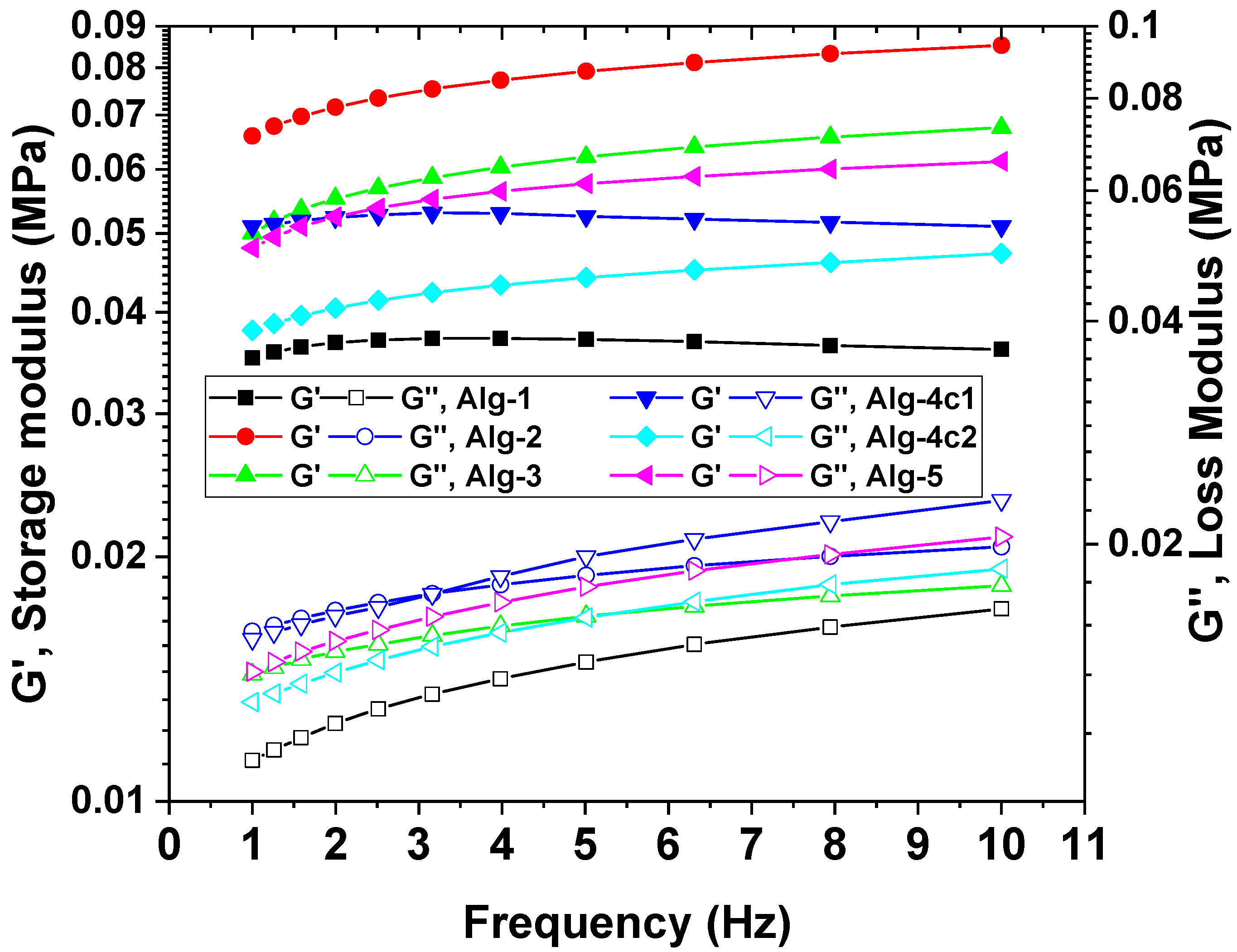
2.5. Mechanical Properties of the Hydrogels
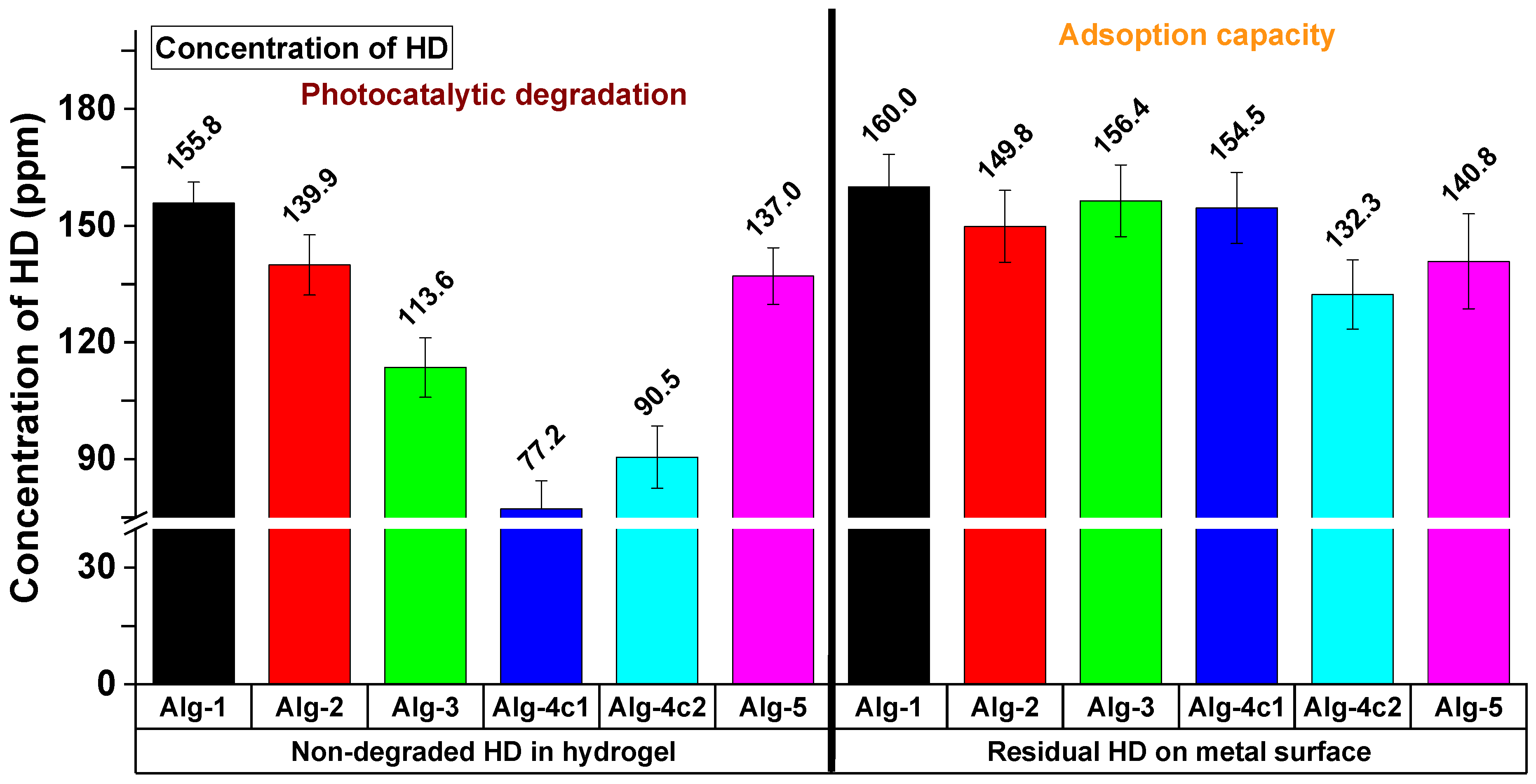
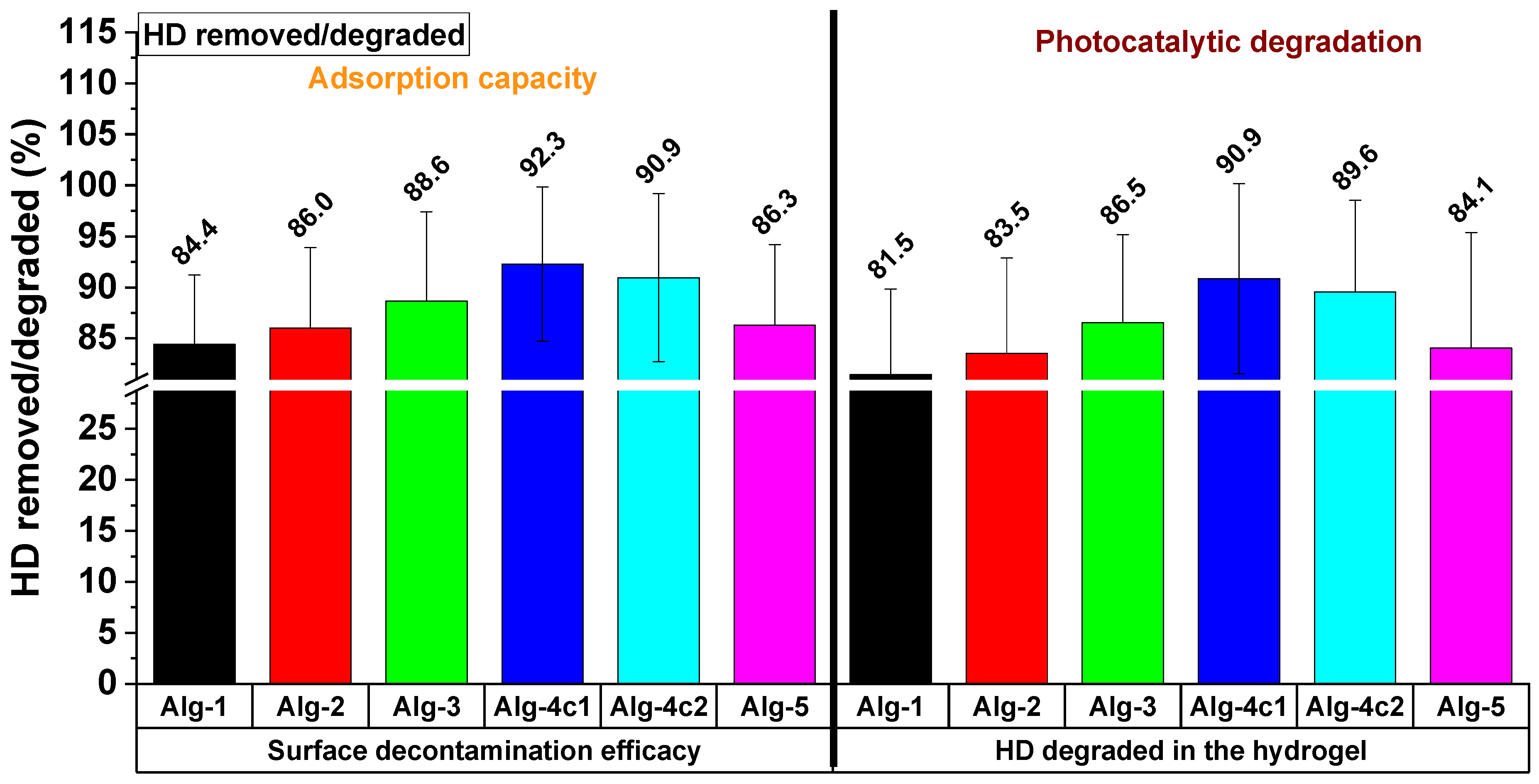
2.6. Decontamination Survey
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Formulation and Application of the Hydrogels
4.2.2. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramesh, A.C.; Kumar, S. Triage, monitoring, and treatment of mass casualty events involving chemical, biological, radiological, or nuclear agents. J. Pharm. Bioallied Sci. 2010, 2, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Altmann, H.J.; Jung, M.; Richardt, A. Decontamination of Chemical Warfare Agents—What is Thorough? In CBRN Protection; Wiley: Hoboken, NJ, USA, 2013; pp. 351–382. [Google Scholar]
- Yang, Y.C.; Baker, J.A.; Ward, J.R. Decontamination of chemical warfare agents. Chem. Rev. 1992, 92, 1729–1743. [Google Scholar] [CrossRef]
- Dachir, S.; Fishbine, E.; Meshulam, Y.; Buch, H.; Allon, N.; Kadar, T. Fuller’s Earth: Old and Faithful Skin Decontaminant against Toxic Agents. In Skin Decontamination: A Comprehensive Clinical Research Guide; Zhu, H., Maibach, H.I., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 101–119. [Google Scholar]
- Waysbort, D.; McGarvey, D.J.; Creasy, W.R.; Morrissey, K.M.; Hendrickson, D.M.; Durst, H.D. A decontamination system for chemical weapons agents using a liquid solution on a solid sorbent. J. Hazard. Mater. 2009, 161, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Talmage, S.S.; Watson, P.A.; Hauschild, V.; Munro, B.N.; King, J. Chemical Warfare Agent Degradation and Decontamination. Curr. Org. Chem. 2007, 11, 285–298. [Google Scholar] [CrossRef]
- Wattana, M.; Bey, T. Mustard Gas or Sulfur Mustard: An Old Chemical Agent as a New Terrorist Threat. Prehospital Disaster Med. 2009, 24, 19–29. [Google Scholar] [CrossRef]
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef] [PubMed]
- Klöffel, T.; Gordon, D.; Popiel, S.; Nawala, J.; Meyer, B.; Rodziewicz, P. Understanding the mechanism of the sulfur mustard hydrolysis reaction on the atomistic level from experiment and first-principles simulations. Process Saf. Environ. Prot. 2023, 172, 105–112. [Google Scholar] [CrossRef]
