Influence of Thermal Treatment on the Chemical and Structural Properties of Geopolymer Gels Doped with Nd2O3 and Sm2O3
Abstract
1. Introduction
2. Results and Discussion
2.1. Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS)
2.2. Scanning Electron Microscopy with Energy-Dispersive Spectrometry (SEM-EDS)
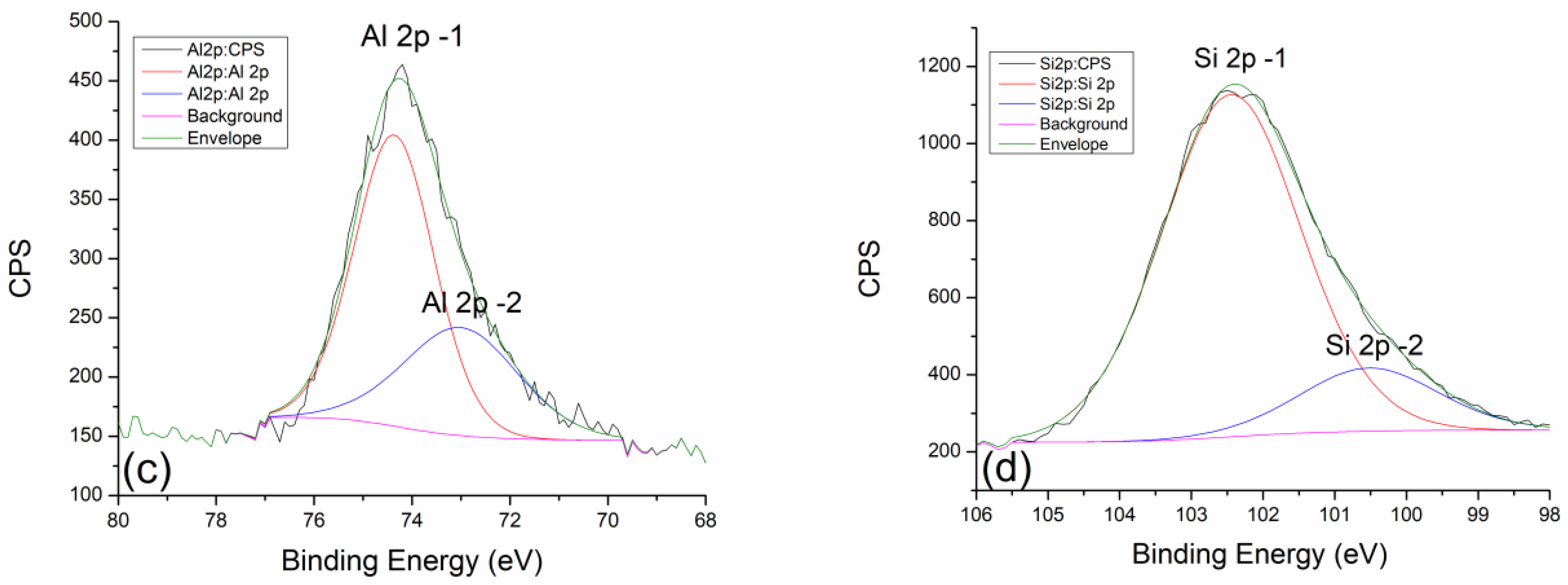
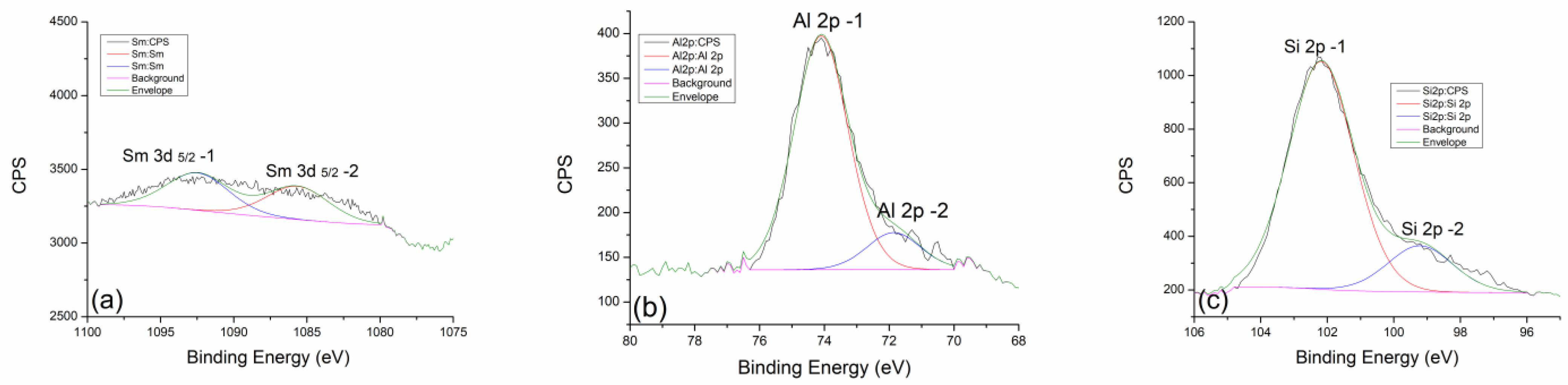
2.3. X-ray Photoelectron Spectroscopy (XPS)
2.4. Sorptivity, Open Porosity, and Archimedean Density
3. Conclusions
4. Materials and Methods
Characterization Techniques
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, H.; Wei, L.; Ji, Y.; Cao, L.; Cheng, F. Heavy metal fixing and heat resistance abilities of coal fly ash-waste glass based geopolymers by hydrothermal hot pressing. Adv. Powder Technol. 2018, 29, 1487–1492. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E.; Lachowski, E.E. Fixing Arsenic in Alkali-Activated Cementitious Matrices. J. Am. Ceram. Soc. 2005, 88, 1122–1126. [Google Scholar] [CrossRef]
- Palomo, A.; López dela Fuente, J.I. Alkali-activated cementitous materials: Alternative matrices for the immobilisation of hazardous wastes: Part I. Stabilisation of boron. Cem. Concr. Res. 2003, 33, 281–288. [Google Scholar] [CrossRef]
- Kljajević, L.M.; Nenadovic, S.S.; Nenadovic, M.T.; Bundaleski, N.K.; Todorovic, B.Ž.; Pavlovic, V.B.; Rakocevic, Z.L. Structural and chemical properties of thermally treated geopolymer samples. Ceram. Int. 2017, 43, 6700–6708. [Google Scholar] [CrossRef]
- Bankowski, P.; Zou, L.; Hodges, R. Reduction of metal leaching in brown coal fly ash using geopolymers. J. Hazard. Mater. 2004, 114, 59–67. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Nenadović, S.S.; Ferone, C.; Nenadović, M.T.; Cioffi, R.; Mirković, M.M.; Vukanac, I.; Kljajević, M. Chemical, physical and radiological evaluation of raw materials and geopolymers for building applications. J. Radioanal. Nucl. Chem. 2020, 325, 435–445. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Macphee, D.E.; Lachowski, E.E.; Palomo, A. Immobilization of cesium in alkaline activated fly ash matrix. J. Nucl. Mater. 2005, 346, 185–193. [Google Scholar] [CrossRef]
