Multi-Layer PVA-PANI Conductive Hydrogel for Symmetrical Supercapacitors: Preparation and Characterization
Abstract
:1. Introduction
2. Results and Discussions
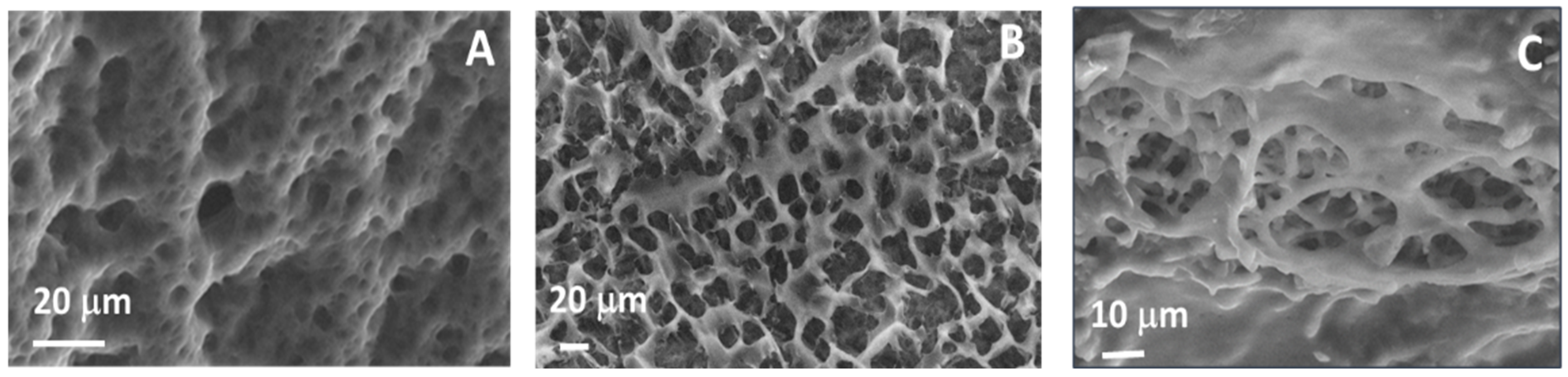
2.1. Hydrogels Characterizations
2.2. Electrochemical Behavior
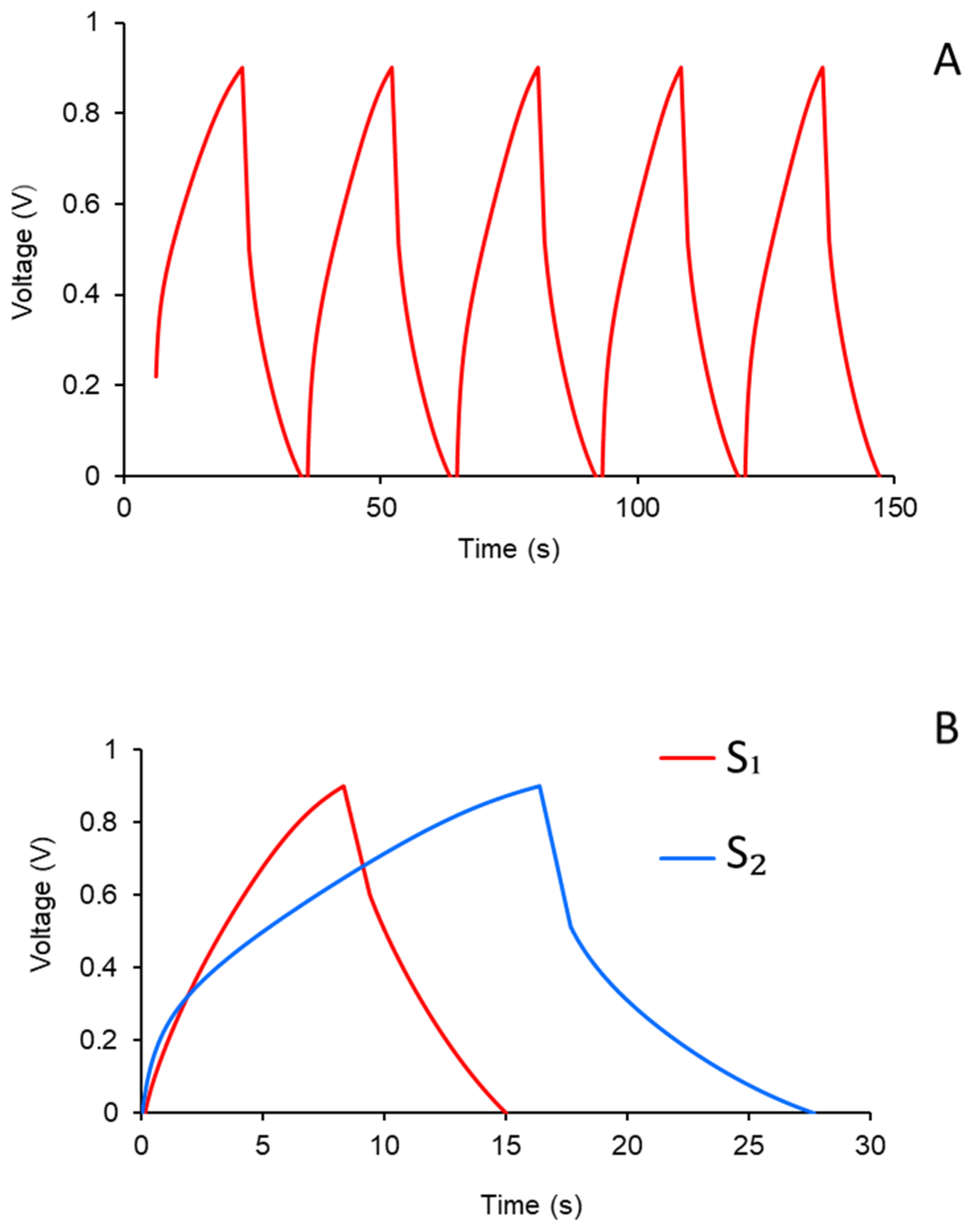
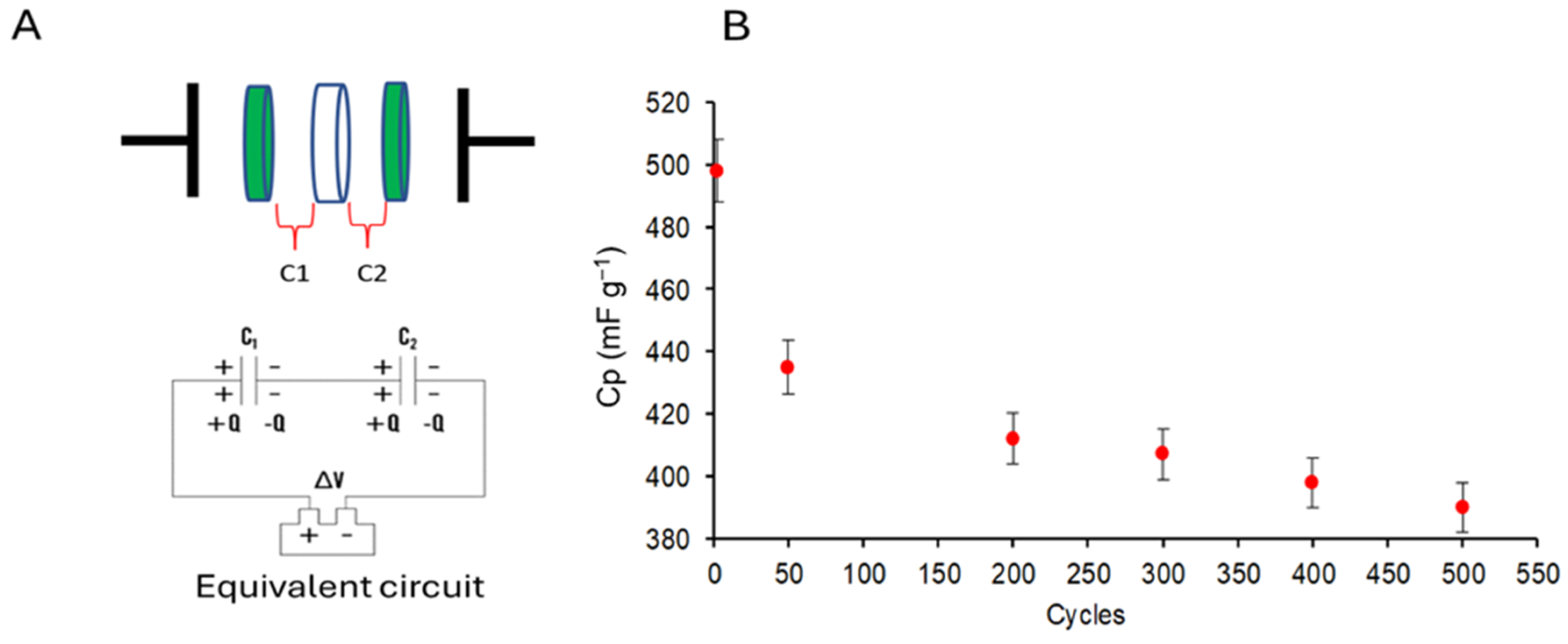
2.2.1. Semi-Cell and Symmetric Cell
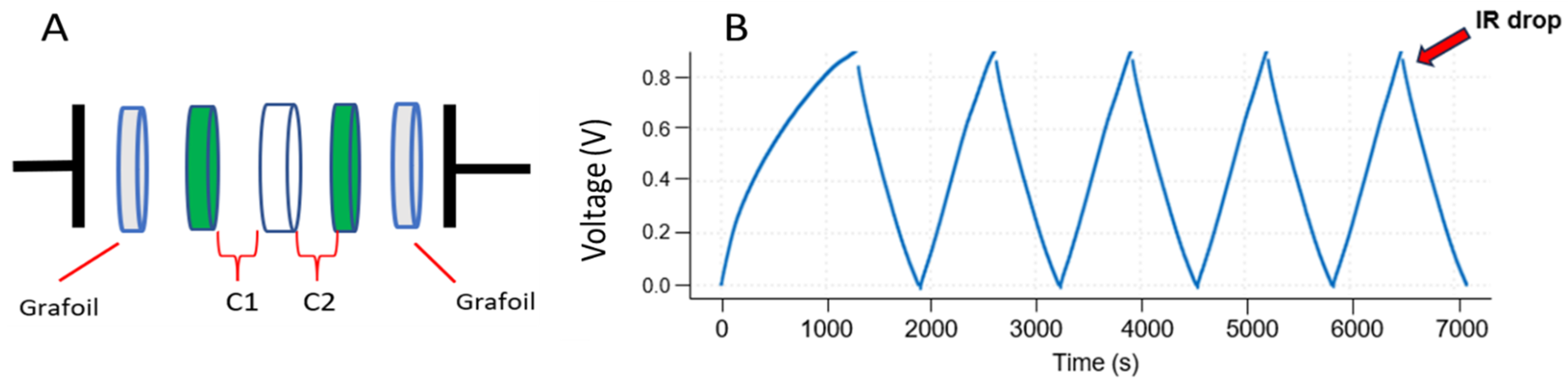
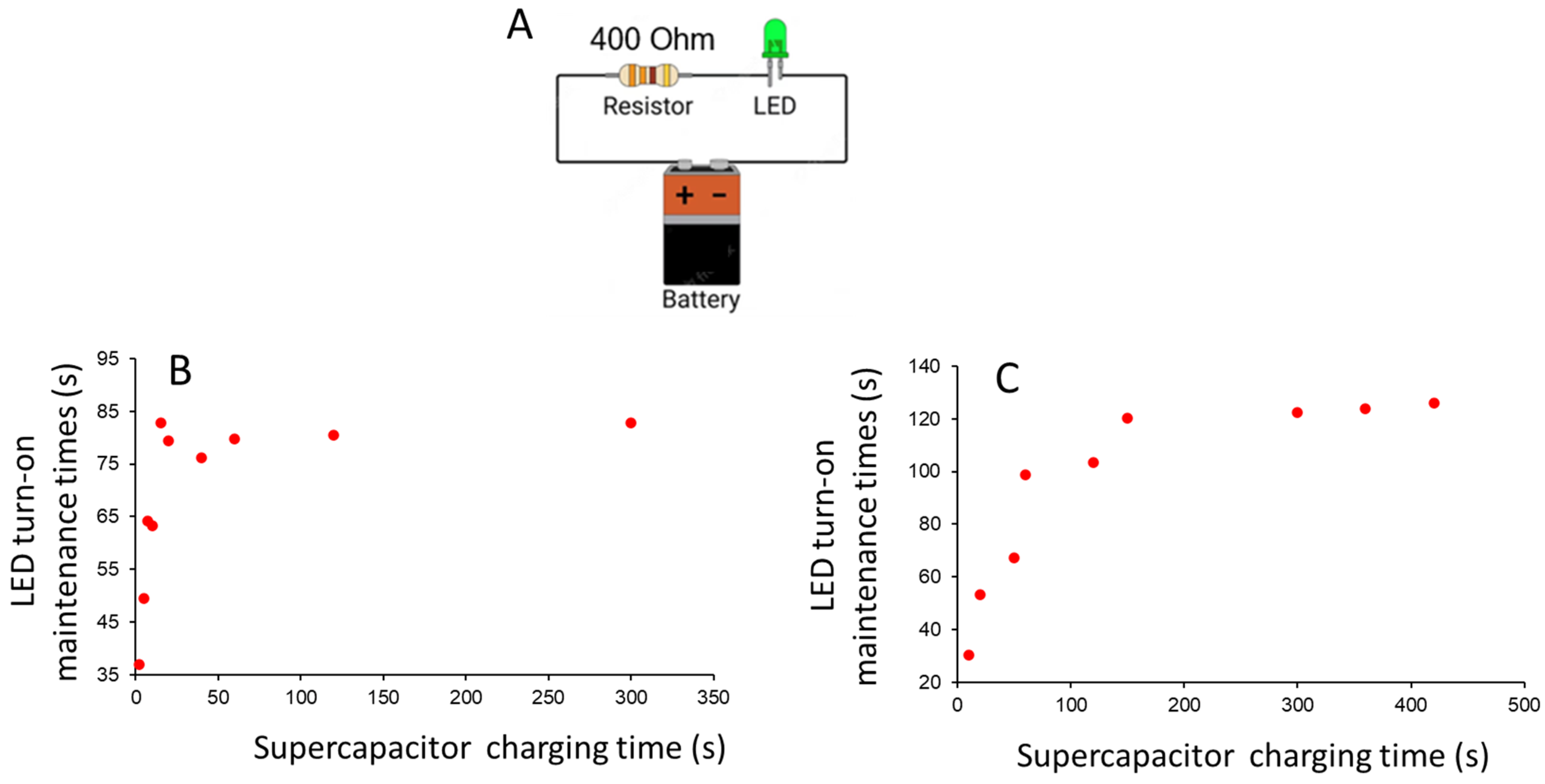
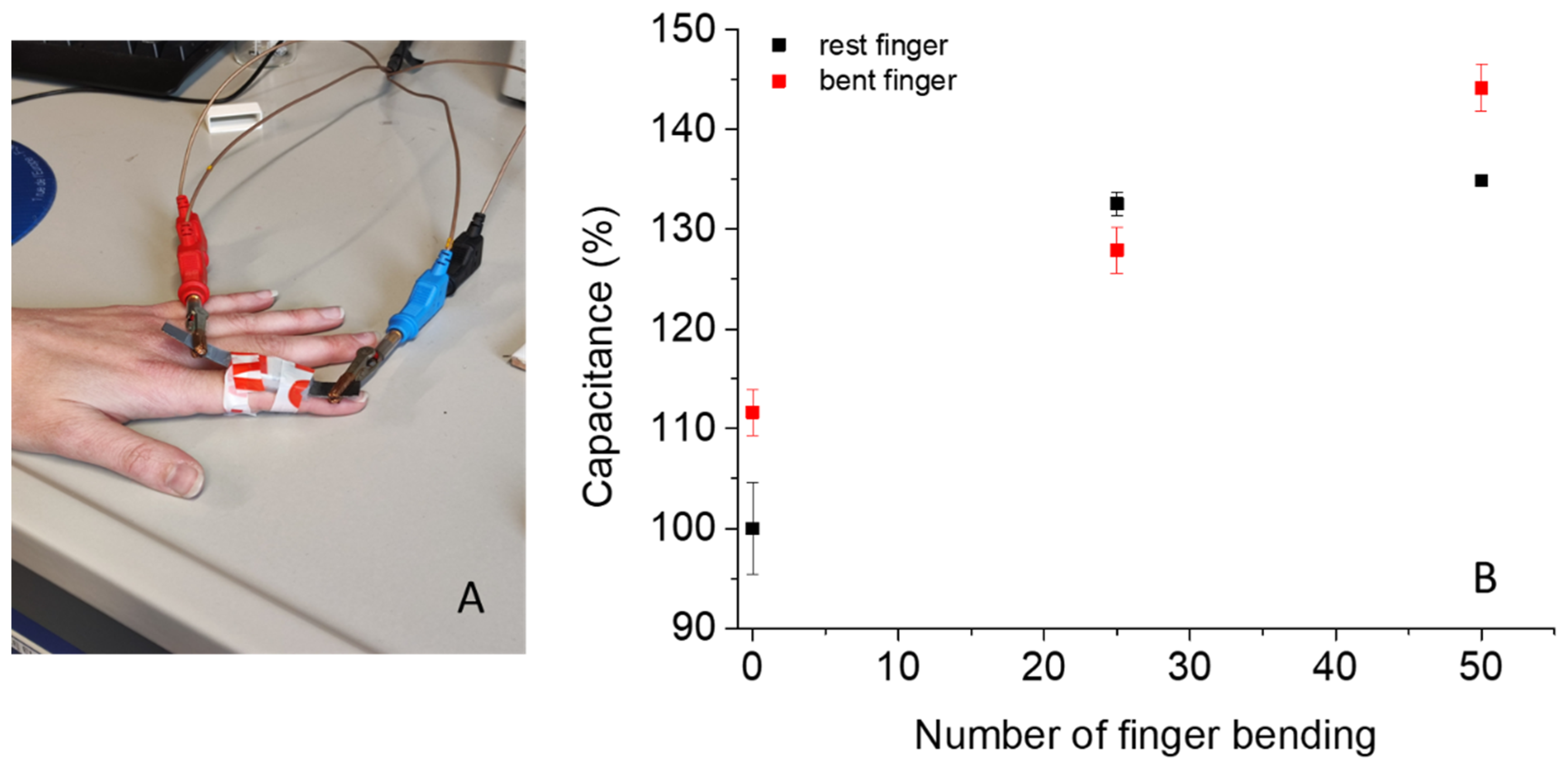
2.2.2. Flexible Supercapacitor Unit Assembly and Application
3. Conclusions
4. Materials and Methods
4.1. Materials and Characterizations
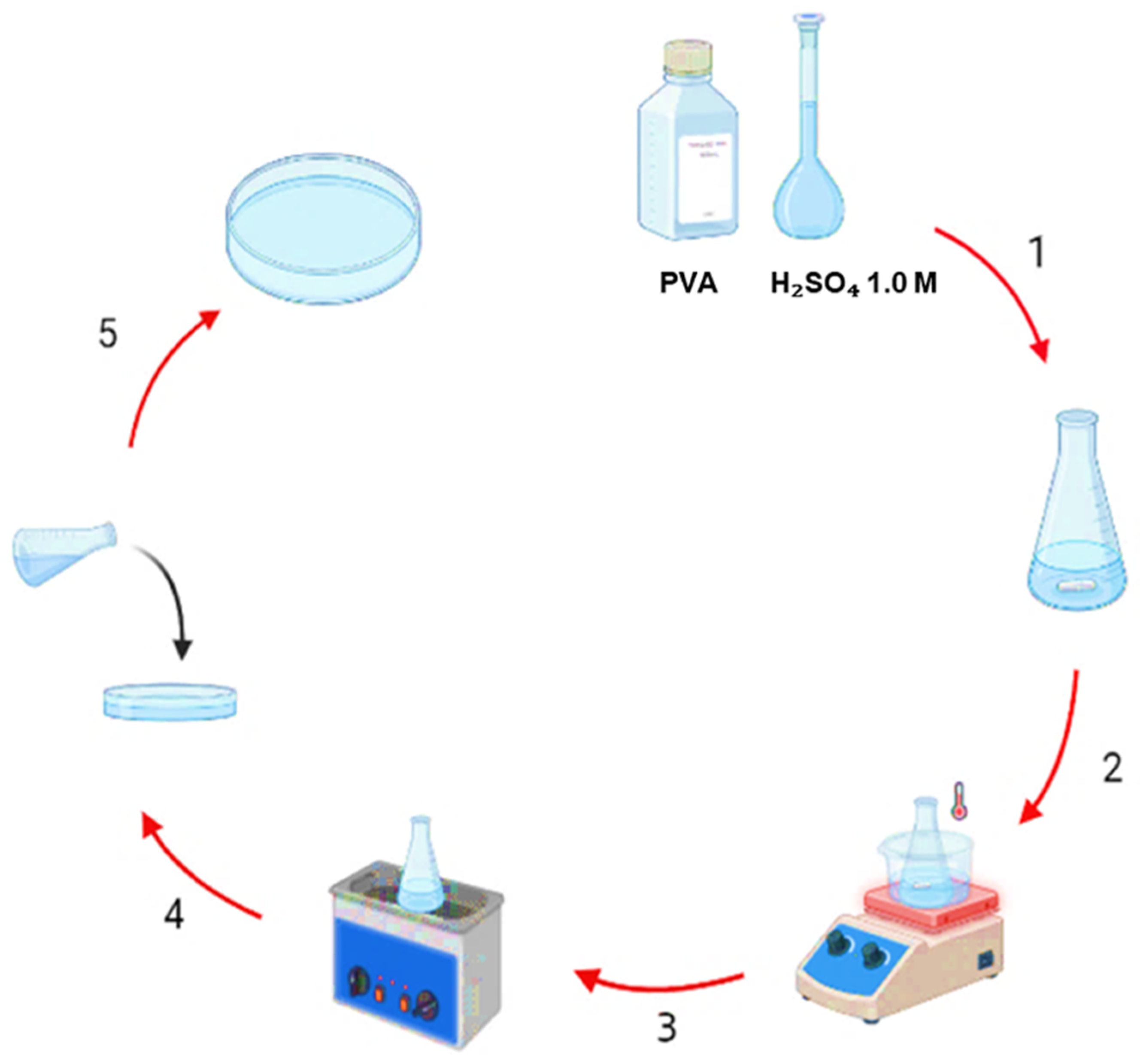
4.2. Preparation of PVA-H2SO4 Hydrogel
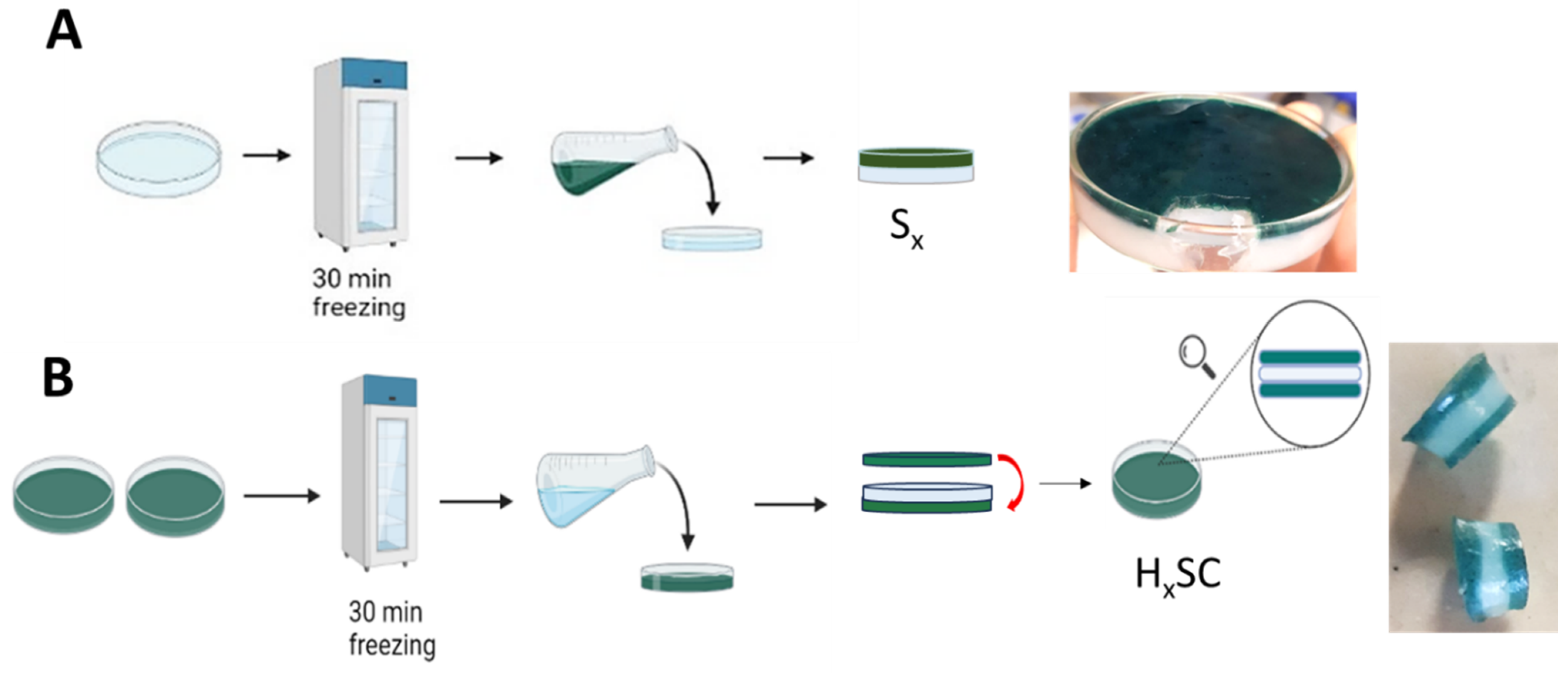
4.3. Preparation of PVA-H2SO4-PANI_PAMPSA Hydrogel, Semi-Cell, and Integrated Hydrogel Supercapacitors
4.4. Material Characterizations
4.5. Electrochemical Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Q.; Dong, B.; He, T.; Sun, Z.; Zhu, J.; Zhang, Z.; Lee, C. Progress in Wearable Electronics/Photonics—Moving toward the Era of Artificial Intelligence and Internet of Things. InfoMat 2020, 2, 1131–1162. [Google Scholar] [CrossRef]
- Khan, R.; Ur Rehman, N.; Ilyas, N.; Sfina, N.; Barhoumi, M.; Khan, A.; Althubeiti, K.; Al Otaibi, S.; Iqbal, S.; Rahman, N.; et al. Threshold switching in nickel-doped zinc oxide based memristor for artificial sensory applications. Nanoscale 2023, 15, 1900. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.A.; Arnold, L.; Rasheed, A.; Khan, I.A.; Wang, L. Textronics—A Review of Textile-Based Wearable Electronics. Adv. Eng. Mater. 2021, 23, 2100469. [Google Scholar] [CrossRef]
- Lim, H.R.; Kim, H.S.; Qazi, R.; Kwon, Y.T.; Jeong, J.W.; Yeo, W.H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing Flexible, Smart and Self-Sustainable Supercapacitors for Portable/Wearable Electronics: From Conductive Polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Sun, Q.; Wang, Z.L. Self-Powered Sensing in Wearable Electronics—A Paradigm Shift Technology. Chem. Rev. 2023, 123, 12105–12134. