Development and Geometrical Considerations of Unique Conductive and Reversible Carbon-Nanotube Hydrogel without Need for Gelators
Abstract
:1. Introduction
2. Results and Discussion
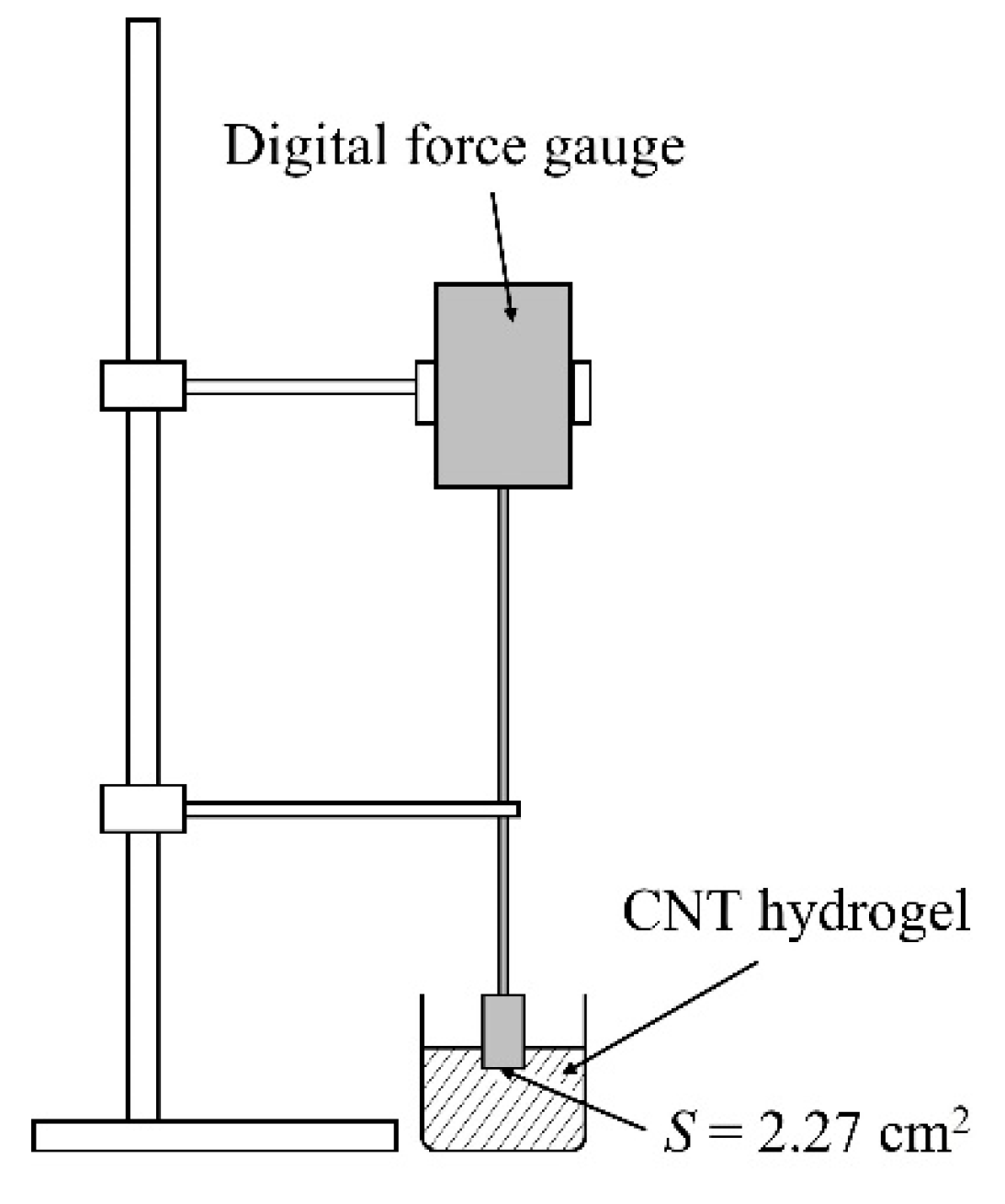
2.1. Experiments on CNT Hydrogelation Consisting Only of CNTs, Dispersants, and Water from CNT Dispersion
2.2. Investigating Response of CNT Hydrogels to Heating Time of CNT Dispersion
2.3. Investigating Influence of Dispersion Concentration
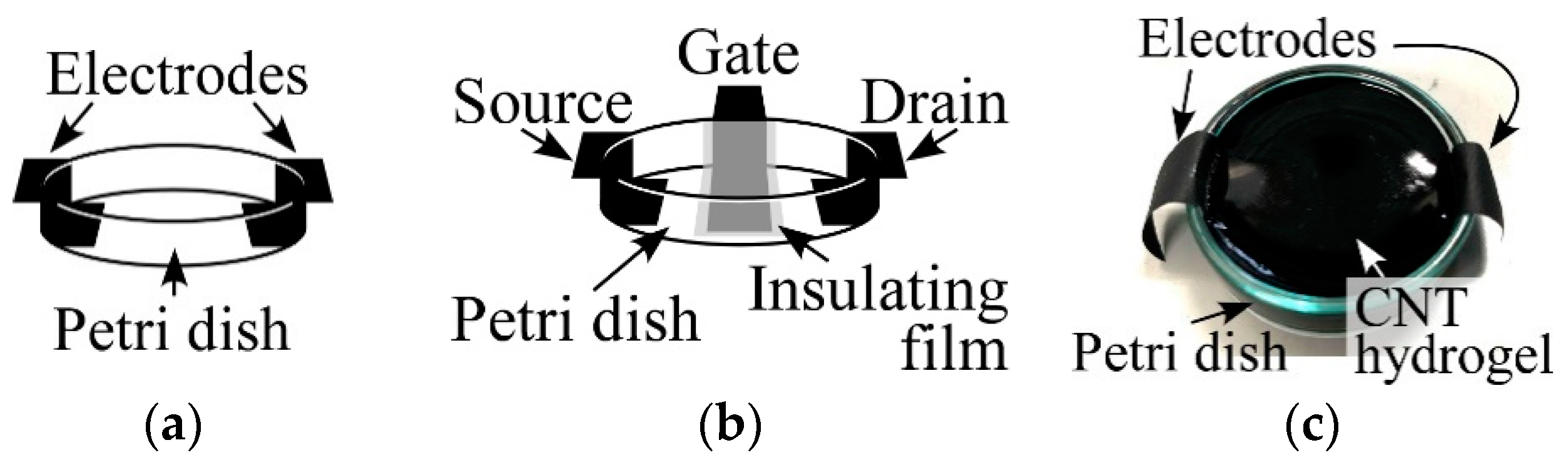
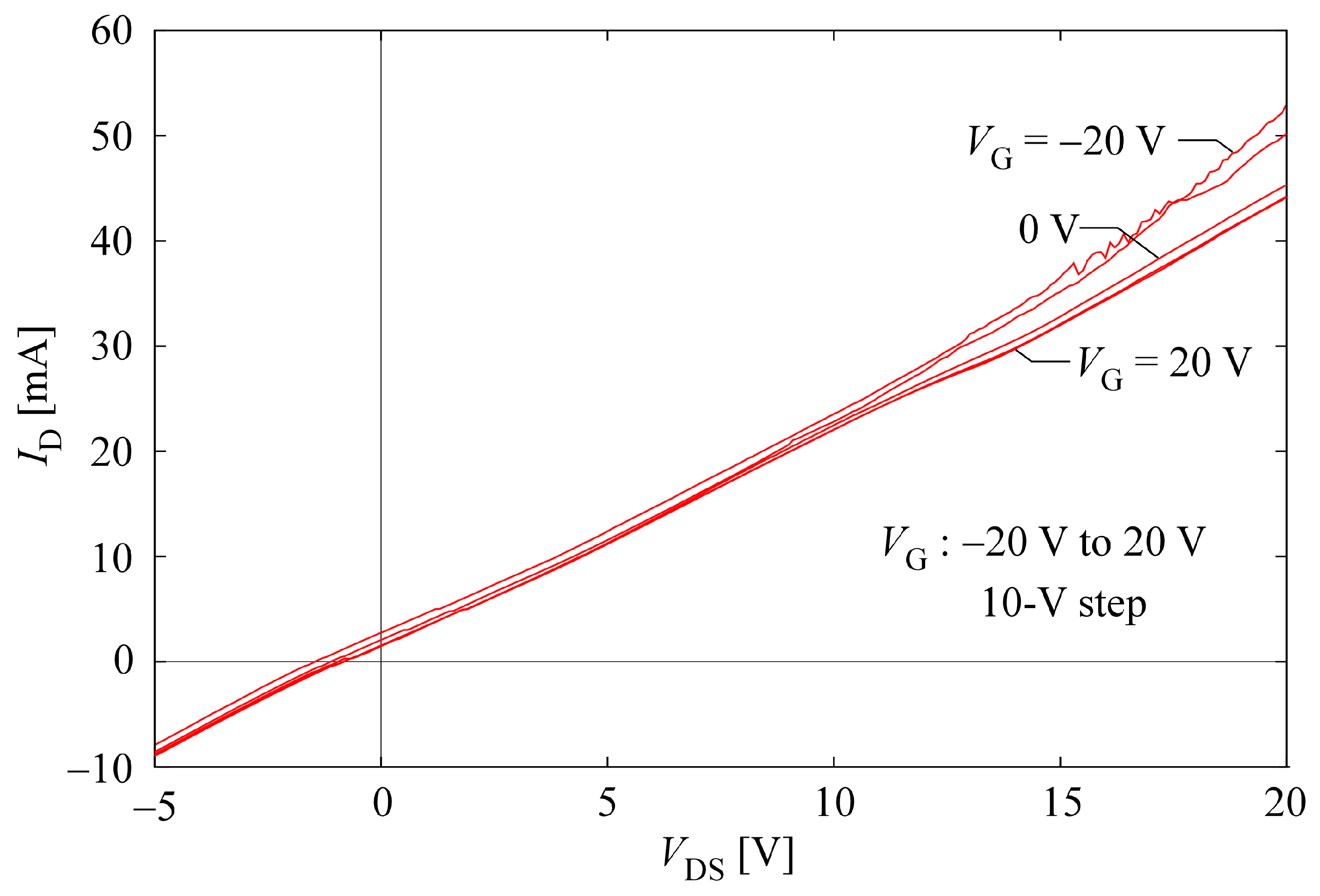
2.4. Investigating Electrical Properties of CNT Hydrogels
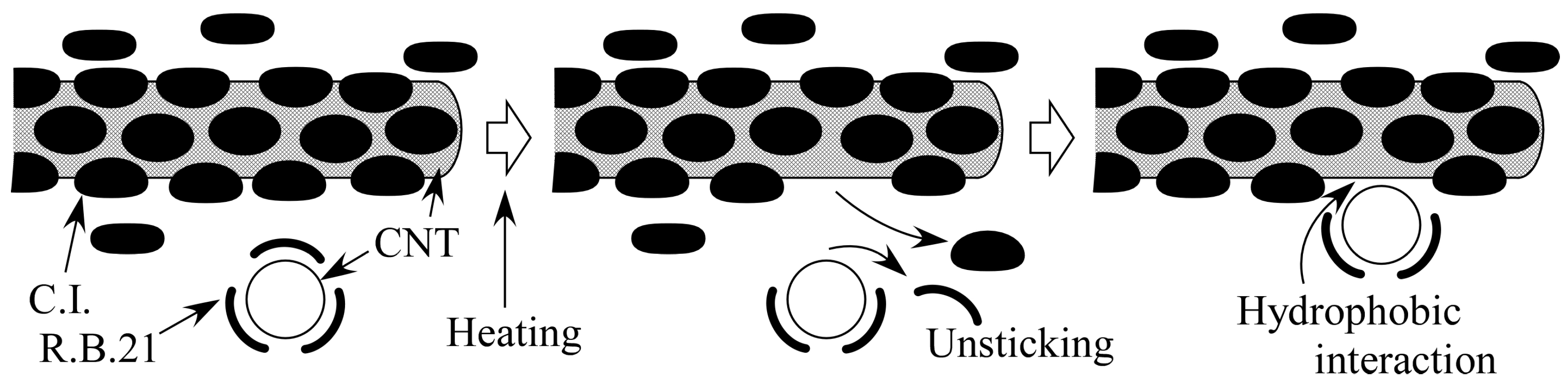
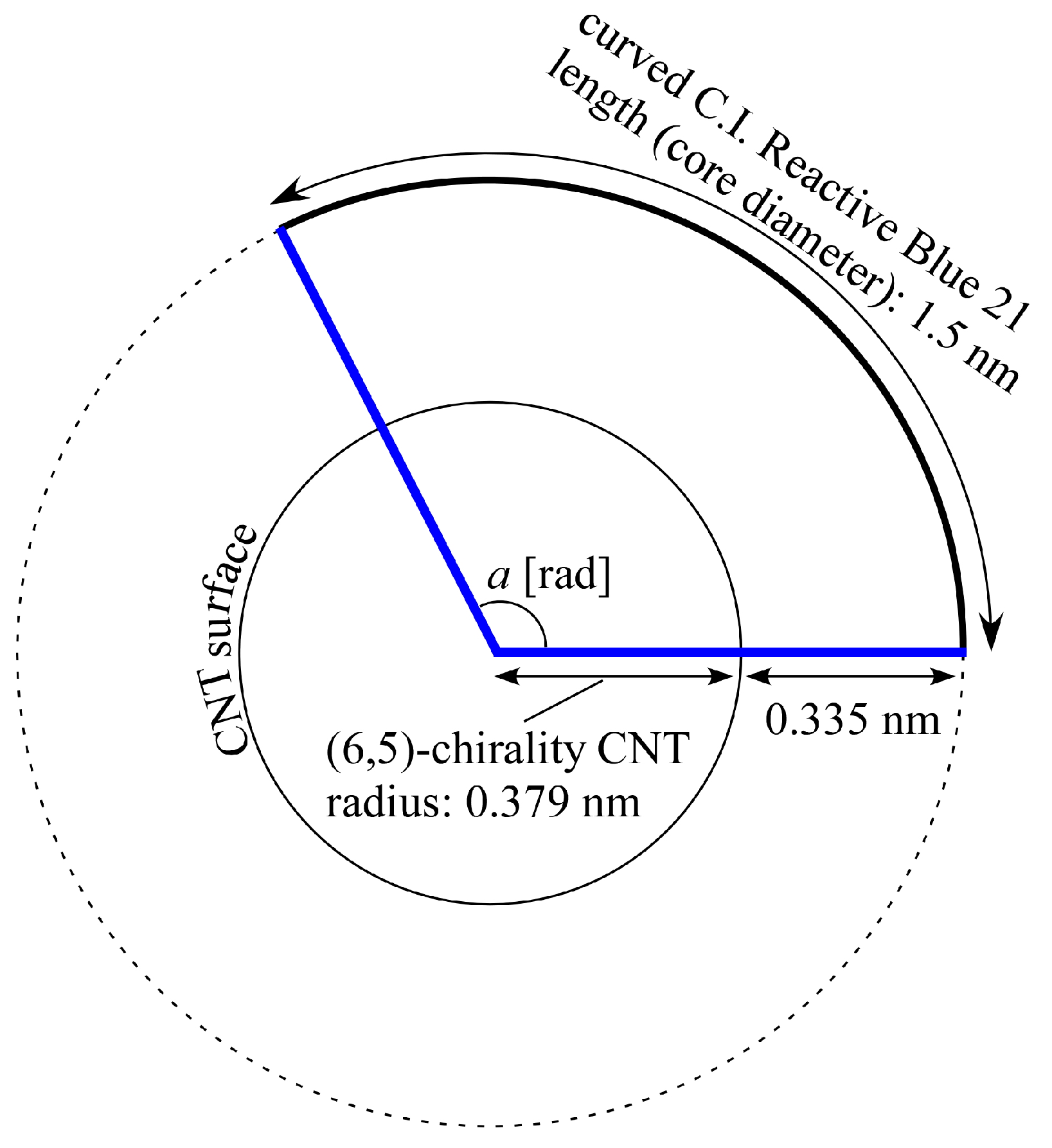
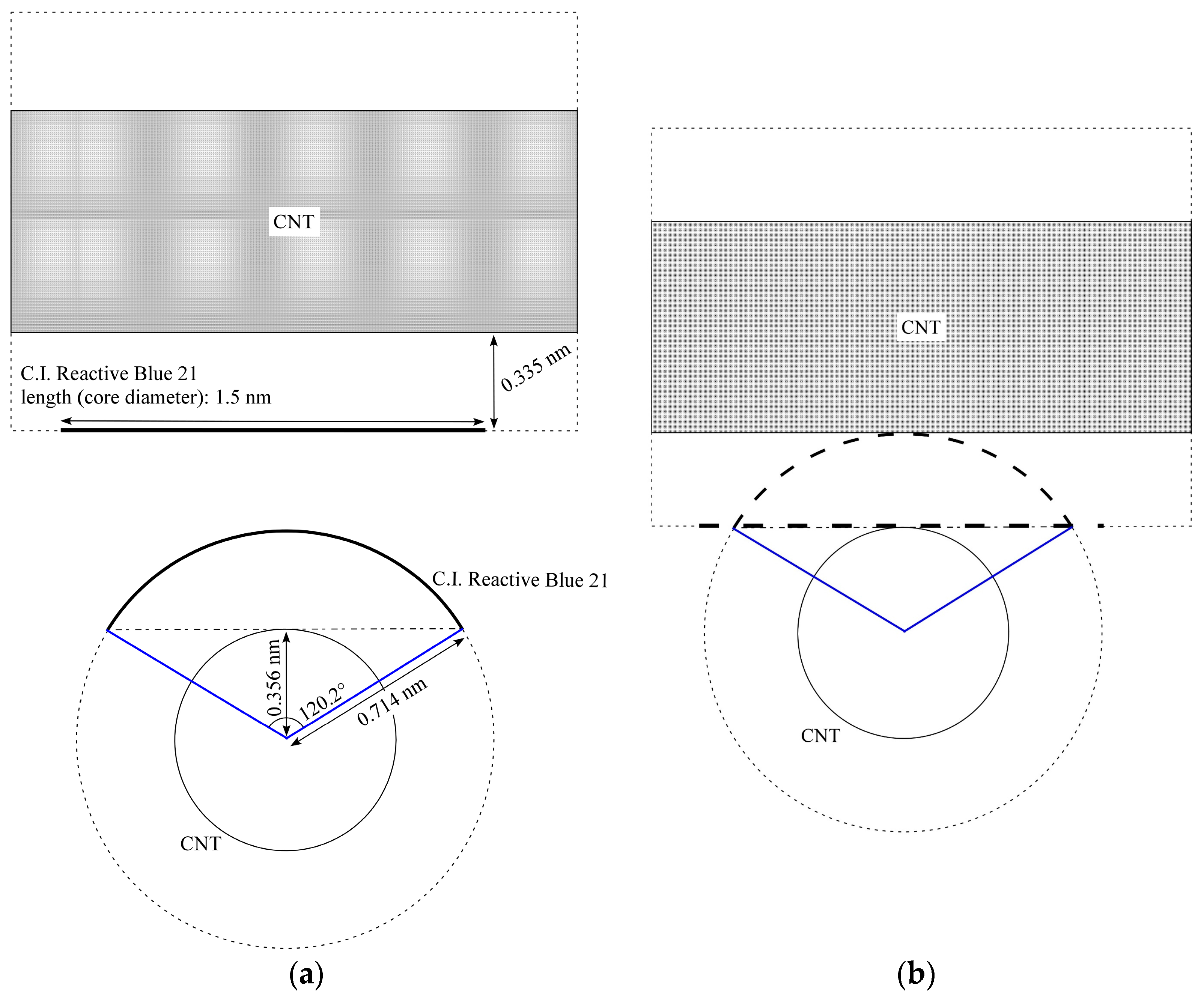
2.5. Discussion with Geometrical Considerations of Changing CNT Dispersion into Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Candidates of CNTs and Dispersants
4.2. Investigating Possibility of Gelation by Combination of CNT and Dispersant Candidates
- CNT dispersion is prepared by dispersing the solution prepared in step 1 for 1 h using an ultrasonic homogenizer (UX-050, Mitsui Electric Co., Ltd., Noda, Japan) while cooling the solution to 0 °C.
- After step 2, the dispersion is heated to 60 °C for 1 h.
- After step 3, the dispersion is checked to see whether it has gelatinized.
4.3. Investigating Response of CNT Hydrogels to Heating Time
4.4. Investigating Effect of Dispersion Concentration on Gelation
4.5. Investigating Electrical Properties of CNT Hydrogels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical micro-tubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. In Materials Science and Engineering: R: Reports; Elsevier: Amsterdam, The Netherlands, 2004; Volume 43, pp. 61–102. [Google Scholar]
