Facile Preparation of Multifunctional Hydrogels with Sustained Resveratrol Release Ability for Bone Tissue Regeneration
Abstract
1. Introduction
2. Results and Discussion
2.1. Fabrication of a Res-Loaded Injectable Hydrogel
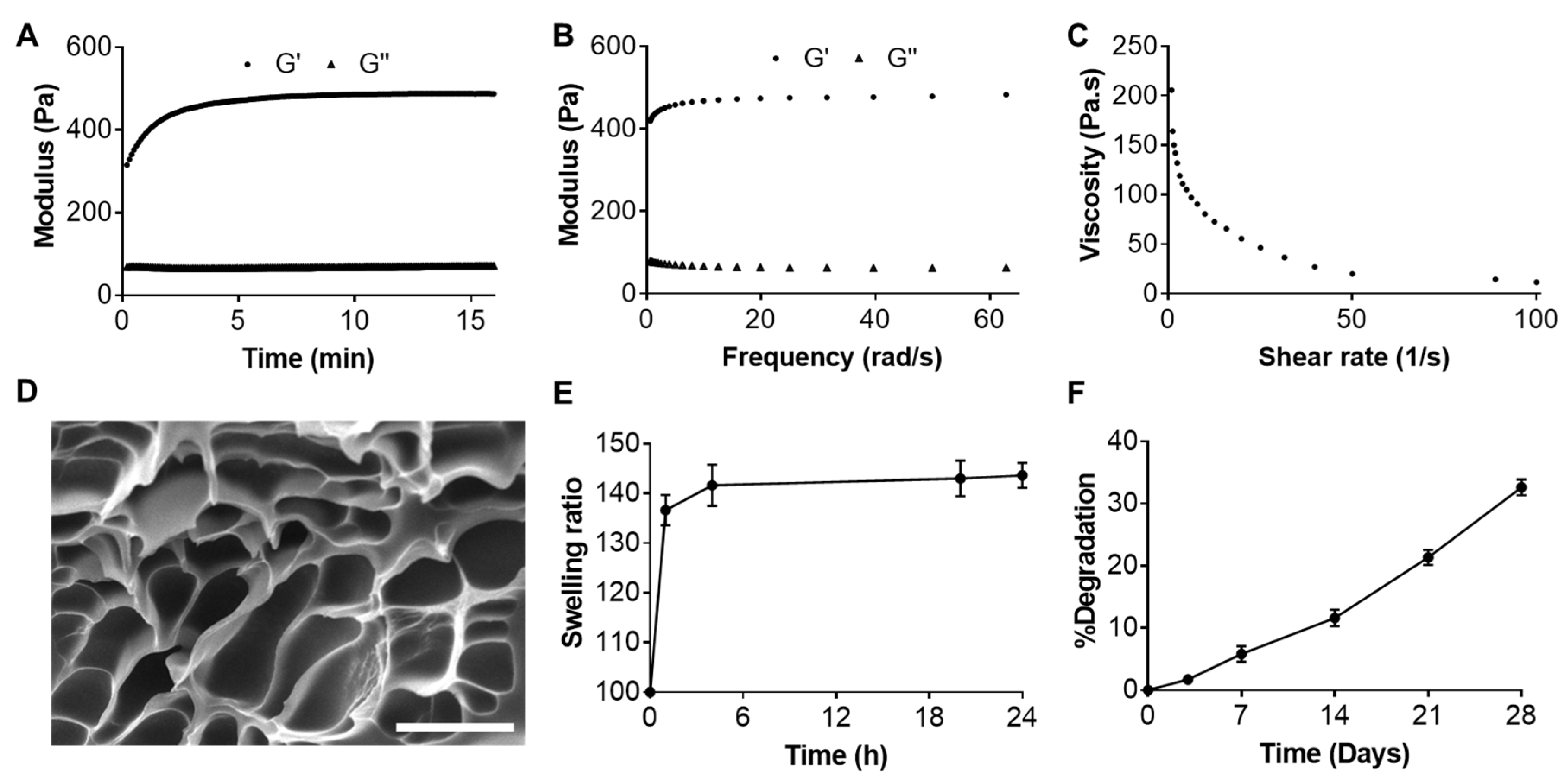
2.2. Characterizations of Resveratrol-Loaded Hydrogel
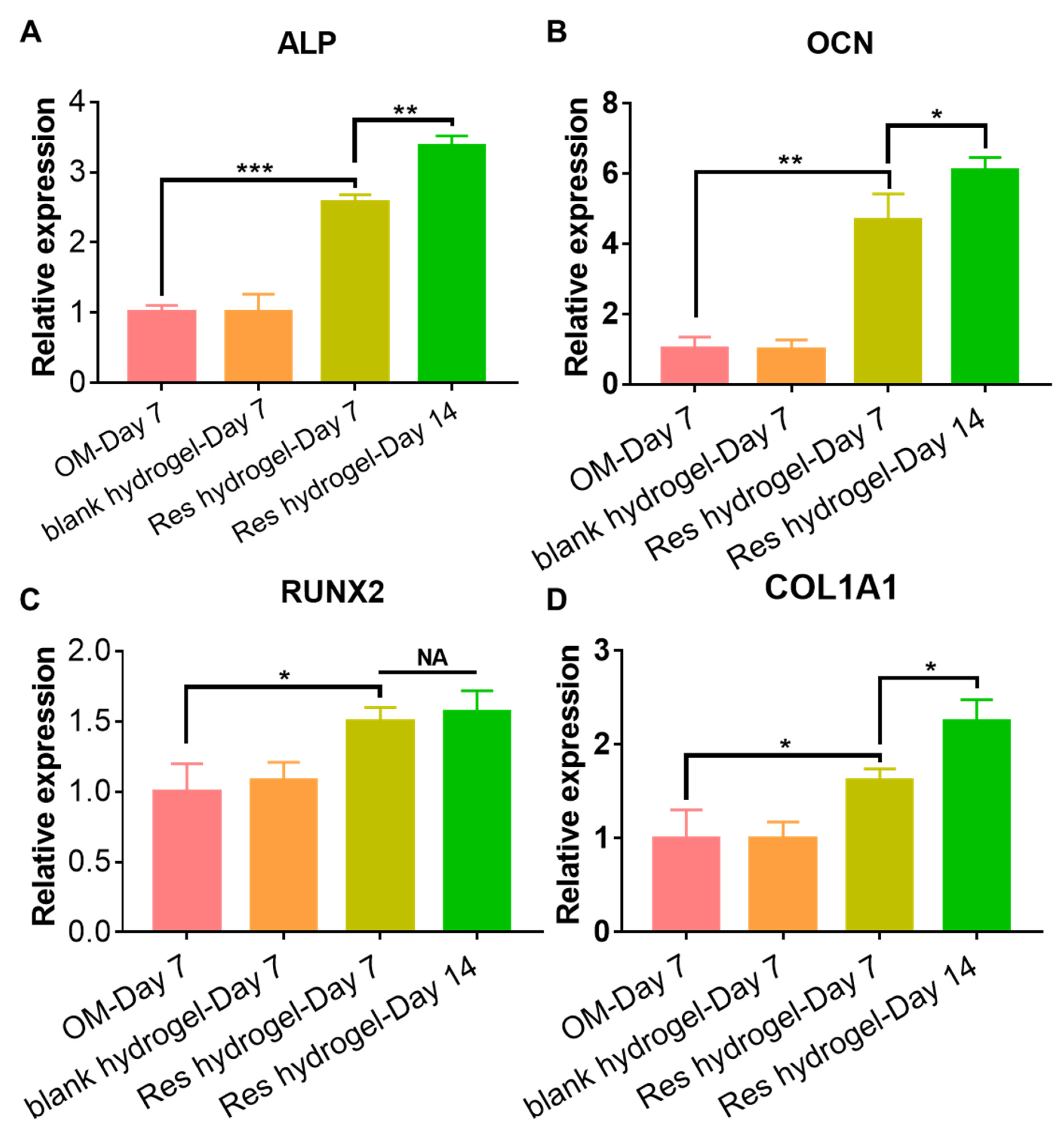
2.3. Cytocompatibility and Osteogenic Activity of the Resveratrol-Loaded Hydrogel
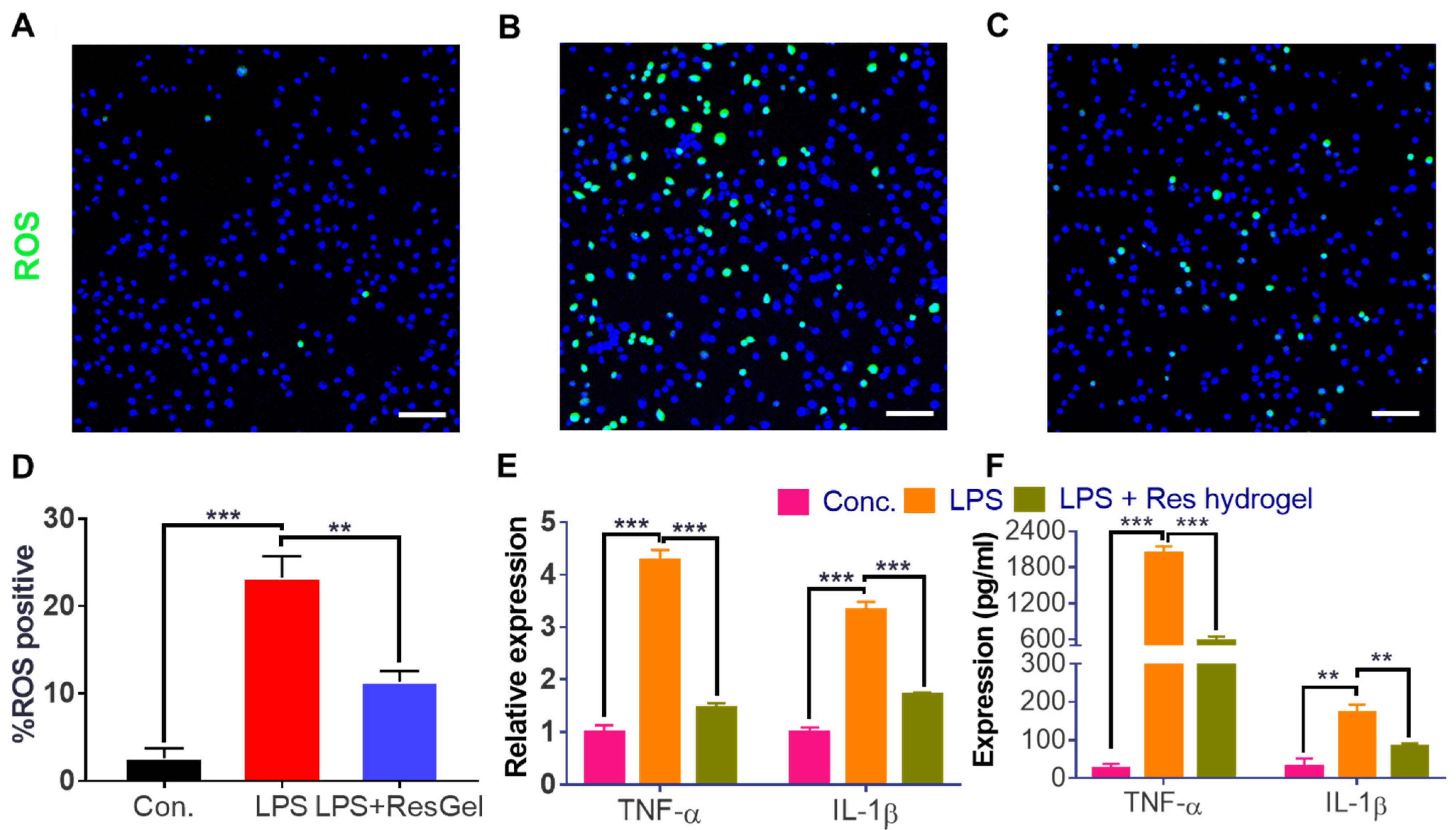
2.4. Anti-ROS and Anti-Inflammation Properties of the Resveratrol-Loaded Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Acrylated Hyaluronic Acid (HA-Acrylate) Precursor
| Agents (commercial) | Vendor & Purity | Amount used | Properties |
| Pluronic F-127 | Sigma, >99% | 3.75 g | Non-ionic copolymer, white powder, Mw ~ 12,700 |
| Hyaluronic acid | Bloomage Biotech, >95% | 50 mg | Polysaccharide, white powder, Mw ~ 290 kDa |
| Acrylic Anhydride | TCI, >95% | 30/60 µL | Volatile liquid, Mw 126.1 |
| p-Nitrophenyl chloroformate | Sigma, 96% | 0.48 g | White powder, Mw 201.6 |
| Cysteamine hydrochloride | Sigma, ≥98% | 11.3 mg | Colorless to very faintly yellow powder, Mw 113.6 |
| Agents (synthesized) | Purity | Amount used | Properties |
| Thiolated Pluronic F-127 | >90% pure, 70% grafting ratio | 10% (w/v), 50 µL | White lyophilized powder |
| Hyaluronic acid-acrylate | >90% pure, 24% grafting ratio | 2% (w/v), 50 µL | White lyophilized powder |
4.2. Proton Nuclear Magnetic Resonance (1H NMR) Characterization
4.3. Preparation of Resveratrol-Loaded F-127 Micelles
4.4. Fabrication of a Resveratrol-Loaded Injectable Hydrogel
4.5. Rheological Characterization of the Resveratrol-Loaded Hydrogel
4.6. Swelling and Degradation Study
4.7. In Vitro Drug Release Study
4.8. Scanning Electron Microscopy (SEM) Imaging
4.9. Cell Culture, Treatment, and Viability Studies
4.10. Mesenchymal Stem Cell Differentiation and RT-PCR Analysis
4.11. Evaluation of Anti-Oxidative and Anti-Inflammatory Properties