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Sahlan, S.; Osman, K.; Mohamed Ali, M.S. Energy Harvesters for Wearable Electronics and Biomedical Devices. Adv. Mater. Technol. 2021, 6, 2000771. [Google Scholar] [CrossRef]
- Liu, Z.; Mo, F.; Li, H.; Zhu, M.; Wang, Z.; Liang, G.; Zhi, C. Advances in Flexible and Wearable Energy-Storage Textiles. Small Methods 2018, 2, 1800124. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Jia, H.; Ng, P.F.; Chow, L.; Fei, B. Recent Advances of Hydrogel Electrolytes in Flexible Energy Storage Devices. J. Mater. Chem. A Mater. 2021, 9, 2043–2069. [Google Scholar] [CrossRef]
- Keum, K.; Kim, J.W.; Hong, S.Y.; Son, J.G.; Lee, S.S.; Ha, J.S. Flexible/Stretchable Supercapacitors with Novel Functionality for Wearable Electronics. Adv. Mater. 2020, 32, 2002180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A Review of Supercapacitor Modeling, Estimation, and Applications: A Control/Management Perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- Govindarajan, D.; Chinnakutti, K.K. Chapter 3—Fundamentals, Basic Components and Performance Evaluation of Energy Storage and Conversion Devices. In Oxide Free Nanomaterials for Energy Storage and Conversion Applications; Arunachalam, P., Theerthagiri, J., Al-Mayouf, A.M., Choi, M.Y., Jagannathan, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 51–74. ISBN 978-0-12-823936-0. [Google Scholar]
- Wu, Z.; Li, L.; Yan, J.M.; Zhang, X.B. Materials Design and System Construction for Conventional and New-Concept Supercapacitors. Adv. Sci. 2017, 4, 1600382. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A Review of Electrolyte Materials and Compositions for Electrochemical Supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in Sensing Applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jiang, C.; Sun, N.; Tan, D.; Li, Q.; Bi, S.; Song, J. Recent Progress in Multifunctional Hydrogel-Based Supercapacitors. J. Sci. Adv. Mater. Devices 2021, 6, 338–350. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, e50376. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A Review of Polyvinyl Alcohol and Its Uses in Cartilage and Orthopedic Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl Alcohol: A Review of Research Status and Use of Polyvinyl Alcohol Based Nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked Poly(Vinyl Alcohol) Membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Pang, L.; Shen, Y.; Hu, H.; Zeng, X.; Huang, W.; Gao, H.; Wang, H.; Wang, D. Chemically and Physically Cross-Linked Polyvinyl Alcohol-Borosilicate Gel Hybrid Scaffolds for Bone Regeneration. Mater. Sci. Eng. C 2019, 105, 110076. [Google Scholar] [CrossRef] [PubMed]
- Guiseppi-Elie, A. Electroconductive Hydrogels: Synthesis, Characterization and Biomedical Applications. Biomaterials 2010, 31, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Bian, J.; Peng, F.; Zhang, X.M.; Sun, R.C. High Strength of Hemicelluloses Based Hydrogels by Freeze/Thaw Technique. Carbohydr. Polym. 2014, 101, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q.; Ma, Y.; Wei, Z. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef] [PubMed]
- Prunet, G.; Pawula, F.; Fleury, G.; Cloutet, E.; Robinson, A.J.; Hadziioannou, G.; Pakdel, A. A Review on Conductive Polymers and Their Hybrids for Flexible and Wearable Thermoelectric Applications. Mater. Today Phys. 2021, 18, 100402. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/Thawed Polyvinyl Alcohol Hydrogels: Present, Past and Future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Ma, W.B.; Zhu, K.H.; Ye, S.F.; Wang, Y.; Guo, L.; Tao, X.Y.; Guo, L.T.; Fan, H.L.; Liu, Z.S.; Zhu, Y.B.; et al. A Self-Healing Hydrogel Electrolyte towards All-in-One Flexible Supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 20445–20460. [Google Scholar] [CrossRef]
- Peng, H.; Lv, Y.; Wei, G.; Zhou, J.; Gao, X.; Sun, K.; Ma, G.; Lei, Z. A Flexible and Self-Healing Hydrogel Electrolyte for Smart Supercapacitor. J. Power Sources 2019, 431, 210–219. [Google Scholar] [CrossRef]