- Odom, T.W.; Huang, J.L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Yu, M.F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile loading of ropes of single wall carbon nanotubes and their mechanical properties. Phys. Rev. Lett. 2000, 84, 5552–5555. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Dresselhaus, G.; Avouris, P. (Eds.) Carbon Nanotubes: Synthesis, Structure, Properties, and Applications; Springer: Berlin, Germany, 2000. [Google Scholar]
- Jorio, A.; Dresselhaus, M.S.; Dresselhaus, G. (Eds.) Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties, and Applications; Springer: Berlin, Germany, 2008. [Google Scholar]
- Hata, K. Great expectations for a dream material, 21st century industrial revolution. AIST Stories 2013, 1, 18–19. [Google Scholar]
- Lau, A.K.-T.; Hui, D. The revolutionary creation of new advanced materials—Carbon nanotube composites. Compos. Part B Eng. 2002, 33, 263–277. [Google Scholar] [CrossRef]
- Khare, R.; Bose, S. Carbon nanotube based composites—A review. J. Miner. Mater. Charact. Eng. 2005, 4, 31–46. [Google Scholar] [CrossRef]
- Byrne, M.T.; Gun’ko, Y.K. Recent advances in research on carbon nanotube–polymer composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Esawi, A.M.K.; Farag, M.M. Carbon nanotube reinforced composites: Potential and current challenges. Mater. Des. 2007, 28, 2394–2401. [Google Scholar] [CrossRef]
- Hermans, P.H. Gels in Colloid Science; Krust, H.R., Ed.; Elsevier: New York, NY, USA, 1949; Volume II. [Google Scholar]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef]
- Fukushima, T.; Aida, T. Ionic Liquids for Soft Functional Materials with Carbon Nanotubes. Chem. A Eur. J. 2007, 13, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Asaka, K.; Kosaka, A.; Aida, T. Fully plastic actuator through layer-by-layer casting with ionic-liquid-based Bucky gel. Angew. Chem. Int. Ed. 2005, 44, 2305–2457. [Google Scholar] [CrossRef]