- Raber, E.; Jin, A.; Noonan, K.; McGuire, R.; Kirvel, R.D. Decontamination issues for chemical and biological warfare agents: How clean is clean enough? Int. J. Environ. Health Res. 2001, 11, 128–148. [Google Scholar] [CrossRef]
- Toader, G.; Diacon, A.; Rotariu, T.; Alexandru, M.; Rusen, E.; Ginghină, R.E.; Alexe, F.; Oncioiu, R.; Zorila, F.L.; Podaru, A.; et al. Eco–Friendly Peelable Active Nanocomposite Films Designed for Biological and Chemical Warfare Agents Decontamination. Polymers 2021, 13, 3999. [Google Scholar] [CrossRef]
- Ma, B.; Zuo, G.; Dong, B.; Gao, S.; You, L.; Wang, X. Optical detection of sulfur mustard contaminated surfaces based on a sprayable fluorescent probe. New J. Chem. 2021, 45, 20569–20574. [Google Scholar] [CrossRef]
- Ashmore, M.H.; Nathanail, C.P. A critical evaluation of the implications for risk based land management of the environmental chemistry of Sulphur Mustard. Environ. Int. 2008, 34, 1192–1203. [Google Scholar] [CrossRef]
- Ungureanu, C.; Răileanu, S.; Zgârian, R.; Tihan, G.; Burnei, C. State-of-the-Art Advances and Current Applications of Gel-Based Membranes. Gels 2024, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Toader, G.; Ginghina, R.E.; Diacon, A.; Rusen, E.; Bratu, A.E.; Podaru, A.; Rotariu, T. Design and Application of Photocrosslinkable Hydrogel Films for Fast and Efficient Decontamination of Chemical Warfare Agents. ACS Appl. Polym. Mater. 2023, 5, 877–891. [Google Scholar] [CrossRef]
- Elgegren, M.; Nakamatsu, J.; Galarreta, B.; Kim, S. Three-Dimensional Membranes of Natural Polymer Complex Nanoparticle for Potential Medical Applications. Gels 2023, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Asthana, N.; Pal, K.; Aljabali, A.A.A.; Tambuwala, M.M.; de Souza, F.G.; Pandey, K. Polyvinyl alcohol (PVA) mixed green–clay and aloe vera based polymeric membrane optimization: Peel-off mask formulation for skin care cosmeceuticals in green nanotechnology. J. Mol. Struct. 2021, 1229, 129592. [Google Scholar] [CrossRef]