- D’Altri, G.; Yeasmin, L.; Di Matteo, V.; Scurti, S.; Giovagnoli, A.; Di Filippo, M.F.; Gualandi, I.; Cassani, M.C.; Caretti, D.; Panzavolta, S.; et al. Preparation and Characterization of Self-Healing PVA-H2SO4 Hydrogel for Flexible Energy Storage. ACS Omega 2023, 9, 6391. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal Oxide-Based Supercapacitors: Progress and Prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, Properties, and Application. Polym. Sci.-Ser. C 2014, 56, 144–153. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoha, K.G.; Tanh, K.L. Polyaniline: A Polymer with many interesting intrinsic redox state. Prog. Polym. Sci. 1998, 23, 211–324. [Google Scholar] [CrossRef]
- Saraswat, A.; Kumar, S. A Topical Study of Electrochemical Response of Functionalized Conducting Polyaniline: An Overview. Eur. Polym. J. 2023, 182, 111714. [Google Scholar] [CrossRef]
- Somani, P.R.; Radhakrishnan, S. Electrochromic Materials and Devices: Present and Future. Mater. Chem. Phys. 2002, 77, 117–133. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic Organic and Polymeric Materials for Display Applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef]
- Huerta, F.; Quijada, C.; Montilla, F.; Morallón, E. Revisiting the Redox Transitions of Polyaniline. Semiquantitative Interpretation of Electrochemically Induced IR Bands. J. Electroanal. Chem. 2021, 897, 115593. [Google Scholar] [CrossRef]
- Kaempgen, M.; Roth, S. Transparent and Flexible Carbon Nanotube/Polyaniline PH Sensors. J. Electroanal. Chem. 2006, 586, 72–76. [Google Scholar] [CrossRef]
- Li, Y.; Mao, Y.; Xiao, C.; Xu, X.; Li, X. Flexible PH Sensor Based on a Conductive PANI Membrane for PH Monitoring. RSC Adv. 2019, 10, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ragazzini, I.; Gualandi, I.; D’Altri, G.; Di Matteo, V.; Yeasmin, L.; Cassani, M.C.; Scavetta, E.; Bernardi, E.; Ballarin, B. Polyaniline/Poly (2-Acrylamido-2-Methyl-1-Propanesulfonic Acid) Modified Cellulose as Promising Material for Sensors Design. Carbohydr. Polym. 2023, 316, 121079. [Google Scholar] [CrossRef]