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Sözen, T.; Özışık, L.; Başaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Geng, Z.; Su, J. The “Three in One” Bone Repair Strategy for Osteoporotic Fractures. Front. Endocrinol. 2022, 13, 910602. [Google Scholar] [CrossRef]
- Gehrke, B.; Alves Coelho, M.C.; Brasil d’Alva, C.; Madeira, M. Long-term consequences of osteoporosis therapy with bisphosphonates. Arch. Endocrinol. Metab. 2023, 68, e220334. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Kalinkovich, A. Targeting chronic inflammation as a potential adjuvant therapy for osteoporosis. Life Sci. 2022, 306, 120847. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, S.; Liu, X.; Zhao, Y.; Yang, J.; Chai, G.; Wang, N.; Ma, S.; Liu, W.; Ding, C. Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review. Molecules 2023, 28, 7039. [Google Scholar] [CrossRef]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Gong, Y.; Bu, Y.; Li, Y.; Hao, D.; He, B.; Kong, L.; Huang, W.; Gao, X.; Zhang, B.; Qu, Z.; et al. Hydrogel-based delivery system applied in the local anti-osteoporotic bone defects. Front. Bioeng. Biotechnol. 2022, 10, 1058300. [Google Scholar] [CrossRef]
- Zheng, Z.; Yu, C.; Wei, H. Injectable Hydrogels as Three-Dimensional Network Reservoirs for Osteoporosis Treatment. Tissue Eng. Part B Rev. 2021, 27, 430–454. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, J.; Han, F.; Meng, Q.; Wang, H.; Wei, Q.; Li, Z.; Li, F.; Xie, E.; Qin, X.; et al. A Multifunctional Composite Hydrogel That Rescues the ROS Microenvironment and Guides the Immune Response for Repair of Osteoporotic Bone Defects. Adv. Funct. Mater. 2022, 32, 2201067. [Google Scholar] [CrossRef]
- Sun, B.; Wang, H.; Xiao, B.; Yan, H.; Wu, H.; Zhang, R.; Zhang, Y.; Yuan, W.; Wang, X.; Shi, C. Bioactive composite hydrogel with effects of robust promoting osteogenesis and immunomodulation for osteoporotic bone regeneration. Chem. Eng. J. 2023, 476, 146743. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhang, L.; Xu, C.; Xu, H.; Chen, Z. Reprogramming Macrophage Polarization, Depleting ROS by Astaxanthin and Thioketal-Containing Polymers Delivering Rapamycin for Osteoarthritis Treatment. Adv. Sci. 2024, 11, 2305363. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Wu, T.; Shi, K.; Bei, Z.; Wang, M.; Chu, B.; Xu, K.; Pan, M.; Li, Y.; et al. An Injectable Thermosensitive Hydrogel Containing Resveratrol and Dexamethasone-Loaded Carbonated Hydroxyapatite Microspheres for the Regeneration of Osteoporotic Bone Defects. Small Methods 2024, 8, 2300843. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Gilarska, A.; Hinz, A.; Bzowska, M.; Dyduch, G.; Kamiński, K.; Nowakowska, M.; Lewandowska-Łańcucka, J. Addressing the Osteoporosis Problem—Multifunctional Injectable Hybrid Materials for Controlling Local Bone Tissue Remodeling. ACS Appl. Mater. Interfaces 2021, 13, 49762–49779. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; You, X.; Zhou, H.; He, W.; Li, B.; Xia, J.; Zhu, H.; Zhao, Y.; Yu, G.; et al. Resveratrol promotes osteogenesis and alleviates osteoporosis by inhibiting p53. Aging 2020, 12, 10359–10369. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability is the Problem, What is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Duffy, P.; McMahon, S.; Wang, X.; Lyu, J.; Xu, Q.; Sigen, A.; Chen, N.N.; Bi, V.; et al. Resveratrol-Loaded Poly(d,l-Lactide-Co-Glycolide) Microspheres Integrated in a Hyaluronic Acid Injectable Hydrogel for Cartilage Regeneration. Adv. NanoBiomed Res. 2022, 2, 2100070. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Wei, B.; Wang, W.; Liu, X.; Xu, C.; Wang, Y.; Wang, Z.; Xu, J.; Guan, J.; Zhou, P.; Mao, Y. Gelatin methacrylate hydrogel scaffold carrying resveratrol-loaded solid lipid nanoparticles for enhancement of osteogenic differentiation of BMSCs and effective bone regeneration. Regen. Biomater. 2021, 8, rbab044. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Huang, H.-B.; Gong, W.; He, W.-Y.; Li, X.; Xu, Y.; Gong, X.-J.; Hu, J.