- Toader, G.; Diacon, A.; Rusen, E.; Mangalagiu, I.I.; Alexandru, M.; Zorilă, F.L.; Mocanu, A.; Boldeiu, A.; Gavrilă, A.M.; Trică, B.; et al. Peelable Alginate Films Reinforced by Carbon Nanofibers Decorated with Antimicrobial Nanoparticles for Immediate Biological Decontamination of Surfaces. Nanomaterials 2023, 13, 2775. [Google Scholar] [CrossRef] [PubMed]
- Andrle, M.; Trousil, V.; Černý, J.; Štreblová, A.; Kořínková, R. Photodegradation of chemical warfare agents and their simulants using zinc phthalocyanine sulfonamide in solutions and embedded in a polymer matrix. J. Photochem. Photobiol. A Chem. 2023, 435, 114281. [Google Scholar] [CrossRef]
- Gephart, R.T., III; Coneski, P.N.; Wynne, J.H. Decontamination of Chemical-Warfare Agent Simulants by Polymer Surfaces Doped with the Singlet Oxygen Generator Zinc Octaphenoxyphthalocyanine. ACS Appl. Mater. Interfaces 2013, 5, 10191–10200. [Google Scholar] [CrossRef] [PubMed]
- Hernon-Kenny, L.A.; Behringer, D.L.; Crenshaw, M.D. Comparison of latex body paint with wetted gauze wipes for sampling the chemical warfare agents VX and sulfur mustard from common indoor surfaces. Forensic Sci. Int. 2016, 262, 143–149. [Google Scholar] [CrossRef]
- Gao, N.; Li, J.; Zhang, W.; Ahmed, S.; Liu, F.; Han, E.-H. Analysis of Permeation Behaviors of Dimethyl Methyl Phosphonate Solutions and Influence on Mechanical Properties of Designed Double-Layer Peelable Coating. ACS Appl. Polym. Mater. 2022, 4, 7330–7339. [Google Scholar] [CrossRef]
- Thomas, E.; Bordes, C.; Chaput, F.; Arquier, D.; Briançon, S.; Bolzinger, M.-A. CeO2-based peelable gel for neutralization and skin decontamination toward chemical warfare agents. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133520. [Google Scholar] [CrossRef]
- Durmaz, K.; Misbach, M.; Danoy, A.; Salvi, J.-P.; Bloch, E.; Bourrelly, S.; Verrier, B.; Sohier, J. An innovative Fuller’s earth-based film-forming formulation for skin decontamination, through removal and entrapment of an organophosphorus compound, paraoxon-ethyl. J. Hazard. Mater. 2024, 470, 134190. [Google Scholar] [CrossRef] [PubMed]
