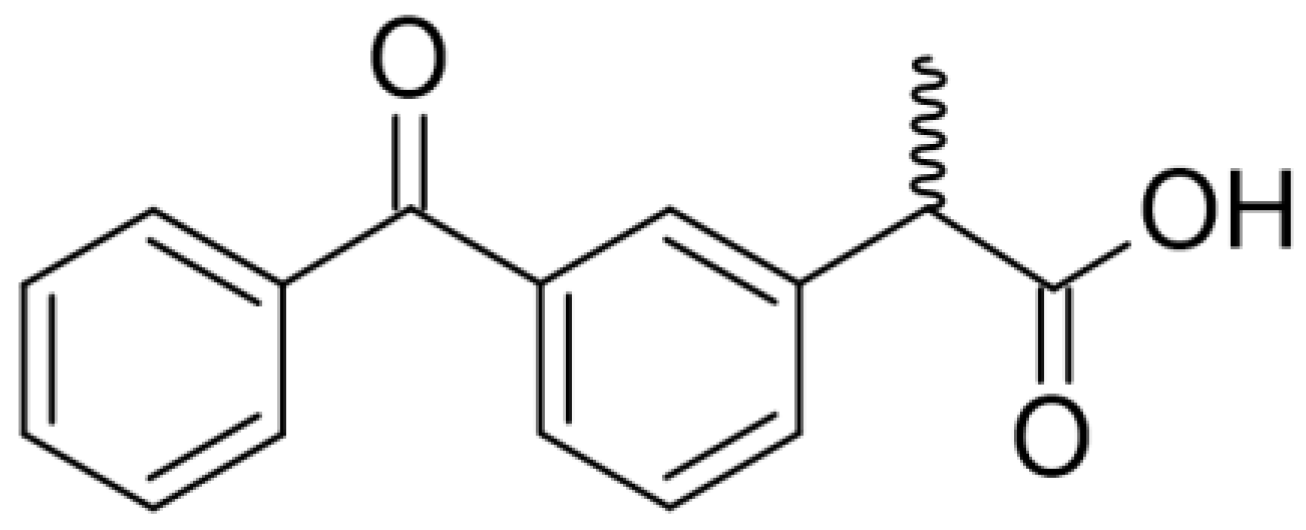
Microemulsion-Based Polymer Gels with Ketoprofen and Menthol: Physicochemical Properties and Drug Release Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Microemulsion Preparation and Characterization
2.2. Microemulsion-Based Gels: Preparation and Characterization
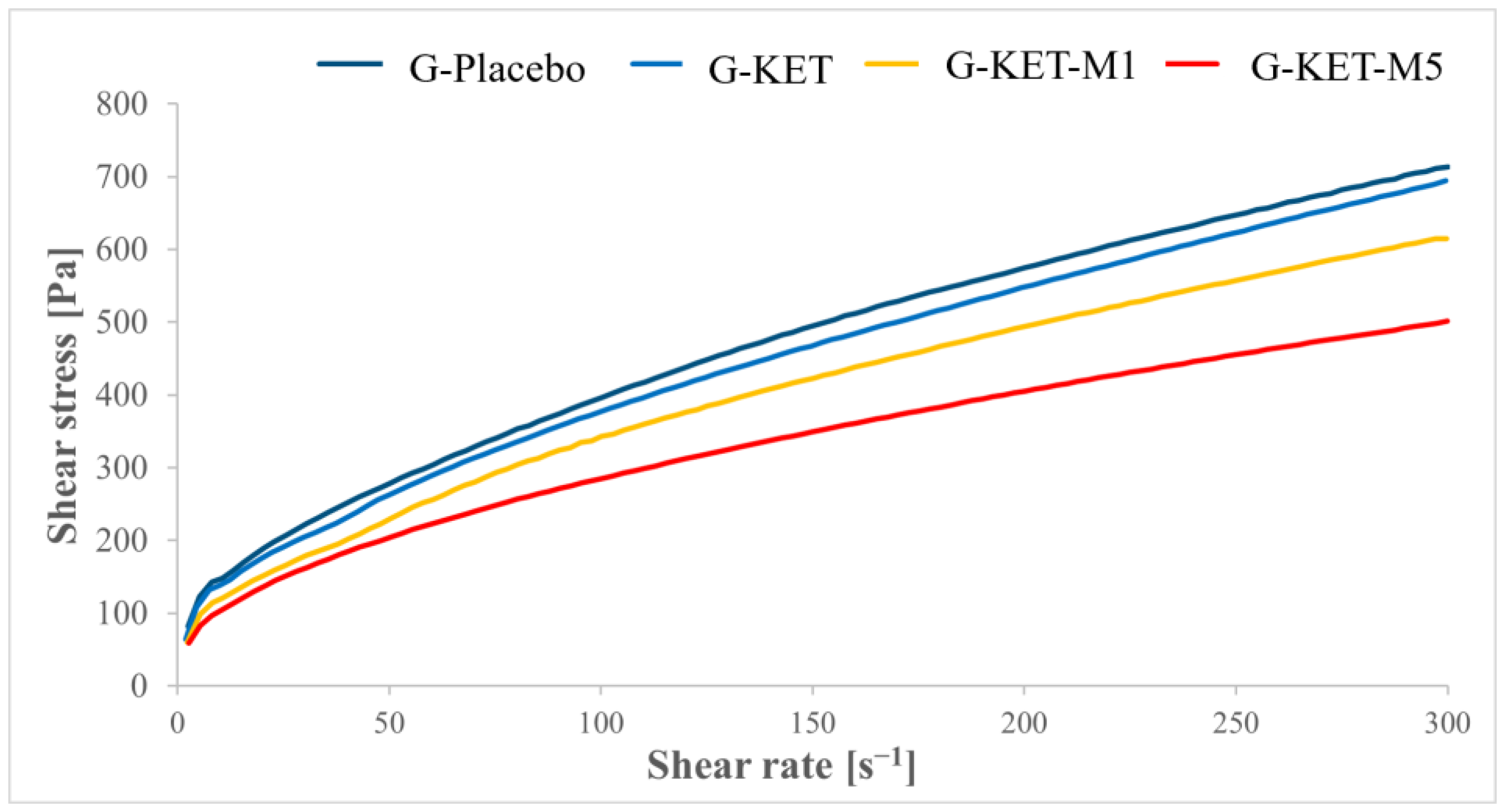
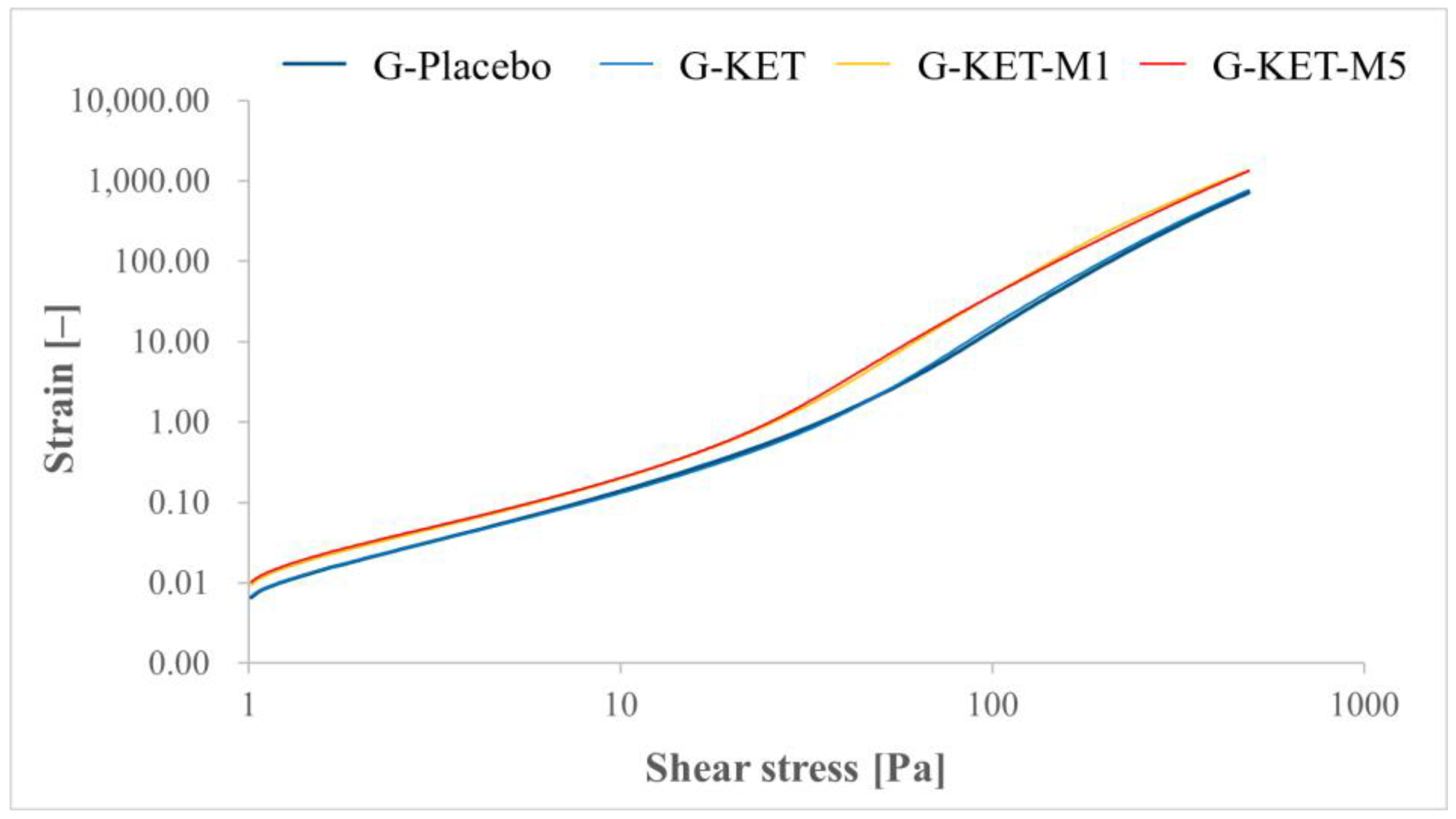
2.2.1. Rheological Studies
2.2.2. Texture Profile Analysis (TPA)
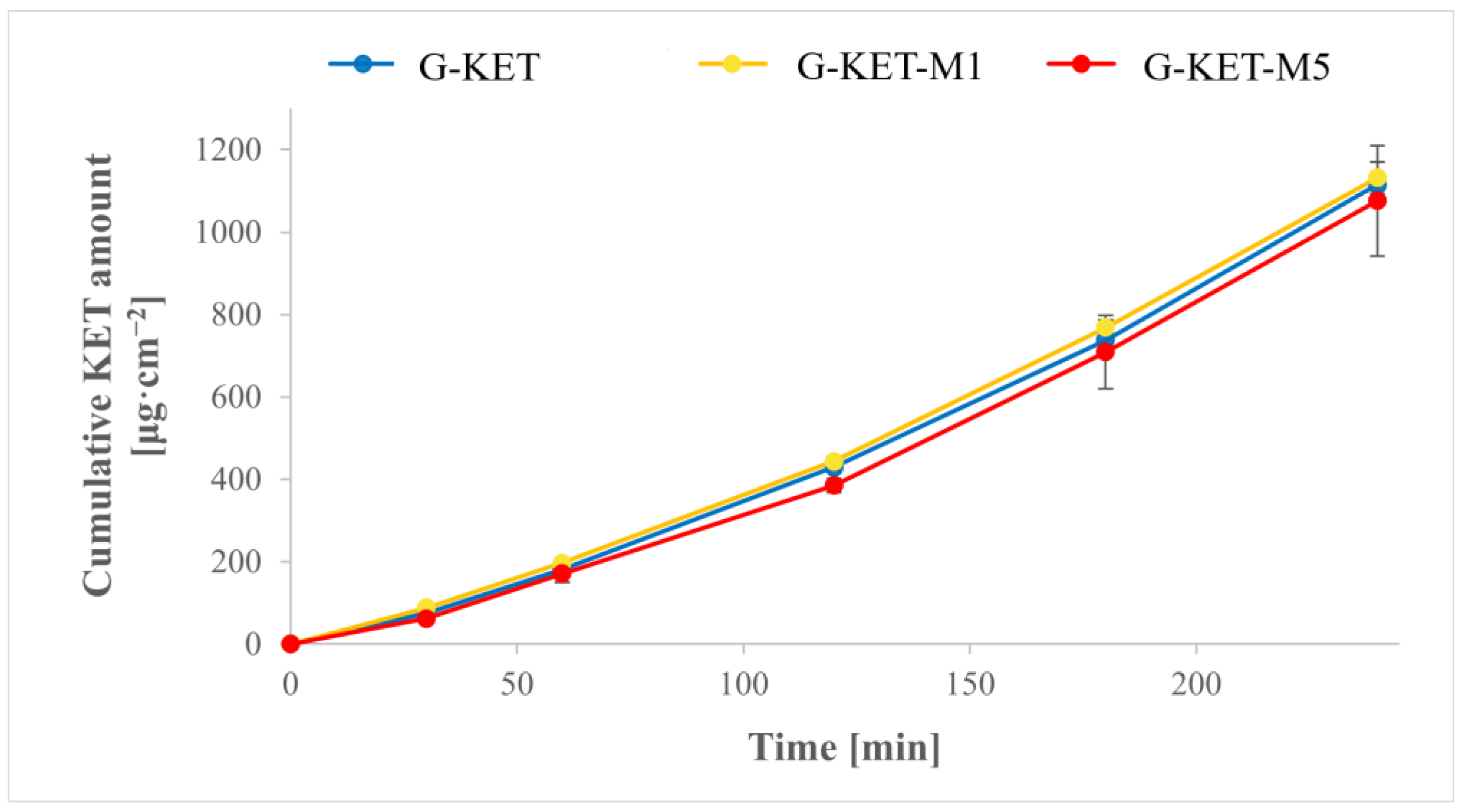
2.2.3. The Drug Release Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Microemulsion Preparation and Characterization
4.2.2. Microemulsion-Based Gels: Preparation and Characterization
Rheological Studies
- Flow behavior study in controlled shear rate mode (CR; shear rate: 1.0–300.0 s−1, measurement time: 60 s);
- Flow behavior study in controlled shear stress mode (CS; shear stress: 1.0–500.0 Pa, measurement time: 60 s);
- Oscillatory stress sweep (SS; oscillatory stress: 0.1–500.0 Pa; frequency was kept constant at 1.0 Hz);
- Oscillatory frequency sweep (FS; oscillatory frequency: 0.1–10.0 Hz; stress was kept constant at 1.0 Pa, which was based on the results of SS tests).
Texture Profile Analysis (TPA)
Drug Release Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Patients with COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti-inflammatory-drugs-(nsaids)-in-patients-with-covid-19 (accessed on 23 April 2024).