-N. Resveratrol Triggered the Quick Self-Assembly of Gallic Acid into Therapeutic Hydrogels for Healing of Bacterially Infected Wounds. Biomacromolecules 2022, 23, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Balakrishnan, A.; Shanmughan, P.; Maliakel, B.; Illathu Madhavamenon, K. Micelle/Hydrogel Composite as a “Natural Self-Emulsifying Reversible Hybrid Hydrogel (N’SERH)” Enhances the Oral Bioavailability of Free (Unconjugated) Resveratrol. ACS Omega 2022, 7, 12835–12845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, W.; Wu, S.; Kuss, M.; Jiang, X.; Untrauer, J.B.; Reid, S.P.; Duan, B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 2020, 12, 035020. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Kong, Y.; Shi, W.; Zheng, L.; Zhang, D.; Jiang, X.; Liu, B.; Xue, W.; Kuss, M.; Li, Y.; Sorgen, P.L.; et al. In situ delivery of a curcumin-loaded dynamic hydrogel for the treatment of chronic peripheral neuropathy. J. Control. Release 2023, 357, 319–332. [Google Scholar] [CrossRef]
- Zheng, L.; Shi, W.; Liu, B.; Duan, B.; Sorgen, P.L. Evaluation of Tyrosine Kinase Inhibitors Loaded Injectable Hydrogels for Improving Connexin43 Gap Junction Intercellular Communication. ACS Appl. Mater. Interfaces 2024, 16, 1985–1998. [Google Scholar] [CrossRef]
- Vivero-Lopez, M.; Sparacino, C.; Quelle-Regaldie, A.; Sánchez, L.; Candal, E.; Barreiro-Iglesias, A.; Huete-Toral, F.; Carracedo, G.; Otero, A.; Concheiro, A.; et al. Pluronic®/casein micelles for ophthalmic delivery of resveratrol: In vitro, ex vivo, and in vivo tests. Int. J. Pharm. 2022, 628, 122281. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, L.; Tang, E.K.Y.; Zhang, Z.; Chen, W.; Liu, G.; Mo, J. Synthesis of TPGS/Curcumin Nanoparticles by Thin-Film Hydration and Evaluation of Their Anti-Colon Cancer Efficacy In Vitro and In Vivo. Front. Pharmacol. 2019, 10, 769. [Google Scholar] [CrossRef]
- Almeida, T.C.; Seibert, J.B.; de Souza Almeida, S.H.; Amparo, T.R.; de Medeiros Teixeira, L.F.; Barichello, J.M.; Postacchini, B.B.; Santos, O.D.H.D.; da Silva, G.N. Polymeric micelles containing resveratrol: Development, characterization, cytotoxicity on tumor cells and antimicrobial activity. Braz. J. Pharm. Sci. 2020, 56, e18411. [Google Scholar] [CrossRef]