- Yang, D.; Wang, J.; Cao, Y.; Tong, X.; Hua, T.; Qin, R.; Shao, Y. Polyaniline-Based Biological and Chemical Sensors: Sensing Mechanism, Configuration Design, and Perspective. ACS Appl. Electron. Mater. 2023, 5, 593–611. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) Based Electrode Materials for Energy Storage and Conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Guo, H.; He, W.; Lu, Y.; Zhang, X. Self-Crosslinked Polyaniline Hydrogel Electrodes for Electrochemical Energy Storage. Carbon 2015, 92, 133–141. [Google Scholar] [CrossRef]
- Li, W.; Lu, H.; Zhang, N.; Ma, M. Enhancing the Properties of Conductive Polymer Hydrogels by Freeze-Thaw Cycles for High-Performance Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 20142–20149. [Google Scholar] [CrossRef] [PubMed]
- Alipoori, S.; Torkzadeh, M.M.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Performance-Tuning of PVA-Based Gel Electrolytes by Acid/PVA Ratio and PVA Molecular Weight. SN Appl. Sci. 2021, 3, 310. [Google Scholar] [CrossRef]
- Patil, D.S.; Shaikh, J.S.; Dalavi, D.S.; Kalagi, S.S.; Patil, P.S. Chemical Synthesis of Highly Stable PVA/PANI Films for Supercapacitor Application. Mater. Chem. Phys. 2011, 128, 449–455. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O. Crosslinked PVA/SSA Proton Exchange Membranes: Correlation between Physiochemical Properties and Free Volume Determined by Positron Annihilation Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Carrillo, A.; Mota, M.L.; Ambrosio, R.C.; Aguirre, F.S. Purification and Glutaraldehyde Activation Study on HCl-Doped PVA-PANI Copolymers with Different Aniline Concentrations. Molecules 2019, 24, 63. [Google Scholar] [CrossRef] [PubMed]