- Redy Keisar, O.; Nahum, V.; Yehezkel, L.; Marcovitch, I.; Columbus, I.; Fridkin, G.; Chen, R. Active and strippable PVA/Borax/NaBO3 hydrogel for effective containment and decontamination of chemical warfare agents. ACS Omega 2021, 6, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Voss, B.A.; Noble, R.D.; Gin, D.L. Ionic Liquid Gel-Based Containment and Decontamination Coating for Blister Agent-Contacted Substrates. Chem. Mater. 2012, 24, 1174–1180. [Google Scholar] [CrossRef]
- Voss, B.A.M. Applications and Properties of Ionic Liquid-Based Gels and Soft Solid Composites. Ph.D. Thesis, University of Colorado at Boulder, Boulder, CO, USA, 2011. [Google Scholar]
- Tran, V.T.; Mredha, M.T.I.; Pathak, S.K.; Yoon, H.; Cui, J.; Jeon, I. Conductive Tough Hydrogels with a Staggered Ion-Coordinating Structure for High Self-Recovery Rate. ACS Appl. Mater. Interfaces 2019, 11, 24598–24608. [Google Scholar] [CrossRef] [PubMed]
- Tušek, D.; Ašperger, D.; Bačić, I.; Ćurković, L.; Macan, J. Environmentally acceptable sorbents of chemical warfare agent simulants. J. Mater. Sci. 2017, 52, 2591–2604. [Google Scholar] [CrossRef]
- Mambrini, R.V.; Saldanha, A.L.M.; Ardisson, J.D.; Araujo, M.H.; Moura, F.C.C. Adsorption of sulfur and nitrogen compounds on hydrophobic bentonite. Appl. Clay Sci. 2013, 83–84, 286–293. [Google Scholar] [CrossRef]
- Nawała, J.; Jóźwik, P.; Popiel, S. Thermal and catalytic methods used for destruction of chemical warfare agents. Int. J. Environ. Sci. Technol. 2019, 16, 3899–3912. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Sharma, P.K.; Sikarwar, B.; Gupta, G.; Nigam, A.K.; Tripathi, B.K.; Pandey, P.; Boopathi, M.; Ganesan, K.; Singh, B. A simple degradation method for sulfur mustard at ambient conditions using nickelphthalocyanine incorporated polypyrrole modified electrode. Appl. Nanosci. 2014, 4, 37–46. [Google Scholar] [CrossRef][Green Version]
- Gamelas, S.R.D.; Tomé, J.P.C.; Tomé, A.C.; Lourenço, L.M.O. Advances in photocatalytic degradation of organic pollutants in wastewaters: Harnessing the power of phthalocyanines and phthalocyanine-containing materials. RSC Adv. 2023, 13, 33957–33993. [Google Scholar] [CrossRef] [PubMed]
- Aljar, M.A.A.; Rashdan, S.; Abd El-Fattah, A. Environmentally Friendly Polyvinyl Alcohol−Alginate/Bentonite Semi-Interpenetrating Polymer Network Nanocomposite Hydrogel Beads as an Efficient Adsorbent for the Removal of Methylene Blue from Aqueous Solution. Polymers 2021, 13, 4000. [Google Scholar] [CrossRef] [PubMed]
