Microfluidic Rheology: An Innovative Method for Viscosity Measurement of Gels and Various Pharmaceuticals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Time and Sample Quantity
2.2. Parameters of the Applicators, Force Required for Extrusions
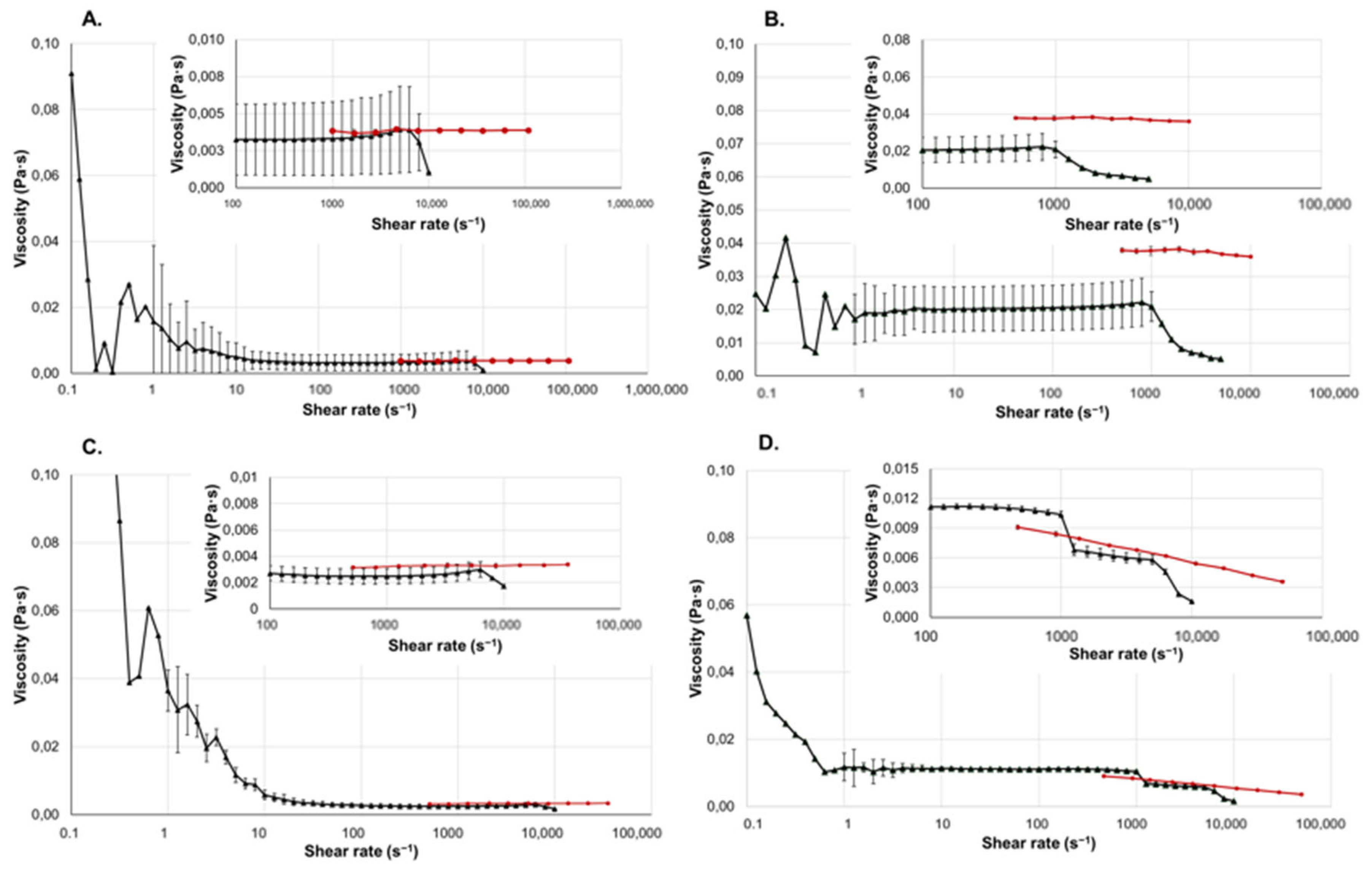
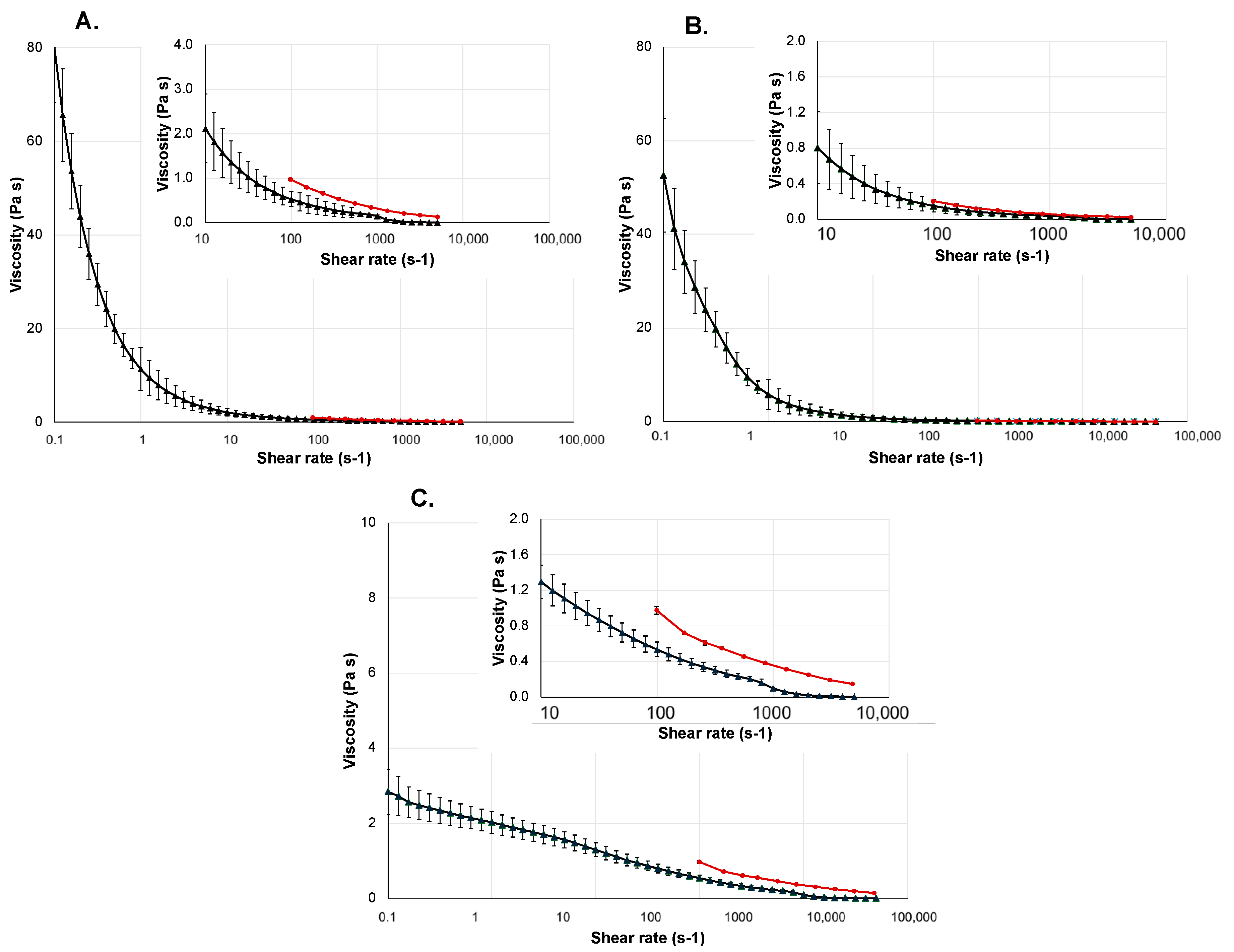
2.3. Liquid Dosage Forms
2.4. Gel-Based Dosage Forms
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
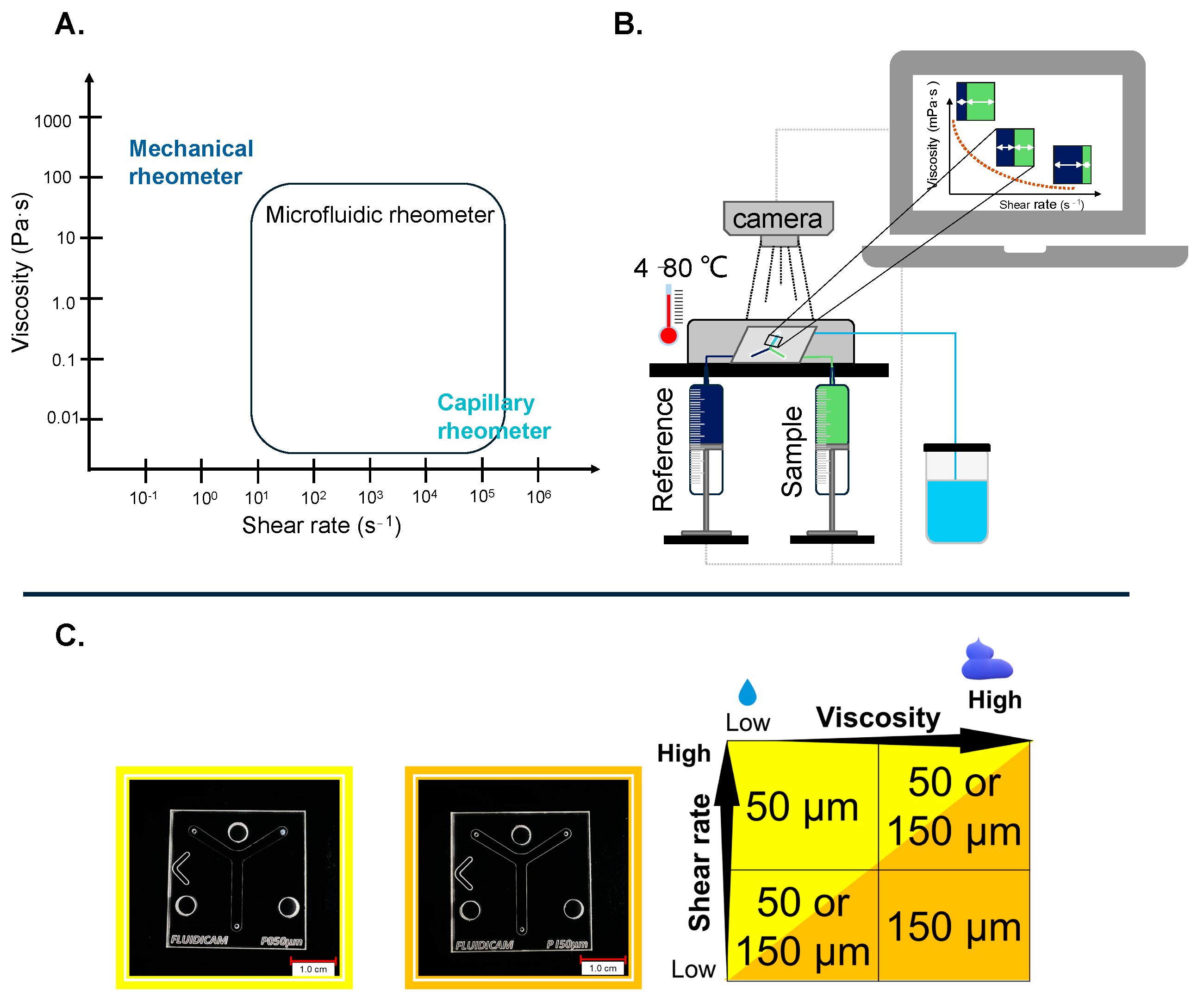
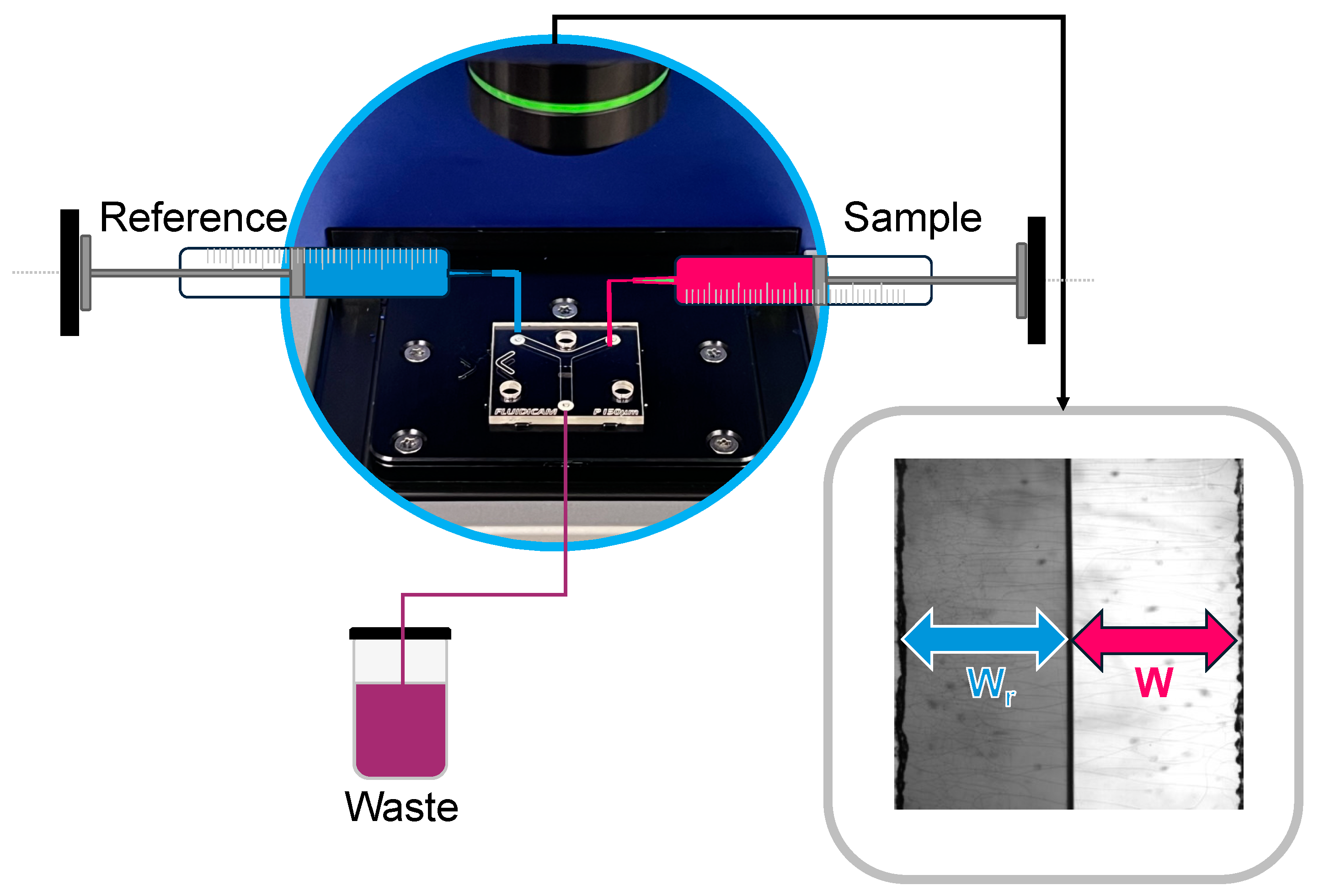
4.2.1. Viscosity Measurements with Kinexus Pro+
4.2.2. Viscosity Measurements with FluidicamTM RHEO
4.2.3. Digital Caliper
4.2.4. Extrudability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carnicer, V.; Alcázar, C.; Orts, M.J.; Sánchez, E.; Moreno, R. Microfluidic rheology: A new approach to measure viscosity of ceramic suspensions at extremely high shear rates. Open Ceram. 2021, 5, 100052. [Google Scholar] [CrossRef]