- Abureesh, M.A.; Oladipo, A.A.; Gazi, M. Facile Synthesis of Glucose-Sensitive Chitosan–Poly(Vinyl Alcohol) Hydrogel: Drug Release Optimization and Swelling Properties. Int. J. Biol. Macromol. 2016, 90, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Scurti, S.; Ortolani, J.; Ghirri, A.; Maccaferri, E.; Caretti, D.; Mazzocchetti, L. Phosphorylated Poly(Vinyl Alcohol) Surface Coatings as Intumescent Flame Inhibitor for Polymer Matrix Composites. Prog. Org. Coat. 2023, 177, 107457. [Google Scholar] [CrossRef]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of Molecular Weight and Molecular Weight Distribution on Mechanical Properties of Polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- Shin, M.; Shin, S.H.; Lee, M.; Kim, H.J.; Jeong, J.H.; Choi, Y.H.; Oh, D.X.; Park, J.; Jeon, H.; Eom, Y. Rheological Criteria for Distinguishing Self-Healing and Non-Self-Healing Hydrogels. Polymer 2021, 229, 123969. [Google Scholar] [CrossRef]
- Ghenaatian, H.R.; Mousavi, M.F.; Kazemi, S.H.; Shamsipur, M. Electrochemical Investigations of Self-Doped Polyaniline Nanofibers as a New Electroactive Material for High Performance Redox Supercapacitor. Synth. Met. 2009, 159, 1717–1722. [Google Scholar] [CrossRef]
- Chen, W.C.; Wen, T.C. Electrochemical and Capacitive Properties of Polyaniline-Implanted Porous Carbon Electrode for Supercapacitors. J. Power Sources 2003, 117, 273–282. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Fan, S. Flexible Carbon Nanotube/Polyaniline Paper-like Films and Their Enhanced Electrochemical Properties. Electrochem. Commun 2009, 11, 186–189. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, L.B.; Wang, B.; Luo, Y.C.; Kang, L. In-Situ Electrochemical Polymerization of Multi-Walled Carbon Nanotube/Polyaniline Composite Films for Electrochemical Supercapacitors. Synth. Met. 2009, 159, 260–266. [Google Scholar] [CrossRef]
- Dou, P.; Liu, Z.; Cao, Z.; Zheng, J.; Wang, C.; Xu, X. Rapid Synthesis of Hierarchical Nanostructured Polyaniline Hydrogel for High Power Density Energy Storage Application and Three-Dimensional Multilayers Printing. J. Mater. Sci. 2016, 51, 4274–4282. [Google Scholar] [CrossRef]