- Liparoti, S.; Speranza, V.; Marra, F. Alginate hydrogel: The influence of the hardening on the rheological behaviour. J. Mech. Behav. Biomed. Mater. 2021, 116, 104341. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhan, W.; Zuo, W.; Li, L.; Zhang, J.; Cai, G.; Tian, Y. Elastic, tough and switchable swelling hydrogels with high entanglements and low crosslinks for water remediation. Chem. Eng. J. 2022, 450, 138417. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological Study of Genipin Cross-Linked Chitosan Hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.-H.; Atluri, P.; Burdick, J.A. Methods To Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef] [PubMed]
- Mamand, D.M.; Anwer, T.M.K.; Qadr, H.M.; Mussa, C.H. Investigation of Spectroscopic and Optoelectronic Properties of Phthalocyanine Molecules. Russ. J. Gen. Chem. 2022, 92, 1827–1838. [Google Scholar] [CrossRef]
- Haider, M.; Zhen, C.; Wu, T.; Wu, J.; Jia, C.; Liu, G.; Cheng, H.-M. Nickel phthalocyanine as an excellent hole-transport material in inverted planar perovskite solar cells. Chem. Commun. 2019, 55, 5343–5346. [Google Scholar] [CrossRef] [PubMed]
- Claessens, C.G.; Hahn, U.; Torres, T. Phthalocyanines: From outstanding electronic properties to emerging applications. Chem. Rec. 2008, 8, 75–97. [Google Scholar] [CrossRef]
- Raber, E.; McGuire, R. Oxidative decontamination of chemical and biological warfare agents using L-Gel. J. Hazard. Mater. 2002, 93, 339–352. [Google Scholar] [CrossRef]
- Brickhouse, M.D.; Lalain, T.; Hall, M.R.; Mantooth, B.A.; Henderson, V.D.; Procell, L.R.; Rastogi, V.K.; Sumpter, K.B.; Wallace, L. Laboratory Evaluation of the Clean Earth Technologies Decontamination Solutions for Chemical and Biological Agents; Defense Technical Information Center: Fort Belvoir, VA, USA, 2008. [Google Scholar]
- Oudejans, L. Decontamination of Agent Yellow, a Lewisite and Sulfur Mustard Mixture; US Environmental Protection Agency: Washington, DC, USA, 2015. Available online: https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=522026&Lab=NHSRC (accessed on 16 June 2024).