- Chen, G.-X.; Zhang, S.; Zhou, Z.; Li, Q. Dielectric properties of poly(vinylidene fluoride) composites based on Bucky gels of carbon nanotubes with ionic liquids. Polym. Compos. 2015, 36, 94–101. [Google Scholar] [CrossRef]
- Yoon, S.W.; Ren, X.; Chen, G. Preparation and dielectric properties of poly(ε-caprolactone) compounded with “bucky gels”-like mixture. J. Appl. Polym. Sci. 2014, 131, 40231. [Google Scholar] [CrossRef]
- Katakabe, T.; Kaneko, T.; Watanabe, M.; Fukushima, T.; Aida, T. Electric double-layer capacitors using “Bucky gels” consisting of an ionic liquid and carbon nanotubes. J. Electrochem. Soc. 2005, 152, A1913. [Google Scholar] [CrossRef]
- Ogoshi, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. Chemically-responsive sol−gel transition of supramolecular single-walled carbon nanotubes (SWNTs) hydrogel made by hybrids of SWNTs and cyclodextrins. J. Am. Chem. Soc. 2007, 129, 4878–4879. [Google Scholar] [CrossRef]
- Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Photochemically controlled supramolecular curdlan/single-walled carbon nanotube composite gel: Preparation of molecular distaff by cyclodextrin modified curdlan and phase transition control. Eur. J. Org. Chem. 2011, 2011, 2801–2806. [Google Scholar] [CrossRef]
- Liu, J.; Mendoza, S.; Román, E.; Lynn, M.J.; Xu, R.; Kaifer, A.E. Cyclodextrin-modified gold nanospheres. Host−guest interactions at work to control colloidal properties. J. Am. Chem. Soc. 1999, 121, 4304–4305. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Hetzer, M.; Ritter, H.; Barner-Kowollik, C. Complex macromolecular architecture design via cyclodextrin host/guest complexes. Prog. Polym. Sci. 2014, 39, 235–249. [Google Scholar] [CrossRef]