- Simões, A.; Miranda, M.; Cardoso, C.; Veiga, F.; Vitorino, C. Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Allahham, A.; Mainwaring, D.; Stewart, P.; Marriott, J. Development and application of a micro-capillary rheometer for in-vitro evaluation of parenteral injectability. J. Pharm. Pharmacol. 2004, 56, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, A.; Amsden, B. Biodegradable injectable in situ forming drug delivery systems. J. Control Release 2002, 80, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Phan-Thien, N.; Mai-Duy, N. Rheological Properties. In Understanding Viscoelasticity: An Introduction to Rheology; Springer International Publishing: Cham, Switzerland, 2017; pp. 29–41. [Google Scholar]
- Liu, H.; Fu, K.; Cui, X.; Zhu, H.; Yang, B. Shear Thickening Fluid and Its Application in Impact Protection: A Review. Polymers 2023, 15, 2238. [Google Scholar] [CrossRef] [PubMed]
- Neupert, T.; Bartel, D. Evaluation of Various Shear-Thinning Models for Squalane Using Traction Measurements, TEHD and NEMD Simulations. Lubricants 2023, 11, 178. [Google Scholar] [CrossRef]
- Budai, L.; Budai, M.; Fulopne Papay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Budai, M.; Fulopne Papay, Z.E.; Szalkai, P.; Niczinger, N.A.; Kijima, S.; Sugibayashi, K.; Antal, I.; Kallai-Szabo, N. Viscoelasticity of Liposomal Dispersions. Nanomaterials 2023, 13, 2340. [Google Scholar] [CrossRef] [PubMed]
- Zupančič Valant, A.; Žiberna, L.; Papaharilaou, Y.; Anayiotos, A.; Georgiou, G.C. The influence of temperature on rheological properties of blood mixtures with different volume expanders—Implications in numerical arterial hemodynamics simulations. Rheol. Acta 2011, 50, 389–402. [Google Scholar] [CrossRef]
- Barnes, H.A. The yield stress—A review or ‘panta roi’—Everything flows? J. Non-Newton. Fluid. Mech. 1998, 81, 133–178. [Google Scholar] [CrossRef]
- Winter, M.M.a.H.H. Relaxation Patterns of Nearly Critical Gels. Macromolecules 1996, 29, 7221–7229. [Google Scholar]