- Wang, H.; Wagner, G.W.; Lu, A.X.; Nguyen, D.L.; Buchanan, J.H.; McNutt, P.M.; Karwacki, C.J. Photocatalytic Oxidation of Sulfur Mustard and Its Simulant on BODIPY-Incorporated Polymer Coatings and Fabrics. ACS Appl. Mater. Interfaces 2018, 10, 18771–18777. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-C.; Geng, X.; Li, S.-S.; Wu, J.-N.; Xu, C.-L.; Xin, Y.; Cui, Y.; Zhao, C.-L.; Ye, L.; Chen, L.-K. Decontamination of Mustard Gas with Processable Dry Reactive Polymers via Oxidation–Chlorination. ACS Appl. Polym. Mater. 2021, 3, 5452–5459. [Google Scholar] [CrossRef]
- NATO; AEP. 58—Combined Operational Characteristics Technical Specifications, Test Procedures and Evaluation Criteria for Chemical, Biological, Radiological and Nuclear Decontamination Equipment; EDSTAR: Brussels, Belgium, 2014. [Google Scholar]
- Hao, Y.; Papazyan, E.K.; Ba, Y.; Liu, Y. Mechanism-Guided Design of Metal–Organic Framework Composites for Selective Photooxidation of a Mustard Gas Simulant under Solvent-Free Conditions. ACS Catal. 2022, 12, 363–371. [Google Scholar] [CrossRef]
- Viana, M.M.; Soares, V.F.; Mohallem, N.D.S. Synthesis and characterization of TiO2 nanoparticles. Ceram. Int. 2010, 36, 2047–2053. [Google Scholar] [CrossRef]
- Ginghina, R.-E.; Toader, G.; Purica, M.; Bratu, A.-E.; Lazaroaie, C.; Tiganescu, T.-V.; Oncioiu, R.-E.; Iorga, G.-O.; Zorila, F.-L.; Constantin, M.; et al. Antimicrobial Activity and Degradation Ability Study on Nanoparticle-Enriched Formulations Specially Designed for the Neutralization of Real and Simulated Biological and Chemical Warfare Agents. Pharmaceuticals 2022, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Macron, J.; Bresson, B.; Tran, Y.; Hourdet, D.; Creton, C. Equilibrium and Out-of-Equilibrium Adherence of Hydrogels against Polymer Brushes. Macromolecules 2018, 51, 7556–7566. [Google Scholar] [CrossRef]
- Ninciuleanu, C.M.; Ianchiş, R.; Alexandrescu, E.; Mihăescu, C.I.; Scomoroşcenco, C.; Nistor, C.L.; Preda, S.; Petcu, C.; Teodorescu, M. The effects of monomer, crosslinking agent, and filler concentrations on the viscoelastic and swelling properties of poly (methacrylic acid) hydrogels: A comparison. Materials 2021, 14, 2305. [Google Scholar] [CrossRef]