- Research, C. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- Musculoskeletal Health. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 23 April 2024).
- Liu, S.; Wang, B.; Fan, S.; Wang, Y.; Zhan, Y.; Ye, D. Global Burden of Musculoskeletal Disorders and Attributable Factors in 204 Countries and Territories: A Secondary Analysis of the Global Burden of Disease 2019 Study. BMJ Open 2022, 12, e062183. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Ye, Z.; Shao, Z.; Fan, B.; Huang, C.; Zhang, Y.; Kuang, X.; Miao, L.; Wu, X.; Zhao, R.; et al. Multidisciplinary Guidelines for the Rational Use of Topical Non-Steroidal Anti-Inflammatory Drugs for Musculoskeletal Pain (2022). J. Clin. Med. 2023, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.K.; Pergolizzi, J.V.; Christo, P.J. Management of Musculoskeletal Pain: An Update with Emphasis on Chronic Musculoskeletal Pain. Pain. Ther. 2021, 10, 181–209. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Identifying Disabling Medical Conditions Likely to Improve with Treatment. Musculoskeletal Disorders. In Selected Health Conditions and Likelihood of Improvement with Treatment; National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.C.; Ang, A.T.W.; Kadir, H.B.A.; Lee, P.H.; Goh, B.Q.; Harikrishnan, S.; Kwek, J.L.; Gan, S.S.W.; Choo, J.C.J.; Tan, N.C. Short-Course Systemic and Topical Non-Steroidal Anti-Inflammatory Drugs: Impact on Adverse Renal Events in Older Adults with Co-Morbid Disease. Drugs Aging 2021, 38, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wei, J.; Persson, M.S.M.; Sarmanova, A.; Doherty, M.; Xie, D.; Wang, Y.; Li, X.; Li, J.; Long, H.; et al. Relative Efficacy and Safety of Topical Non-Steroidal Anti-Inflammatory Drugs for Osteoarthritis: A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials and Observational Studies. Br. J. Sports Med. 2018, 52, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Hawthorn, C. A Narrative Review: The Use of the Topical NSAID Ibuprofen for the Treatment of Knee Osteoarthritis. Supporting Clinician Decision-Making in the First-Line Treatment of Osteoarthritis. Rehabil. Process Outcome 2020, 9, 1179572720914945. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, M.; Wang, H.; You, Y.; Wei, C.; Liu, M.; Luo, A.; Xu, X.; Duan, X. Relative Safety and Efficacy of Topical and Oral NSAIDs in the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis. Medicine 2022, 101, e30354. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.G.; Christophersen, C.; Brown, S.M.; Mulcahey, M.K. Topical Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Physician Sportsmed. 2021, 49, 381–391. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Xia, J. Systematic Review of Topical Diclofenac for the Treatment of Acute and Chronic Musculoskeletal Pain. Curr. Med. Res. Opin. 2020, 36, 637–650. [Google Scholar] [CrossRef]
- Maloney, J.; Pew, S.; Wie, C.; Gupta, R.; Freeman, J.; Strand, N. Comprehensive Review of Topical Analgesics for Chronic Pain. Curr. Pain Headache Rep. 2021, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska, J.; Nieradko-Iwanicka, B. Future Prospects of Ketoprofen in Improving the Safety of the Gastric Mucosa. Biomed. Pharmacother. 2021, 139, 111608. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Dargan, P.; Lanas, A.; Wiffen, P. The Burden of Musculoskeletal Pain and the Role of Topical Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Its Treatment. Ten Underpinning Statements a Global Pain Faculty. Curr. Med. Res. Opin. 2021, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Szumała, P.; Macierzanka, A. Topical Delivery of Pharmaceutical and Cosmetic Macromolecules Using Microemulsion Systems. Int. J. Pharm. 2022, 615, 121488. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Nishchaya, K.; Rai, V.K. Nanoemulsion-Based Dosage Forms for the Transdermal Drug Delivery Applications: A Review of Recent Advances. Expert Opin. Drug Deliv. 2022, 19, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid Vesicles: A Versatile Drug Delivery Platform for Dermal and Transdermal Applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.V.; Van, M.C.; Thi, H.P.; Thanh, C.Đ.; Ngoc, B.T.; Van, B.N.; Le Thien, G.; Van, B.N.; Nguyen, C.N. Development of Ibuprofen-Loaded Solid Lipid Nanoparticle-Based Hydrogels for Enhanced in Vitro Dermal Permeation and in Vivo Topical Anti-Inflammatory Activity. J. Drug Deliv. Sci. Technol. 2020, 57, 101758. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Parmar, P.K.; Wadhawan, J.; Bansal, A.K. Pharmaceutical Nanocrystals: A Promising Approach for Improved Topical Drug Delivery. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef]