- Mackley, M.; Marshall, R.; Smeulders, J.; Zhao, F. The rheological characterization of polymeric and colloidal fluids. Chem. Eng. Sci. 1994, 49, 2551–2565. [Google Scholar] [CrossRef]
- Barber, E.M.; Muenger, J.R.; Villforth, F.J. A High Rate of Shear Rotational Viscometer. Anal. Chem. 1955, 27, 5. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J. Complex Fluids and Rheometry in Microfluidics. In Complex Fluid-Flows in Microfluidics; Galindo-Rosales, F.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–23. [Google Scholar]
- Gupta, S.; Wang, W.S.; Vanapalli, S.A. Microfluidic viscometers for shear rheology of complex fluids and biofluids. Biomicrofluidics 2016, 10, 043402. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, D.; Lam, R.H.W. Microfluidic Viscometer Using a Suspending Micromembrane for Measurement of Biosamples. Micromachines 2020, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ke, Y.; Zhao, H.; Tang, Q.; Zhang, Z.; Bai, C. Application of microfluidic technology in oil industry—A new quick test method of fluid viscosity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 452, 022040. [Google Scholar] [CrossRef]
- Formulaction. Fluidicam RHEO Instrument Note. Available online: https://www.ulprospector.com/documents/1527662.pdf?bs=5964&b=724579&st=20&r=na&ind=personalcare (accessed on 12 July 2024).
- Eneo. Phyteneo Occusept—Instruction fo Use. Available online: https://im9.cz/product-docs/5grza3la6de1kx76/N%C3%A1vod%20k%20pou%C5%BEit%C3%AD%20-%20Phyteneo%20Occusept%20o%C4%8Dn%C3%AD%20kapky%202%20x%2020%20ml.pdf (accessed on 12 July 2024).
- Kozuch, B.; Weber, J.; Buske, J.; Mader, K.; Garidel, P.; Diederichs, T. Comparative Stability Study of Polysorbate 20 and Polysorbate 80 Related to Oxidative Degradation. Pharmaceutics 2023, 15, 2332. [Google Scholar] [CrossRef] [PubMed]
- DesitinPharma. Diazepam Desitin 10 mg Rectal solution SmPC. Available online: https://www.medicines.org.uk/emc/product/3001/smpc (accessed on 12 July 2024).