- Dong, H.; Li, Y.; Yu, J.; Song, Y.; Cai, X.; Liu, J.; Zhang, J.; Ewing, R.C.; Shi, D. A versatile multicomponent assembly via β-cyclodextrin host–guest chemistry on graphene for biomedical applications. Small 2013, 9, 446–456. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Zhang, H.; Luo, L.; Yao, S. A sensitive and selective sensor-coated molecularly imprinted sol–gel film incorporating β-cyclodextrin-multi-walled carbon nanotubes and cobalt nanoparticles-chitosan for oxacillin determination. Surf. Interface Anal. 2012, 44, 334–341. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Hu, L.; Stoddart, J.F.; Grüner, G. Pyrenecyclodextrin-decorated single-walled carbon nanotube field-effect transistors as chemical sensors. Adv. Mater. 2008, 20, 1910–1915. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.-L.; Zhang, Y.-M.; Guo, D.-S.; Liu, Y.-P. Supramolecular architectures of β-cyclodextrin-modified chitosan and pyrene derivatives mediated by carbon nanotubes and their DNA condensation. J. Am. Chem. Soc. 2008, 130, 10431–10439. [Google Scholar] [CrossRef]
- Shigeta, M.; Komatsu, M.; Nakashima, N. Individual solubilization of single-walled carbon nanotubes using totally aromatic polyimide. Chem. Phys. Lett. 2006, 418, 115–118. [Google Scholar] [CrossRef]
- Shaffer, M.S.P.; Windle, A.H. Analogies between polymer solutions and carbon nanotube dispersions. Macromolecules 1999, 32, 6864–6866. [Google Scholar] [CrossRef]
- Ha, M.; Xia, Y.; Green, A.A.; Zhang, W.; Renn, M.J.; Kim, C.H.; Hersam, M.C.; Frisbie, C.D. Printed, sub-3V digital circuits on plastic from aqueous carbon nanotube inks. ACS Nano 2010, 4, 4388–4395. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Y.; Rui, X.; Qi, D.; Luo, Y.; Leow, W.R.; Chen, S.; Guo, J.; Wei, J.; Li, W.; et al. Conductive inks based on a lithium titanate nanotube gel for high-rate lithium-ion batteries with customized configuration. Adv. Mater. 