- Rao, D.; Cote, B.; Stammet, M.; Al Fatease, A.; Alani, A. Evaluation of the Stability of Resveratrol Pluronic® Micelles Prepared by Solvent Casting and Simple Equilibrium Methods. Pharm. Nanotechnol. 2016, 4, 120–125. [Google Scholar] [CrossRef]
- Shi, W.; Fang, F.; Kong, Y.; Greer, S.E.; Kuss, M.; Liu, B.; Xue, W.; Jiang, X.; Lovell, P.; Mohs, A.M.; et al. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication 2022, 14, 014107. [Google Scholar] [CrossRef] [PubMed]
- Spearman, B.S.; Agrawal, N.K.; Rubiano, A.; Simmons, C.S.; Mobini, S.; Schmidt, C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. A 2020, 108, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Sigen, A.; Xu, Q.; McMichael, P.; Gao, Y.; Li, X.; Wang, X.; Greiser, U.; Zhou, D.; Wang, W. A facile one-pot synthesis of acrylated hyaluronic acid. Chem. Commun. 2018, 54, 1081–1084. [Google Scholar] [CrossRef]
- Chauhan, N.; Gupta, P.; Arora, L.; Pal, D.; Singh, Y. Dexamethasone-loaded, injectable pullulan-poly(ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions. Mater. Sci. Eng. C 2021, 130, 112463. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-C.; Hou, S.-M.; Chen, R.-J.; Peng, H.-W.; Hsieh, C.-F.; Kuo, M.-L.; Yen, M.-L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef]
- Deng, X.; Liu, Q.; Fu, Y.; Luo, X.; Hu, M.; Ma, F.; Wang, Q.; Lai, X.; Zhou, L. Effects of Lycium barbarum polysaccharides with different molecular weights on function of RAW264.7 macrophages. Food Agric. Immunol. 2018, 29, 808–820. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, M.; Li, Y.; Li, Q.; Yang, H.; Zheng, M.; Han, Y.; Lu, D.; Lu, S.; Gui, L. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact. Mater. 2021, 6, 1255–1266. [Google Scholar] [CrossRef]
- Mancuso, A.; Tarsitano, M.; Cavaliere, R.; Fresta, M.; Cristiano, M.C.; Paolino, D. Gelled Liquid Crystal Nanocarriers for Improved Antioxidant Activity of Resveratrol. Gels 2023, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F., Jr. Antioxidant and anti-inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 2022, 13, 967193. [Google Scholar] [CrossRef]