- Kao, T.-T.; Wang, M.-C.; Chen, Y.-H.; Chung, Y.-T.; Hwang, P.-A. Propylene Glycol Improves Stability of the Anti-Inflammatory Compounds in Scutellaria baicalensis Extract. Processes 2021, 9, 894. [Google Scholar] [CrossRef]
- Zhai, J.; Mantaj, J.; Vllasaliu, D. Ascorbyl Palmitate Hydrogel for Local, Intestinal Delivery of Macromolecules. Pharmaceutics 2018, 10, 188. [Google Scholar] [CrossRef]
- OpellaHealthcare. Algopyrin 1 g/2 mL SmPC-HUN. Available online: https://ogyei.gov.hu/kiseroirat/ah/ah_0000018952_20230417100407.doc (accessed on 12 July 2024).
- Hu, F.; Lu, H.; Ye, Z.; Zhang, S.; Wang, W.; Gao, L. Slow-release lubrication of artificial joints using self-healing polyvinyl alcohol/polyethylene glycol/graphene oxide hydrogel. J. Mech. Behav. Biomed. Mater. 2021, 124, 104807. [Google Scholar] [CrossRef]
- Germanova, V.N.; Karlova, E.V.; Volova, L.T.; Zolotarev, A.V.; Rossinskaya, V.V.; Zakharov, I.D.; Korigodskiy, A.R.; Boltovskaya, V.V.; Nefedova, I.F.; Radaykina, M.V. PLA-PEG Implant as a Drug Delivery System in Glaucoma Surgery: Experimental Study. Polymers 2022, 14, 3419. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, K.M.; Kim, J.H.; Choi, Y.J.; Park, H.I.; Jung, H.C.; Roh, H.J.; Han, J.H.L.; Kim, J.R.; Lee, B.K. Biocompatibility evaluation of peo-treated magnesium alloy implants placed in rabbit femur condyle notches and paravertebral muscles. Biomater. Res. 2022, 26, 29. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ang, M.; Foo, S.; Lee, W.S.; Ma, Z.; Venkatraman, S.S.; Wong, T.T. Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS ONE 2011, 6, e22507. [Google Scholar] [CrossRef] [PubMed]
- Exeltis. Lactofeel Information Leaflet. Available online: https://www.lejdyeshop.cz/files/product_file/8_4312_LACTOFEEL-1.pdf (accessed on 12 July 2024).
- Mendling, W.; Shazly, M.A.E.; Zhang, L. The Role of Lactic Acid in the Management of Bacterial Vaginosis: A Systematic Literature Review. Future Pharmacol. 2022, 2, 198–213. [Google Scholar] [CrossRef]
- van der Veer, C.; Bruisten, S.M.; van Houdt, R.; Matser, A.A.; Tachedjian, G.; van de Wijgert, J.; de Vries, H.J.C.; van der Helm, J.J. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019, 19, 168. [Google Scholar] [CrossRef]
- Palmieri, E.; Pescosolido, F.; Montaina, L.; Carcione, R.; Petrella, G.; Cicero, D.O.; Tamburri, E.; Battistoni, S.; Orlanducci, S. A Sustainable Hydroxypropyl Cellulose-Nanodiamond Composite for Flexible Electronic Applications. Gels 2022, 8, 783. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- RichterGedeonPLC. Curiosa Gel Information Leaflet. Available online: https://rgwebsite-uat-cm.allwin.hu/-/media/sites/hq/documents/our-products/curiosa-gel-ifu-hu.pdf (accessed on 12 July 2024).
- Dekic, L.; Milinkovic Budincic, J.; Stanic, D.; Fraj, J.; Petrovic, L. Carbomer Hydrogels with Microencapsulated alpha-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study. Pharmaceutics 2024, 16, 628. [Google Scholar] [CrossRef]
- Rheology for Beginners—Determining the Viscosity of a Hand Cream. Available online: https://analyzing-testing.netzsch.com/en/training-know-how/tips-tricks/rheology/rheology-for-beginners-determining-the-viscosity-of-a-hand-cream (accessed on 20 June 2024).
- Gloup Information Leaflet. Available online: https://www.apo-direkt.de/app/download/25935563/GLOUP%C2%AE+Produktinformationen.pdf (accessed on 12 July 2024).