2016, 28, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Richtering, W.; Saunders, B.R. Gel architectures and their complexity. Soft Matter 2014, 10, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Cao, X.; Wen, M.; Chen, C.; Wen, Q.; Fu, Q.; Deng, H. Highly electrical conductive PEDOT:PSS/SWCNT flexible thermoelectric films fabricated by a high-velocity non-solvent turbulent secondary doping approach. ACS Appl. Mater. Interfaces 2023, 15, 10947–10957. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for flexible and stretchable electronics: Modifications, strategies, and applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef]
- Oya, T.; Ogino, T. Production of electrically conductive paper by adding carbon nanotubes. Carbon 2008, 46, 169–171. [Google Scholar] [CrossRef]
- Yoshida, M.; Oya, T. Development of carbon-nanotube composite thread and its application to “thread transistor”. Adv. Sci. Technol. 2014, 95, 38–43. [Google Scholar]
- Kou, Y.; Oya, T. Unique dye-sensitized solar cell using carbon nanotube composite-papers with gel electrolyte. J. Compos. Sci. 2023, 7, 232. [Google Scholar] [CrossRef]
- Kamekawa, Y.; Arai, K.; Oya, T. Development of transpiration-type thermoelectric power generating material using carbon-nanotube-composite papers with capillary action and heat of vaporization. Energies 2023, 16, 8032. [Google Scholar] [CrossRef]
- Arakaki, R.; Oya, T. Development and evaluation of “thermoelectric power generating thread” using carbon nanotube-coated threads. Jpn. J. Appl. Phys. 2019, 58, SDDD06. [Google Scholar] [CrossRef]
- Ampo, T.; Oya, T. Development of paper actuator based on carbon-nanotube-composite paper. Molecules 2021, 26, 1463. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, N.; Fujigaya, T. Fundamentals and applications of soluble carbon nanotubes. Chem. Lett. 2007, 36, 692–697. [Google Scholar] [CrossRef]
- Rastogi, R.; Kaushal, R.; Tripathi, S.K.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef]
- Hilding, J.; Grulke, E.A.; George Zhang, Z.; Lockwood, F. Dispersion of carbon nanotubes in liquids. J. Dispers. Sci. Technol. 2003, 24, 1–41. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef]