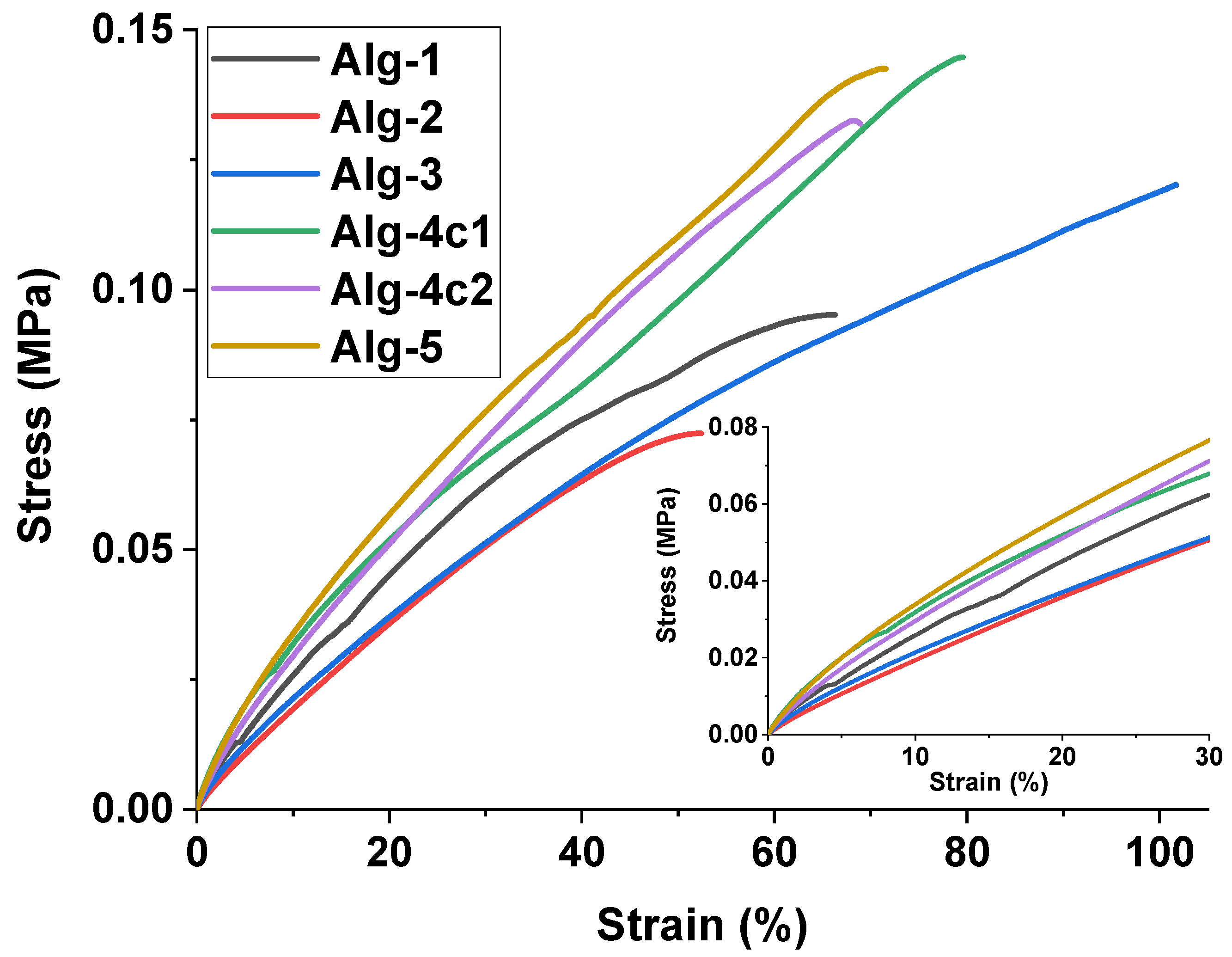
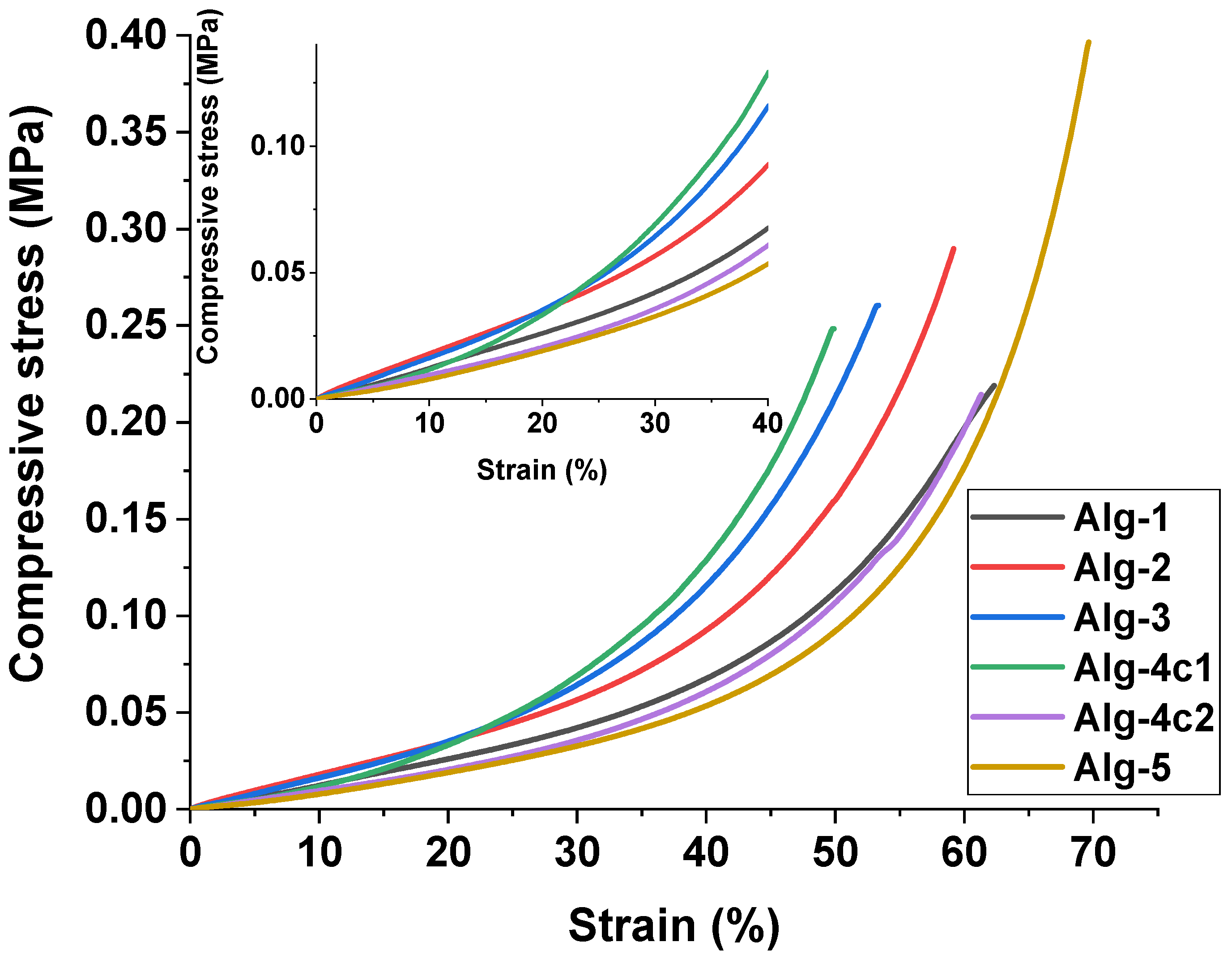
| Sample | Stress (Pa) at 30% Strain | Maximum Stress (Pa) | Maximum Strain (%) |
|---|---|---|---|
| Alg-1 | 62,500 | 95,200 | 66.2 |
| Alg-2 | 50,700 | 72,400 | 52.4 |
| Alg-3 | 51,300 | 120,200 | 101.7 |
| Alg-4c1 | 68,000 | 144,700 | 79.5 |
| Alg-4c2 | 71,200 | 132,000 | 68.9 |
| Alg-5 | 76,600 | 142,500 | 71.5 |
| Sample | Stress (Pa) at 20% Strain | Stress (Pa) at 30% Strain | Maximum Stress (Pa) | Maximum Strain (%) |
|---|---|---|---|---|
| Alg-1 | 260,000 | 42,200 | 218,900 | 62.3 |
| Alg-2 | 35,000 | 56,800 | 289,700 | 59.2 |
| Alg-3 | 35,300 | 64,300 | 260,500 | 53.3 |
| Alg-4c1 | 33,300 | 69,100 | 248,400 | 49.9 |
| Alg-4c2 | 20,700 | 35,800 | 214,300 | 61.3 |
| Alg-5 | 19,100 | 32,700 | 396,500 | 69.6 |
| Main Characteristics of the Decontaminating Formulation | Type of Contaminated Surface | Sulfur Mustard Entrapping Efficacy * | Degraded Sulfur Mustard | The Time Necessary for the Completion of the Decontamination Process | Refs. |
|---|---|---|---|---|---|
| An aqueous solution of commercial oxidizer (OxoneTM) and fumed silica | Acrylic painted metal | N/A (Not available) | 50% | 30 min | [43] |
| Chemical Decontamination Solution (CDS): hydrogen peroxide and sodium hydroxide | Aluminium | N/A | 99% | 60 min | [44] |
| Chemical Agent Resistant Coating painted aluminum | N/A | 97% | 60 min | ||
| EasyDECON® DF200 (Surfactant, Peroxide, and Booster) | Glass | 57% | N/A | 30 min | [45] |
| Concrete | 23% | N/A | 30 min | ||
| Galvanized metal | no significant effect | N/A | 30 min | ||
| BODIPY-embedded polymer coatings and fabrics for photocatalytic oxidation of sulfur mustard simulant CEES | PMMA | N/A | 57% | 60 min | [46] |
| PS | N/A | 60% | 60 min | ||
| Army combat uniforms | N/A | 30–100% | 20–120 min | ||
| Dry Reactive Polymers: chlorinated poly (4-vinylbenzenesulfonamide-co-methyl methacrylate) (PVMCl) | Polymeric substrate obtained via electrospinning | N/A | 95% | 60 min | [47] |