- Komisarska, P.; Pinyosinwat, A.; Saleem, M.; Szczuko, M. Carrageenan as a Potential Factor of Inflammatory Bowel Diseases. Nutrients 2024, 16, 1367. [Google Scholar] [CrossRef]
- Ong, J.J.; Steele, C.M.; Duizer, L.M. Challenges to assumptions regarding oral shear rate during oral processing and swallowing based on sensory testing with thickened liquids. Food Hydrocoll. 2018, 84, 173–180. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Polyethylene Glycol Monograph. In Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; pp. 517–522. [Google Scholar]
- From Low to High Shear Rates: NETZSCH Reaches Them All. Available online: https://analyzing-testing.netzsch.com/en/blog/2022/from-low-to-high-shear-rates-netzsch-reaches-them-all (accessed on 12 July 2024).
- Agwu, O.E.; Akpabio, J.U.; Ekpenyong, M.E.; Inyang, U.G.; Asuquo, D.E.; Eyoh, I.J.; Adeoye, O.S. A critical review of drilling mud rheological models. J. Pet. Sci. Eng. 2021, 203, 108659. [Google Scholar] [CrossRef]
- Not a Dry Eye in the House: Eye Drop Rheology. Available online: https://www.rheologylab.com/articles/pharmaceutical-rheology/rheology-lubricating-eye-drops/ (accessed on 17 June 2024).
- Kapadia, W.; Qin, N.; Zhao, P.; Phan, C.M.; Haines, L.; Jones, L.; Ren, C.L. Shear-Thinning and Temperature-Dependent Viscosity Relationships of Contemporary Ocular Lubricants. Transl. Vis. Sci. Technol. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.P. Oscillatory Shear Rheology for Probing Nonlinear Viscoelasticity of Complex Fluids: Large Amplitude Oscillatory Shear. In Rheology of Complex Fluids; Krishnan, J.M., Deshpande, A.P., Kumar, P.B.S., Eds.; Springer: New York, NY, USA, 2010; pp. 87–110. [Google Scholar]
- Dyvik, K.; Dyrstad, K.; Tronstad, A. Relationship between viscosity and determined injection pressure in angiography catheters for common roentgen contrast media. Acta Radiol. 1995, 36, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Aerospace 6 inch/150 mm Digital Vernier Caliper/Micrometer. Available online: https://thermoindia.com/product/thermo-aerospace-6-inch-150mm-digital-vernier-caliper-micrometer/ (accessed on 12 July 2024).
- Smith, F.D.; Lyndall, G. Apparatus for Testing Pharmaceutical Tablets. U.S. Patent 2041869A, 26 May 1934. [Google Scholar]

| Solution and Gel-Based Product | Used Sample/Measurement (mL) | Time/Measurement (min) | ||
|---|---|---|---|---|
| Kinexus Pro+ | FluidicamTM RHEO | Kinexus Pro+ | FluidicamTM RHEO | |
| Vaginal gel | 1.50 | 2.1 | 31 min ± 2 min | 4 min 58 s ± 27 s |
| Wound gel | 1.50 | 2.6 | 27 min ± 2 min | 5 min 32 s ± 29 s |
| Eyedrop | 1.19 | 1.2 | 48 min ± 4 min | 3 min 12 s ± 12 s |
| Klysma | 1.19 | 0.9 | 35 min ± 2 min | 2 min 53 s ± 21 s |
| Injection | 1.19 | 1.0 | 38 min ± 3 min | 2 min 48 s ± 17 s |
| Lubricant | 1.19 | 1.8 | 30 min ± 2 min | 3 min 8 s ± 24 s |
| Oral gel | 1.50 | 2.16 | 32 min ± 2 min | 5 min 16 s ± 28 s |
| Solution- and Gel-Based Product | Diameter (mm) | Length (mm) | Force (N) |
|---|---|---|---|
| Vaginal gel | 4.02 | 44.49 | 49.05 |
| Klysma | 1.91 | 44.59 | 49.05 |
| Injection | Gauge | Gauge | - |