- McLean, R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, R.-L.; He, P.; Chen, R. MAR1 suppresses inflammatory response in LPS-induced RAW 264.7 macrophages and human primary peripheral blood mononuclear cells via the SIRT1/PGC-1α/PPAR-γ pathway. J. Inflamm. 2021, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in LPS-Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hass, B.; Kuss, M.A.; Zhang, H.; Ryu, S.; Zhang, D.; Li, T.; Li, Y.-L.; Duan, B. Fabrication of versatile dynamic hyaluronic acid-based hydrogels. Carbohydr. Polym. 2020, 233, 115803. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, W.; Kuss, M.; Mirza, S.; Qi, D.; Krasnoslobodtsev, A.; Zeng, J.; Band, H.; Band, V.; Duan, B. 3D Bioprinting of Breast Cancer Models for Drug Resistance Study. ACS Biomater. Sci. Eng. 2018, 4, 4401–4411. [Google Scholar] [CrossRef]
- Zhang, W.; Kuss, M.; Yan, Y.; Shi, W. Dynamic Alginate Hydrogel as an Antioxidative Bioink for Bioprinting. Gels 2023, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mirza, S.; Wu, S.; Zeng, J.; Shi, W.; Band, H.; Band, V.; Duan, B. 3D hydrogel breast cancer models for studying the effects of hypoxia on epithelial to mesenchymal transition. Oncotarget 2018, 9, 32191–32203. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zheng, L.; Yan, Y.; Shi, W. Facile Preparation of Multifunctional Hydrogels with Sustained Resveratrol Release Ability for Bone Tissue Regeneration. Gels 2024, 10, 429. https://doi.org/10.3390/gels10070429
Zhang W, Zheng L, Yan Y, Shi W. Facile Preparation of Multifunctional Hydrogels with Sustained Resveratrol Release Ability for Bone Tissue Regeneration. Gels. 2024; 10(7):429. https://doi.org/10.3390/gels10070429
Chicago/Turabian StyleZhang, Wenhai, Li Zheng, Yi Yan, and Wen Shi. 2024. "Facile Preparation of Multifunctional Hydrogels with Sustained Resveratrol Release Ability for Bone Tissue Regeneration" Gels 10, no. 7: 429. https://doi.org/10.3390/gels10070429
APA StyleZhang, W., Zheng, L., Yan, Y., & Shi, W. (2024). Facile Preparation of Multifunctional Hydrogels with Sustained Resveratrol Release Ability for Bone Tissue Regeneration. Gels, 10(7), 429. https://doi.org/10.3390/gels10070429