- Virani, A.; Puri, V.; Mohd, H.; Michniak-Kohn, B. Effect of Penetration Enhancers on Transdermal Delivery of Oxcarbazepine, an Antiepileptic Drug Using Microemulsions. Pharmaceutics 2023, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Virani, A.; Dholaria, N.; Matharoo, N.; Michniak-Kohn, B. A Study of Microemulsion Systems for Transdermal Delivery of Risperidone Using Penetration Enhancers. J. Pharm. Sci. 2023, 112, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ye, D.; Jing, P.; Tan, X.; Qiu, L.; Li, T.; Shen, L.; Sun, Y.; Hou, H.; Zhang, Y.; et al. Design, Optimization and Evaluation of Co-Surfactant Free Microemulsion-Based Hydrogel with Low Surfactant for Enhanced Transdermal Delivery of Lidocaine. Int. J. Pharm. 2020, 586, 119415. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Abla, K.K.; Domiati, S.; Elmaradny, H. Superiority of Microemulsion-Based Hydrogel for Non-Steroidal Anti-Inflammatory Drug Transdermal Delivery: A Comparative Safety and Anti-Nociceptive Efficacy Study. Int. J. Pharm. 2022, 622, 121830. [Google Scholar] [CrossRef] [PubMed]
- Shewaiter, M.A.; Hammady, T.M.; El-Gindy, A.; Hammadi, S.H.; Gad, S. Formulation and Characterization of Leflunomide/Diclofenac Sodium Microemulsion Base-Gel for the Transdermal Treatment of Inflammatory Joint Diseases. J. Drug Deliv. Sci. Technol. 2021, 61, 102110. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Kunstman, P.; Jadach, B.; Brzostowska, M.; Białas, W. Design and Study of Poloxamer-Based Microemulsion Gels with Naproxen. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 101–112. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Zahid, M.I.; Walsh, T.; Shah, J.; Gates, D.; Yeoh, T.; Nurunnabi, M. Topical Drug Delivery by Sepineo P600 Emulgel: Relationship between Rheology, Physical Stability, and Formulation Performance. Int. J. Pharm. 2024, 658, 124210. [Google Scholar] [CrossRef] [PubMed]
- Lapasin, R.; Grassi, M.; Coceani, N. Effects of Polymer Addition on the Rheology of o/w Microemulsions. Rheol. Acta 2001, 40, 185–192. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Taylor, R.; LeQuang, J.-A.; Raffa, R.B. The Role and Mechanism of Action of Menthol in Topical Analgesic Products. J. Clin. Pharm. Ther. 2018, 43, 313–319. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Hwang, S.W.; Miyamoto, T.; Dubin, A.E.; Patapoutian, A.; Story, G.M. More than Cool: Promiscuous Relationships of Menthol and Other Sensory Compounds. Mol. Cell. Neurosci. 2006, 32, 335–343. [Google Scholar] [CrossRef]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Menthol: A Refreshing Look at This Ancient Compound. J. Am. Acad. Dermatol. 2007, 57, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, L.; Xia, M.; Chu, X. The Evaluations of Menthol and Propylene Glycol on the Transdermal Delivery System of Dual Drug–Loaded Lyotropic Liquid Crystalline Gels. AAPS PharmSciTech 2020, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Olivella, M.S.; Lhez, L.; Pappano, N.B.; Debattista, N.B. Effects of Dimethylformamide and L-Menthol Permeation Enhancers on Transdermal Delivery of Quercetin. Pharm. Dev. Technol. 2007, 12, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, L.; Yang, S.; Wu, X.; Dai, X.; Wang, S.; Shi, X. A Multiscale Study of the Penetration-Enhancing Mechanism of Menthol. J. Tradit. Chin. Med. Sci. 2019, 6, 347–354. [Google Scholar] [CrossRef]
- Tanojo, H.; Junginger, H.E.; Boddé, H.E. In Vivo Human Skin Permeability Enhancement by Oleic Acid: Transepidermal Water Loss and Fourier-Transform Infrared Spectroscopy Studies. J. Control. Release 1997, 47, 31–39. [Google Scholar] [CrossRef]
- Naik, A.; Pechtold, L.A.R.M.; Potts, R.O.; Guy, R.H. Mechanism of Oleic Acid-Induced Skin Penetration Enhancement In Vivo in Humans. J. Control. Release 1995, 37, 299–306. [Google Scholar] [CrossRef]
- Rashid, M.A.; Naz, T.; Abbas, M.; Nazir, S.; Younas, N.; Majeed, S.; Qureshi, N.; Akhtar, M.N. Chloramphenicol Loaded Microemulsions: Development, Characterization and Stability. Colloid Interface Sci. Commun. 2019, 28, 41–48. [Google Scholar] [CrossRef]
- Farghaly, D.A.; Aboelwafa, A.A.; Hamza, M.Y.; Mohamed, M.I. Microemulsion for Topical Delivery of Fenoprofen Calcium: In Vitro and In Vivo Evaluation. J. Liposome Res. 2018, 28, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of Microemulsions of Suitable Viscosity for Cyclosporine Skin Delivery. Int. J. Pharm. 2018, 545, 197–205. [Google Scholar] [CrossRef]
- Tung, N.-T.; Vu, V.-D.; Nguyen, P.-L. DoE-Based Development, Physicochemical Characterization, and Pharmacological Evaluation of a Topical Hydrogel Containing Betamethasone Dipropionate Microemulsion. Colloids Surf. B Biointerfaces 2019, 181, 480–488. [Google Scholar] [CrossRef]