| Dosage Form | Route of Administration | API | Viscosity Modifying Excipient | Indication | Brand Name | Manufacturer |
|---|---|---|---|---|---|---|
| Gel | Vaginal | Acidum lacticum, Glicogen | Methyl-hydroxy-propyl-cellulose (MHPC) | Bacterial vaginosis, Candidiasis | Lactofeel | Exeltis, Florham Park, NJ, USA |
| Gel | Topical | Sodium hyaluronate | Carbomer | Wound care | Curiosa | Richter Gedeon Nyrt., Budapest, Hungary |
| Solution | Eyedrop | - | Polysorbate 80 | Lubricant | Phyteneo Occusept | Neofyt spol. s r.o., Stříbrná Skalice, Czech Republic |
| Klysma (solution) | Rectal | Diazepam | Propylenglycol | Seizure resolution | Diazepam Desitin 10 mg | Desitin Arzneimittel GmbH., Hamburg, Germany |
| Injection | Intramuscular/intravenous | Metamizole-sodium | - | Painkiller | Algopyrin 1 g/2 mL injection | Sanofi-Aventis, Paris, France |
| Excipient | Subcutaneous | - | - | Lubricant for implants | Macrogola 400 | MAGILAB Kft., Budapest, Hungary |
| medical device-gel | Oral | - | Carragenan | Lubricant for solid dosage forms | Gloup | M. Technologies Fr., Tourcoing, France |
| Product | Geometry | Shear Rate Range (s−1) | Temperature (°C) |
|---|---|---|---|
| Vaginal gel | Cone–Plate | 10−1 to 1 × 104 | 37 |
| Wound gel | Cone–Plate | 10−1 to 5 × 103 | 32 |
| Eyedrop | Plate–Plate | 10−1 to 1 × 104 | 34 |
| Klysma | Plate–Plate | 10−1 to 5 × 103 | 37 |
| Injection | Plate–Plate | 10−1 to 1 × 104 | 37 |
| Lubricant | Cone–Plate | 10−1 to 5 × 103 | 37 |
| Oral gel | Cone–Plate | 10−1 to 5 × 103 | 37 |
| Product | Diameter of Gap Microchip (µm) | Shear Rate Range (s−1) | Temperature (°C) | Viscosity of Ready-to-Use Reference (mPa·s) |
|---|---|---|---|---|
| Vaginal gel | 150 | 1 × 102 to 5 × 103 | 37 | 500 |
| Wound gel | 150 | 1 × 102 to 5 × 103 | 32 | 500 |
| Eyedrop | 50 | 5 × 102 to 1 × 104 | 34 | 5 |
| Klysma | 50 | 5 × 102 to 3.5 × 104 | 37 | 5 |
| Injection | 50 | 1 × 103 to 1 × 105 | 37 | 5 |
| Lubricant | 50 | 5 × 102 to 1 × 104 | 37 | 5 |
| Oral gel | 150 | 1 × 102 to 5 × 103 | 37 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilimi, Z.; Pápay, Z.E.; Basa, B.; Orekhova, X.; Kállai-Szabó, N.; Antal, I. Microfluidic Rheology: An Innovative Method for Viscosity Measurement of Gels and Various Pharmaceuticals. Gels 2024, 10, 464. https://doi.org/10.3390/gels10070464
Vilimi Z, Pápay ZE, Basa B, Orekhova X, Kállai-Szabó N, Antal I. Microfluidic Rheology: An Innovative Method for Viscosity Measurement of Gels and Various Pharmaceuticals. Gels. 2024; 10(7):464. https://doi.org/10.3390/gels10070464
Chicago/Turabian StyleVilimi, Zsófia, Zsófia Edit Pápay, Bálint Basa, Xeniya Orekhova, Nikolett Kállai-Szabó, and István Antal. 2024. "Microfluidic Rheology: An Innovative Method for Viscosity Measurement of Gels and Various Pharmaceuticals" Gels 10, no. 7: 464. https://doi.org/10.3390/gels10070464
APA StyleVilimi, Z., Pápay, Z. E., Basa, B., Orekhova, X., Kállai-Szabó, N., & Antal, I. (2024). Microfluidic Rheology: An Innovative Method for Viscosity Measurement of Gels and Various Pharmaceuticals. Gels, 10(7), 464. https://doi.org/10.3390/gels10070464