| Bentonite-supported silver nanoparticles dispersed in PVA/glycerol aqueous solution (film-forming) | Metallic surface | 90.89% | N/A | 20 h | [11] |
| Photocrosslinkable IPN hydrogel peelable films containing TiO2 | Metallic surface | 99.97 | 94.9% | 120 min | [15] |
| Ionic crosslinked hydrogel films (eco-friendly, water-based, hydrolytic and photocatalytic effects, fast and efficient HD decontamination, peelable, safe, and easily disposable) | Metallic surface | 92.3% | 90.9% | 35 min | This study |
| Sample Code | Composition of the Hydrogels | |||||
|---|---|---|---|---|---|---|
| PVA | Alg (Na) | BT | TiO2 | PC-Ni | H2O | |
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | |
| Alg-1 | 2 | 2 | - | - | - | 96 |
| Alg-2 | 2 | 2 | 0.5 | - | - | 95.5 |
| Alg-3 | 2 | 2 | 0.5 | 0.1 | - | 94.8 |
| Alg-4c1 | 2 | 2 | 0.5 | - | 0.005 | 95.5 |
| Alg-4c2 | 2 | 2 | 0.5 | - | 0.01 | 95.5 |
| Alg-5 | 2 | 2 | 0.5 | 0.1 | 0.005 | 95.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toader, G.; Ginghina, R.-E.; Bratu, A.E.; Podaru, A.I.; Pulpea, D.; Rotariu, T.; Gavrilă, A.M.; Diacon, A. Ionic Crosslinked Hydrogel Films for Immediate Decontamination of Chemical Warfare Agents. Gels 2024, 10, 428. https://doi.org/10.3390/gels10070428
Toader G, Ginghina R-E, Bratu AE, Podaru AI, Pulpea D, Rotariu T, Gavrilă AM, Diacon A. Ionic Crosslinked Hydrogel Films for Immediate Decontamination of Chemical Warfare Agents. Gels. 2024; 10(7):428. https://doi.org/10.3390/gels10070428
Chicago/Turabian StyleToader, Gabriela, Raluca-Elena Ginghina, Adriana Elena Bratu, Alice Ionela Podaru, Daniela Pulpea, Traian Rotariu, Ana Mihaela Gavrilă, and Aurel Diacon. 2024. "Ionic Crosslinked Hydrogel Films for Immediate Decontamination of Chemical Warfare Agents" Gels 10, no. 7: 428. https://doi.org/10.3390/gels10070428
APA StyleToader, G., Ginghina, R.-E., Bratu, A. E., Podaru, A. I., Pulpea, D., Rotariu, T., Gavrilă, A. M., & Diacon, A. (2024). Ionic Crosslinked Hydrogel Films for Immediate Decontamination of Chemical Warfare Agents. Gels, 10(7), 428. https://doi.org/10.3390/gels10070428