- Gao, C.; Guo, M.; Liu, Y.; Zhang, D.; Gao, F.; Sun, L.; Li, J.; Chen, X.; Terrones, M.; Wang, Y. Surface modification methods and mechanisms in carbon nanotubes dispersion. Carbon 2023, 212, 118133. [Google Scholar] [CrossRef]
- Arakaki, R.; Oya, T. Development of novel hydrogels using single-walled carbon nanotubes and phthalocyanine derivatives. In Proceedings of the 45th International Conference on Micro & Nano Engineering, Rhodes, Greece, 23–26 September 2019. PC91. [Google Scholar]
- Mazaheri, M.; Payandehpeyman, J.; Jamasb, S. Modeling of effective electrical conductivity and percolation behavior in conductive-polymer nanocomposites reinforced with spherical carbon black. Appl. Compos. Mater. 2022, 29, 695–710. [Google Scholar] [CrossRef]
- Kwon, G.; Heo, Y.; Shin, K.; Sung, B.J. Electrical percolation networks of carbon nanotubes in a shear flow. Phys. Rev. E 2012, 85, 011143. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hu, S.; Li, L.-Y.; Jang, S.-H. Percolation threshold and effective properties of CNTs-reinforced two-phase composite materials. Mater. Today Commun. 2021, 29, 102977. [Google Scholar] [CrossRef]
- Mohan, L.; Kumar, P.N.; Karakkad, S.; Krishnan, S.T. Determination of electrical percolation threshold of carbon nanotube-based epoxy nanocomposites and its experimental validation. IET Sci. Meas. Technol. 2019, 13, 1299–1304. [Google Scholar] [CrossRef]
- Wu, D.; Wei, M.; Li, R.; Xiao, T.; Gong, S.; Xiao, A.; Zhu, Z.; Li, Z. A percolation network model to predict the electrical property of flexible CNT/PDMS composite films fabricated by spin coating technique. Compos. Part B Eng. 2019, 174, 107034. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Grimme, S. Do special noncovalent π–π stacking interactions really exist? Angew. Chem. Int. Ed. 2008, 47, 3430–3434. [Google Scholar] [CrossRef]