- Rhee, Y.-S.; Choi, J.-G.; Park, E.-S.; Chi, S.-C. Transdermal Delivery of Ketoprofen Using Microemulsions. Int. J. Pharm. 2001, 228, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ponce Ponte, M.; Bianco, M.; Longhi, M.; Aloisio, C. Study and Development of Microemulsion Formulations to Increase the Permeability of Acyclovir. J. Mol. Liq. 2022, 348, 118408. [Google Scholar] [CrossRef]
- El Khayat, N.W.; Donia, A.A.; Mady, O.Y.; El Maghraby, G.M. Optimization of Eugenol Microemulsion for Transdermal Delivery of Indomethacin. J. Drug Deliv. Sci. Technol. 2018, 48, 311–318. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Rai, B.; Mitragotri, S. Molecular Mechanism of the Skin Permeation Enhancing Effect of Ethanol: A Molecular Dynamics Study. RSC Adv. 2020, 10, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Perinelli, D.R.; Ciacciarelli, A.; Quassinti, L.; Bramucci, M.; Miano, A.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Properties and Stability of Nanoemulsions: How Relevant Is the Type of Surfactant? J. Drug Deliv. Sci. Technol. 2020, 58, 101772. [Google Scholar] [CrossRef]
- Siddique, M.Y.; Alamgir, I.; Nazar, M.F.; Sumrra, S.H.; Ashfaq, M.; Safdar, M.; Khan, S.U.-D.; Ahmad, A.; Khan, R.; Al Swaidan, H.M.; et al. Structural and Probing Dynamics of Brij-35-Based Microemulsion for Fluoroquinolone Antibiotics. Colloid Polym. Sci. 2021, 299, 1479–1488. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Cazarin, C.A.; da Silva, P.B.; dos Santos, K.P.; da Rocha, M.C.O.; Báo, S.N.; De-Souza, M.M.; Chorilli, M. Intranasal in Situ Gelling Liquid Crystal for Delivery of Resveratrol Ameliorates Memory and Neuroinflammation in Alzheimer’s Disease. Nanomed. Nanotechnol. Biol. Med. 2023, 51, 102689. [Google Scholar] [CrossRef] [PubMed]
- Sarpietro, M.G.; Accolla, M.L.; Puglisi, G.; Castelli, F.; Montenegro, L. Idebenone Loaded Solid Lipid Nanoparticles: Calorimetric Studies on Surfactant and Drug Loading Effects. Int. J. Pharm. 2014, 471, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Śliwa, K.; Śliwa, P. The Accumulated Effect of the Number of Ethylene Oxide Units and/or Carbon Chain Length in Surfactants Structure on the Nano-Micellar Extraction of Flavonoids. J. Funct. Biomater. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Callender, S.P.; Wettig, S.D. Phase Behavior of Non-Ionic Surfactant-Medium Chain Triglyceride-Water Microemulsion Systems. J. Surfactants Deterg. 2021, 24, 603–629. [Google Scholar] [CrossRef]
- Goddeeris, C.; Cuppo, F.; Reynaers, H.; Bouwman, W.G.; Van den Mooter, G. Light Scattering Measurements on Microemulsions: Estimation of Droplet Sizes. Int. J. Pharm. 2006, 312, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Rees, G.D. Microemulsion-Based Media as Novel Drug Delivery Systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef] [PubMed]
- Attwood, D.; Mallon, C.; Ktistis, G.; Taylor, C.J. A Study on Factors Influencing the Droplet Size in Nonionic Oil-in-Water Microemulsions. Int. J. Pharm. 1992, 88, 417–422. [Google Scholar] [CrossRef]
- Aboofazeli, R.; Barlow, D.J.; Lawrence, M.J. Particle Size Analysis of Concentrated Phospholipid Microemulsions: I. Total Intensity Light Scattering. AAPS PharmSci 2000, 2, 13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berardi, A.; Perinelli, D.R.; Bisharat, L.; Sabbatini, B.; Bonacucina, G.; Tiboni, M.; Palmieri, G.F.; Cespi, M. Factors Affecting the Rheological Behaviour of Carbomer Dispersions in Hydroalcoholic Medium: Towards the Optimization of Hand Sanitiser Gel Formulations. Int. J. Pharm. 2022, 616, 121503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, Y.; Xue, Y.; Zhu, Z.; Wu, Y.; Zeng, Q.; Wang, Y.; Han, H.; Zhang, H.; Shen, C.; et al. Mechanism Insight on Licorice Flavonoids Release from Carbopol Hydrogels: Role of “Release Steric Hindrance” and Drug Solubility in the Release Medium. Eur. J. Pharm. Sci. 2022, 179, 106307. [Google Scholar] [CrossRef] [PubMed]
- Shinde, U.A.; Modani, S.H.; Singh, K.H. Design and Development of Repaglinide Microemulsion Gel for Transdermal Delivery. AAPS PharmSciTech 2018, 19, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-Based Commercial Products for Biomedical Applications: A Review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Aggarwal, G.; Nagpal, M. Pharmaceutical Polymer Gels in Drug Delivery. In Polymer Gels: Perspectives and Applications; Thakur, V.K., Thakur, M.K., Voicu, S.I., Eds.; Springer: Singapore, 2018; pp. 249–284. ISBN 978-981-10-6080-9. [Google Scholar]
- Available online: https://www.lubrizol.com/-/media/Lubrizol/Health/TDS/TDS-237_Neutralizing_Carbopol_Pemulen_in_Aqueous_Hydroalcoholic_Systems--PH.pdf (accessed on 10 June 2024).