- Qin, G.; Wang, M.; Fan, L.; Fang, X.; Zhang, D.; Liu, J.; Qin, J.; Shi, J.; Yang, J.; Chen, Q. Multifunctional Supramolecular Gel Polymer Electrolyte for Self-Healable and Cold-Resistant Supercapacitor. J. Power Sources 2020, 474, 1717–1722. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater Chem 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Tanpichai, S.; Oksman, K. Cross-linked nanocomposite hydrogels based on cellulose nanocrystals and PVA: Mechanical properties and creep recovery. Compos. Part A Appl. Sci. Manuf. 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Mecca, T.; Ussia, M.; Caretti, D.; Cunsolo, F.; Dattilo, S.; Scurti, S.; Privitera, V.; Carroccio, S.C. N-methyl-D-glucamine based cryogels as reusable sponges to enhance heavy metals removal from water. Chem. Eng. J. 2020, 399, 125753. [Google Scholar] [CrossRef]

| Semi-Cell | Young’s Modulus (MPa) | Strain (%) |
|---|---|---|
| S1 | 0.18 ± 0.03 | 53 ± 4 |
| S2 | 0.05 ± 0.02 | 170 ± 1 |
| Sample | Wc% | Sw% | SPVA% |
|---|---|---|---|
| Hy1 | 71 ± 2 | 245 ± 1 | 10 |
| Hy2 | 77 ± 1 | 345 ± 9 | 38 |
| Hy1SC | 65 ± 2 | 191 ± 23 | 8 |
| Hy2SC | 72 ± 2 | 264 ± 20 | 29 |
| Semi-Cell | PANI_PAMPSA Amount (g) | Specific Conductance κ (mS/cm) | R (Ohm) | Ionic Conductivity σc (S/cm) | Specific Capacitance Cp (mFg−1) |
|---|---|---|---|---|---|
| S1 | 0.065 | 13.9 ± 0.4 | 4.41 ±0.04 | 0.130 ±0.001 | 242 ± 3 |
| S2 | 0.023 | 5.5 ± 0.9 | 1.25 ± 0.01 | 0.408 ±0.002 | 398.0 ± 0.9 |
| Symmetric Cell | Applied Current Density (mA) | Cp |
|---|---|---|
| Swagelok type cell | ||
| Hy1SC | 0.025 | 94 ± 1 (mFg−1) |
| Hy2SC | 0.025 | 466 ± 2 (mFg−1) |
| Swagelok type cell with Grafoil | ||
| Hy1SC | 0.025 | 13 ± 3 (Fg−1) |
| Hy2SC | 0.025 | 73 ± 1 (Fg−1) |
| Hy2SC | 0.1 | 65 ± 1 (Fg−1) |
| Hy2SC | 0.3 | 48 ± 2 (Fg−1) |
| Symmetric Cell | Capacitance Value | Retain of Specific Capacitance/Number of Cycles | Device | Electrolyte | Ref |
|---|---|---|---|---|---|
| Hy1SC | 26 ± 3 (Fg−1) | Flexible SC | 1 M H2SO4 | This work | |