- Pérez, E.M.; Martín, N. π–π interactions in carbon nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. [Google Scholar] [CrossRef]
- Nakashima, N.; Okuzono, S.; Tomonari, Y.; Murakami, H. Solubilization of carbon nanotubes with a pyrene-carrying polymer in water. Trans. Mater. Res. Soc. Jpn. 2004, 29, 525–528. [Google Scholar]
- Lucas, A.A.; Bruyninckx, V.; Lambin, P.; Bernaerts, D.; Amelinckx, S.; Van Landuyt, J.; Van Tendeloo, G. Electron diffraction by carbon nanotubes. Scanning Microsc. 1998, 12, 415–436. [Google Scholar]
- Jensen, J.H.; Kromann, J.C. The molecule calculator: A web application for fast quantum mechanics-based estimation of molecular properties. J. Chem. Educ. 2013, 90, 1093–1095. [Google Scholar] [CrossRef]

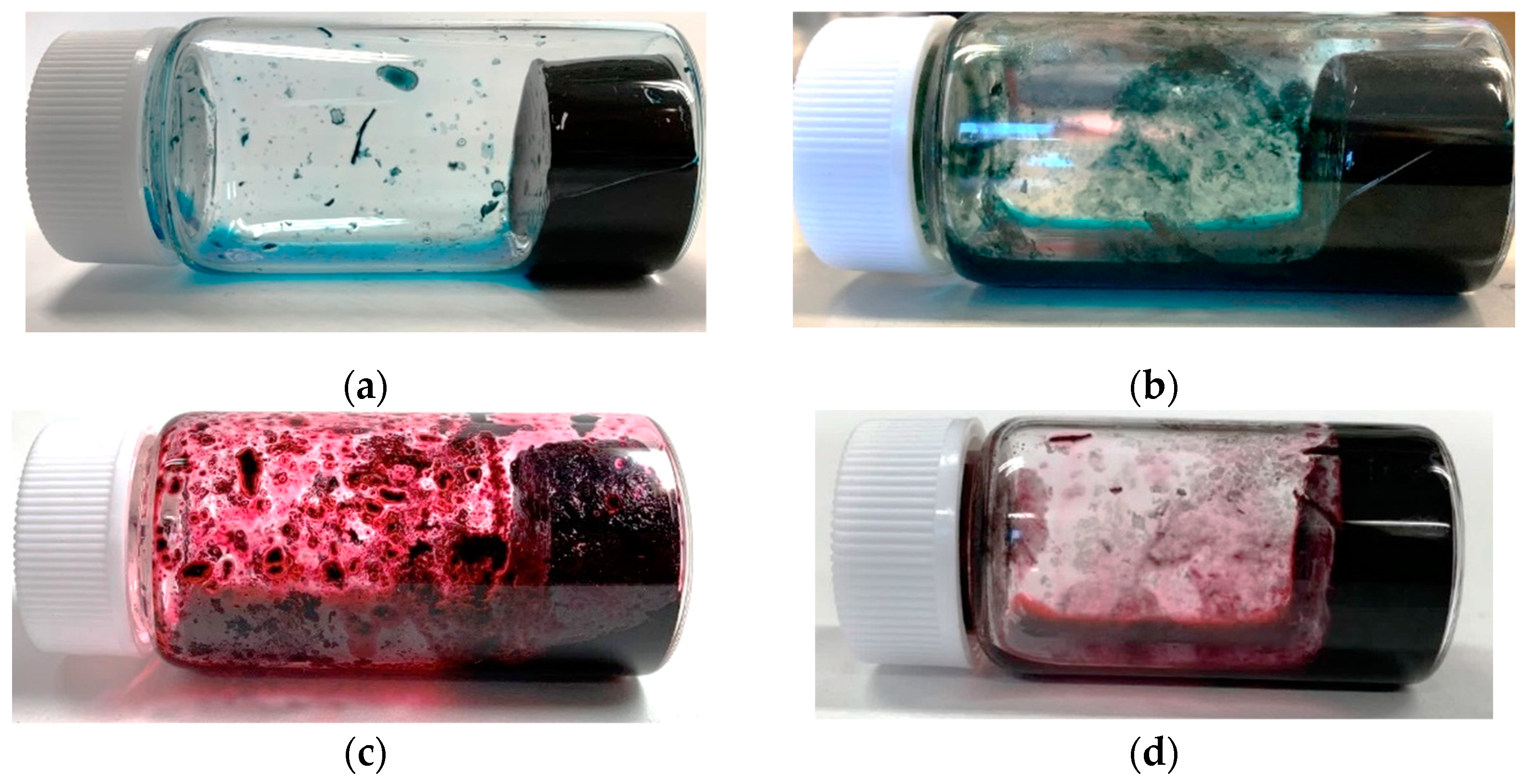
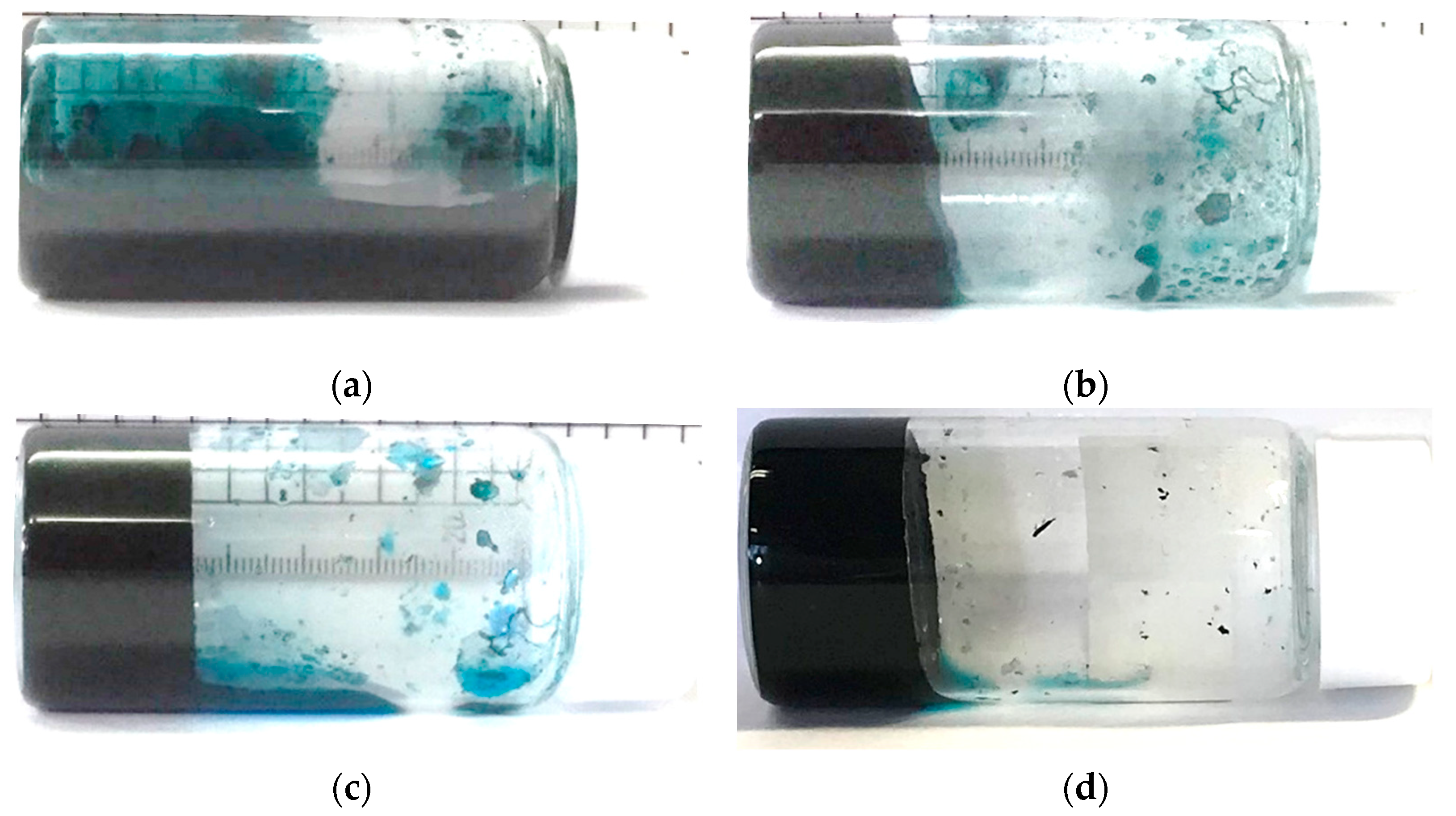


| Name | SG65i | HiPco | CG200 | CG300 | SG101 | NC7000 |
|---|---|---|---|---|---|---|
| C.I. Reactive Blue 21 | ✓ | × | × | ✓ | × | × |
| 5,10,15,20-Tetrakis (4-aminophenyl)porphyrin | × | × | × | × | × | × |
| 5,10,15,20-Tetrakis(4-carboxymethyloxyphenyl)porphyrin | × | × | × | × | × | × |
| Cyanocobalamin (Vitamin B12) | ✓ | × | × | ✓ | × | × |
| SDS | × | × | × | × | × | × |
| Compressive Breaking Stress [kPa] | |
|---|---|
| 0 min heating | — |
| 20 min heating | 0.3 |
| 60 min heating | 2.6 |
| 180 min cooling after 60 min heating | 2.6 |
| CNT (wt%) | C.I. Reactive Blue 21 (wt%) | Gelation |
|---|---|---|
| 0.033 | 0.13 | × |
| 0.067 | 0.27 | × |
| 0.10 | 0.40 | × |
| 0.13 | 0.53 | ✓ |
| 0.17 | 0.67 | ✓ |
| 0.27 | 1.1 | ✓ |
| Gelation | |
|---|---|
| 1 | × |
| 3 | ✓ (at room temperature) |
| 4.5 | ✓ |
| 6 | ✓ |
| 9 | ✓ |
| 12 | ✓ |
| Name | Type | Diameter * | Supplier |
|---|---|---|---|
| SG65i | Single-walled | around 0.78 nm | CHASM, Boston, MA, USA |