- Younes, E.; Himl, M.; Stary, Z.; Bertola, V.; Burghelea, T. On the Elusive Nature of Carbopol Gels: “Model”, Weakly Thixotropic, or Time-Dependent Viscoplastic Materials? J. Non-Newton. Fluid Mech. 2020, 281, 104315. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Madan, P.; Lin, S. Formulation and Rheological Evaluation of Ethosome-Loaded Carbopol Hydrogel for Transdermal Application. Drug Dev. Ind. Pharm. 2016, 42, 1315–1324. [Google Scholar] [CrossRef]
- Kolman, M.; Smith, C.; Chakrabarty, D.; Amin, S. Rheological Stability of Carbomer in Hydroalcoholic Gels: Influence of Alcohol Type. Int. J. Cosmet. Sci. 2021, 43, 748–763. [Google Scholar] [CrossRef]
- Teixeira, A.; Vasconcelos, V.; Teixeira, M.; Almeida, V.; Azevedo, R.; Torres, T.; Sousa Lobo, J.M.; Costa, P.C.; Almeida, I.F. Mechanical Properties of Topical Anti-Psoriatic Medicines: Implications for Patient Satisfaction with Treatment. AAPS PharmSciTech 2019, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K. Some Thoughts on The Definition of a Gel. In Proceedings of the Gels: Structures, Properties, and Functions; Tokita, M., Nishinari, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 87–94. [Google Scholar]
- Clark, A.H.; Ross-Murphy, S.B. Structural and Mechanical Properties of Biopolymer Gels. In Proceedings of the Biopolymers; Springer: Berlin/Heidelberg, Germany, 1987; pp. 57–192. [Google Scholar]
- Kim, J.-Y.; Song, J.-Y.; Lee, E.-J.; Park, S.-K. Rheological Properties and Microstructures of Carbopol Gel Network System. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Snela, A.; Kunstman, P.; Jadach, B.; Olejniczak, M.; Roszak, G.; Białas, W. Novel Microemulsion-Based Gels for Topical Delivery of Indomethacin: Formulation, Physicochemical Properties and In Vitro Drug Release Studies. J. Colloid Interface Sci. 2017, 507, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y. Perception and Measurement of Food Texture: Solid Foods. J. Texture Stud. 2018, 49, 160–201. [Google Scholar] [CrossRef] [PubMed]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of Instrumental Texture Properties from Textural Profile Analysis (TPA) with Eating Behaviours and Macronutrient Composition for a Wide Range of Solid Foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.; Cortizo-Lacalle, D.; Imaz, A.M.; Aldazabal, J.; Vila, M. Application of Texture Analysis Methods for the Characterization of Cultured Meat. Sci. Rep. 2022, 12, 3898. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and Textural Characterization of Cosmetic Emulsions Containing Natural and Synthetic Polymers: Relationships between Both Data. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 150–163. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Djokic, J. Texture Profile Analysis of Bioadhesive Polymeric Semisolids: Mechanical Characterization and Investigation of Interactions between Formulation Components. J. Appl. Polym. Sci. 1996, 61, 2229–2234. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.A.; Marchetti, J.M. Sustained Release of Lidocaine from Poloxamer 407 Gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef]
- Peltola, S.; Saarinen-Savolainen, P.; Kiesvaara, J.; Suhonen, T.M.; Urtti, A. Microemulsions for Topical Delivery of Estradiol. Int. J. Pharm. 2003, 254, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Chang, J.T.; Chang, J.-S.; Huang, C.-T.; Huang, Y.-B.; Wu, P.-C. The Effect of Component of Microemulsions on Transdermal Delivery of Buspirone Hydrochloride. J. Pharm. Sci. 2011, 100, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Alavi, S.E.; Shafiee, A.; Leite-Silva, V.R.; Khosrotehrani, K.; Mohammed, Y. Metamorphosis of Topical Semisolid Products—Understanding the Role of Rheological Properties in Drug Permeation under the “in Use” Condition. Pharmaceutics 2023, 15, 1707. [Google Scholar] [CrossRef] [PubMed]