| Hy2SC | 156 ± 38 (Fg−1) | 78%/500 | Flexible SC | 1 M H2SO4 | This work |
| PANI | 150–606 (Fg−1) | 55%/1000 | Traditional SC | 10−3 M HCl a | [57] |
| PANI + Carbon | 144–160 (Fg−1) | 90%/1000 | Traditional SC | 1 M H2SO4 a | [58] |
| BP/PANI | 347–424 (Fg−1) | 70%/1000 | Flexible electrode | 1 M H2SO4 a | [59] |
| PANI + MWNT | 300–430 (Fg−1) | 68%/1000 | Traditional SC | 0.5 M H2SO4 a | [60] |
| PVA/PANI | 571 (Fg−1) | 75%/2000 | Traditional SC | 1 M H2SO4 a | [50] |
| PVA/PANI | 420 (Fg−1) | 93%/2000 | Flexible hydrogel electrode | ATMP | [61] |
| PVA/TA/PANI/Carbon cloth | 102.7 (Fg−1) | 94.8%/1000 | Flexible SC | 2.8 M H3PO4 | [62] |
| Semi-Cell | Integrated Hydrogel Supercapacitor | PVA/H2SO4 (w/w) | PVA (%) | PVA MW |
|---|---|---|---|---|
| S1 Hy1/Hy1-PANI_PAMPSA | Hy1SC Hy1/Hy1-PANI_PAMPSA/Hy1 | 1/4 | 25 | 31,000–50,000 |
| S2 Hy2/Hy2-PANI_PAMPSA | Hy2SC Hy2/Hy2-PANI_PAMPSA/Hy2 | 1/11 | 9 | 89,000–98,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovagnoli, A.; D’Altri, G.; Yeasmin, L.; Di Matteo, V.; Scurti, S.; Di Filippo, M.F.; Gualandi, I.; Cassani, M.C.; Caretti, D.; Panzavolta, S.; et al. Multi-Layer PVA-PANI Conductive Hydrogel for Symmetrical Supercapacitors: Preparation and Characterization. Gels 2024, 10, 458. https://doi.org/10.3390/gels10070458
Giovagnoli A, D’Altri G, Yeasmin L, Di Matteo V, Scurti S, Di Filippo MF, Gualandi I, Cassani MC, Caretti D, Panzavolta S, et al. Multi-Layer PVA-PANI Conductive Hydrogel for Symmetrical Supercapacitors: Preparation and Characterization. Gels. 2024; 10(7):458. https://doi.org/10.3390/gels10070458
Chicago/Turabian StyleGiovagnoli, Angelica, Giada D’Altri, Lamyea Yeasmin, Valentina Di Matteo, Stefano Scurti, Maria Francesca Di Filippo, Isacco Gualandi, Maria Cristina Cassani, Daniele Caretti, Silvia Panzavolta, and et al. 2024. "Multi-Layer PVA-PANI Conductive Hydrogel for Symmetrical Supercapacitors: Preparation and Characterization" Gels 10, no. 7: 458. https://doi.org/10.3390/gels10070458