| HiPco | Single-walled | 0.8–1.2 nm | NanoIntegris Inc., Boisbriand, QC, Canada |
| CG200 | Single-walled | around 1.3 nm | CHASM, Boston, MA, USA |
| CG300 | Single-walled | around 0.84 nm | CHASM, Boston, MA, USA |
| SG101 | Single-walled | 2–3 nm | ZEON CORPORATION, Tokyo, Japan |
| NC7000 | Multi-walled | 9.5 nm | Nanocyl SA, Sambreville, Belgium |
| Name | Structural Formulas | Core Diameter * | Supplier |
|---|---|---|---|
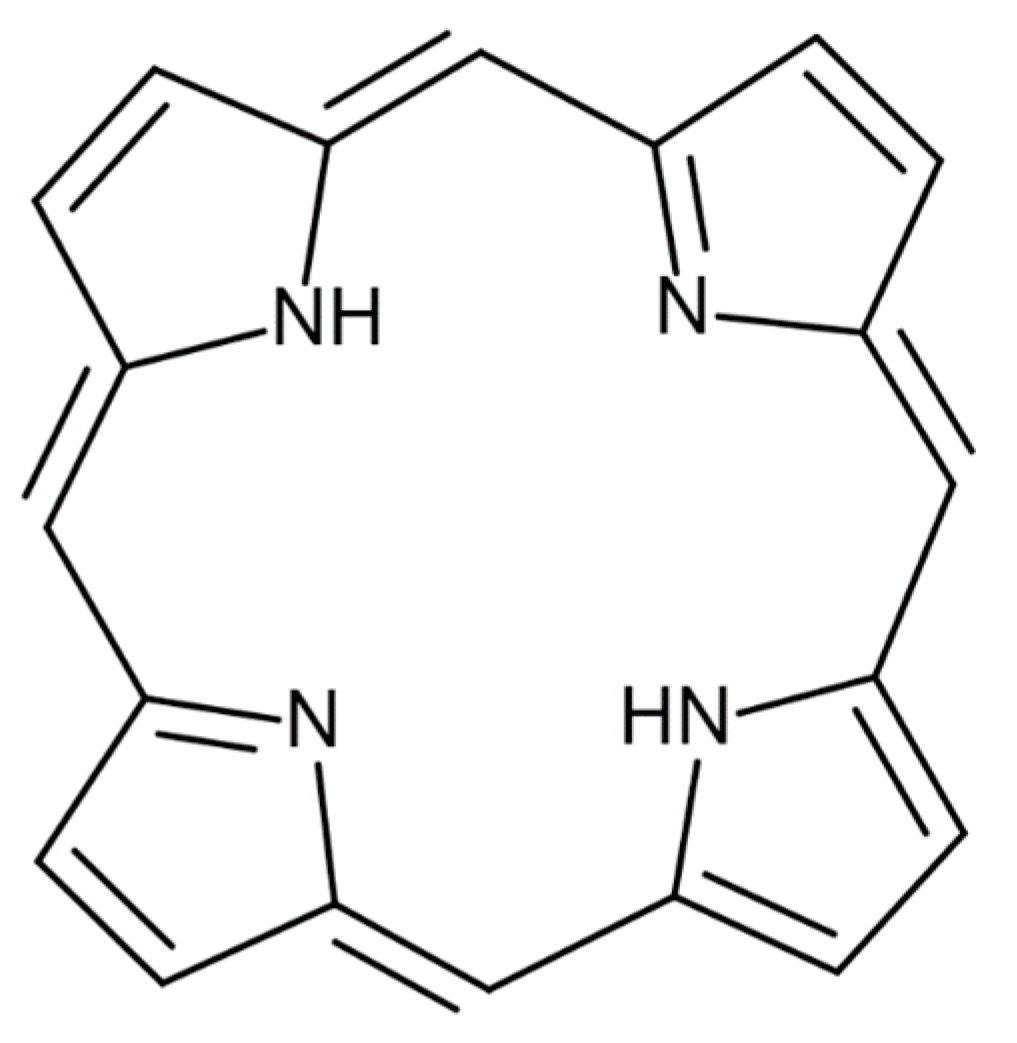
| C.I. Reactive Blue 21 | Figure 10a | about 1.5 nm | Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| 5,10,15,20-Tetrakis (4-aminophenyl)porphyrin | Figure 10b | about 1.9 nm | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 5,10,15,20-Tetrakis(4-carboxymethyloxyphenyl)porphyrin | Figure 10c | about 2.4 nm | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| Cyanocobalamin (Vitamin B12) | Figure 10d | about 1.5 nm | Sigma-Aldrich/Merck, Darmstadt, Germany |
| SDS | — | about 1.8 nm (Length) | Nacalai Tesque Inc., Kyoto, Japan |
| CNT (wt%) | C.I. Reactive Blue 21 (wt%) |
|---|---|
| 0.033 | 0.13 |
| 0.067 | 0.27 |
| 0.10 | 0.40 |
| 0.13 | 0.53 |
| 0.17 | 0.67 |
| 0.27 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, R.; Arakaki, R.; Oya, T. Development and Geometrical Considerations of Unique Conductive and Reversible Carbon-Nanotube Hydrogel without Need for Gelators. Gels 2024, 10, 457. https://doi.org/10.3390/gels10070457
Ogawa R, Arakaki R, Oya T. Development and Geometrical Considerations of Unique Conductive and Reversible Carbon-Nanotube Hydrogel without Need for Gelators. Gels. 2024; 10(7):457. https://doi.org/10.3390/gels10070457
Chicago/Turabian StyleOgawa, Ryo, Ryota Arakaki, and Takahide Oya. 2024. "Development and Geometrical Considerations of Unique Conductive and Reversible Carbon-Nanotube Hydrogel without Need for Gelators" Gels 10, no. 7: 457. https://doi.org/10.3390/gels10070457