- Siemiradzka, W.; Dolińska, B.; Ryszka, F. Influence of Concentration on Release and Permeation Process of Model Peptide Substance-Corticotropin-From Semisolid Formulations. Molecules 2020, 25, 2767. [Google Scholar] [CrossRef]
- Pons, M.; Fiszman, S.M. Instrumental Texture Profile Analysis with Particular Reference to Gelled Systems. J. Texture Stud. 1996, 27, 597–624. [Google Scholar] [CrossRef]
- Salerno, C.; Carlucci, A.M.; Bregni, C. Study of In Vitro Drug Release and Percutaneous Absorption of Fluconazole from Topical Dosage Forms. AAPS PharmSciTech 2010, 11, 986–993. [Google Scholar] [CrossRef]





| Microemulsions | ||||
|---|---|---|---|---|
| Component | Placebo | KET | KET-M1 | KET-M5 |
| KET [%, w/w] | - | 2.5 | 2.5 | 2.5 |
| M [%, w/w] | - | - | 1.0 | 5.0 |
| Microemulsion [%, w/w] | 100.0 | 97.5 | 96.5 | 92.5 |
| pH | 4.89 ± 0.01 | 4.87 ± 0.01 | 4.82 ± 0.02 | 4.73 ± 0.01 |
| Viscosity [mPa s] | 31.29 ± 0.04 | 29.54 ± 0.02 | 29.34 ± 0.08 | 27.91 ± 0.03 |
| Parameter | Placebo | KET | KET-M1 | KET-M5 |
|---|---|---|---|---|
| Peak size [nm] | 3.279 ± 0.169 | 1.877 ± 0.022 | 1.916 ± 0.015 | 2.009 ± 0.029 |
| Peak intensity [%] | 73.2 ± 0.6 | 87.5 ± 1.0 | 90.5 ± 1.5 | 100.0 ± 0.0 |
| Polydispersity index (PDI) | 0.275 ± 0.001 | 0.266 ± 0.004 | 0.276 ± 0.003 | 0.264 ± 0.004 |
| Component | G-Placebo | G-KET | G-KET-M1 | G-KET-M5 |
|---|---|---|---|---|
| KET [%, w/w] | - | 2.5 | 2.5 | 2.5 |
| M [%, w/w] | - | - | 1.0 | 5.0 |
| Carbopol® EZ-3 [%, w/w] | 2.0 | 2.0 | 2.0 | 2.0 |
| TIPA [%, w/w] | 0.2 | 0.2 | 0.2 | 0.2 |
| Microemulsion [%, w/w] | 97.8 | 95.3 | 94.3 | 92.5 |
| pH | 5.10 ± 0.02 | 4.81 ± 0.02 | 4.74 ± 0.01 | 4.71 ± 0.01 |
| Parameter | G-Placebo | G-KET | G-KET-M1 | G-KET-M5 |
|---|---|---|---|---|
| [Pa] | 61.57 ± 2.81 | 56.92 ± 1.77 | 32.46 ± 1.81 | 29.64 ± 1.20 |
| K [Pa sn] | 21.77 ± 1.40 | 17.81 ± 0.61 | 19.19 ± 3.01 | 17.38 ± 3.18 |
| n [-] | 0.60 ± 0.01 | 0.63 ± 0.01 | 0.56 ± 0.02 | 0.61 ± 0.02 |
| Yield point (CS) [Pa] | 33.98 ± 1.45 | 29.67 ± 1.46 | 22.19 ± 2.18 | 18.68 ± 0.63 |
| Crossover point (SS) [Pa] | 84.00 ± 1.78 | 78.47 ± 2.87 | 60.29 ± 0.50 | 46.31 ± 2.29 |
| Parameter | G-Placebo | G-KET | G-KET-M1 | G-KET-M5 |
|---|---|---|---|---|
| Hardness [mN] | 58.37 ± 3.85 | 54.54 ± 2.76 | 44.25 ± 2.25 | 39.72 ± 1.12 |
| Adhesiveness * [mJ] | 568.9 ± 45.1 | 486.8 ± 31.9 | 433.28 ± 43.0 | 433.82 ± 75.0 |
| Cohesiveness [-] | 0.94 ± 0.01 | 0.98 ± 0.01 | 0.96 ± 0.01 | 0.97 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otto, F.; Froelich, A. Microemulsion-Based Polymer Gels with Ketoprofen and Menthol: Physicochemical Properties and Drug Release Studies. Gels 2024, 10, 435. https://doi.org/10.3390/gels10070435
Otto F, Froelich A. Microemulsion-Based Polymer Gels with Ketoprofen and Menthol: Physicochemical Properties and Drug Release Studies. Gels. 2024; 10(7):435. https://doi.org/10.3390/gels10070435
Chicago/Turabian StyleOtto, Filip, and Anna Froelich. 2024. "Microemulsion-Based Polymer Gels with Ketoprofen and Menthol: Physicochemical Properties and Drug Release Studies" Gels 10, no. 7: 435. https://doi.org/10.3390/gels10070435
APA StyleOtto, F., & Froelich, A. (2024). Microemulsion-Based Polymer Gels with Ketoprofen and Menthol: Physicochemical Properties and Drug Release Studies. Gels, 10(7), 435. https://doi.org/10.3390/gels10070435