- Yu, L.; Fang, L.; Zhang, P.; Zhao, S.; Jiao, B.; Li, D. The Utilization of Alkali-Activated Lead–Zinc Smelting Slag for Chromite Ore Processing Residue Solidification/Stabilization. Int. J. Environ. Res. Public Health 2021, 18, 9960. [Google Scholar] [CrossRef]
- Gulicovski, J.; Nenadovic, S.; Kljajevic, L.; Mirkovic, M.; Nisavic, M.; Kragovic, M.; Stojmenovic, M. Geopolymer/CeO2 as solid electrolity for IT-SOFC. Polymers 2020, 12, 248. [Google Scholar] [CrossRef]
- Narasimharao, K.; Ali, T.T. Influence of synthesis conditions on physico-chemical and photocatalytic properties of rare earth (Ho, Nd and Sm) oxides. J. Mater. Res. Technol. 2020, 9, 1819–1830. [Google Scholar] [CrossRef]
- Kljajević, L.M.; Melichova, Z.; Kisić, D.D.; Nenadović, M.T.; Todorović, B.Ž.; Pavlović, V.B.; Nenadović, S.S. The influence of alumino-silicate matrix composition on surface hydrophobic properties. Sci. Sinter. 2019, 51, 163–173. [Google Scholar] [CrossRef]
- Nenadović, S.S.; Kljajević, L.M.; Ivanović, M.M.; Mirković, M.M.; Radmilović, N.; Rakočević, L.Z.; Nenadović, M.T. Structural and chemical properties of geopolymer gels incorporated with neodymium and samarium. Gels 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Trivunac, K.; Ljiljana, K.; Snežana, N.; Jelena, G.; Miljana, M.; Biljana, B.; Slavica, S. Microstructural characterization and adsorption properties of alkali-activated materials based on metakaolin. Sci. Sinter. 2016, 48, 209–220. [Google Scholar] [CrossRef]
- Volokh, A.A.; Gorbunov, A.V.; Gundorina, S.F.; Revich, B.A.; Frontasyeva, M.V.; Pal, C.S. Phosphorus fertilizer production as a source of rare-earth elements pollution of the environment. Sci. Total Environ. 1990, 95, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Parnicka, P.; Mazierski, P.; Grzyb, T.; Lisowski, W.; Kowalska, E.; Ohtani, B.; Zaleska-Medynska, A.; Nadolna, J. Influence of the preparation method on the photocatalytic activity of Nd—Modified TiO2. Beilstein J. Nanotechnol. 2018, 9, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Bachvarova-Nedelcheva, A.; Yordanov, S.; Iordanova, R.; Stambolova, I.; Stoyanova, A.; Georgieva, N.; Nemska, V. The Influence of Nd and Sm on the Structure and Properties of Sol-Gel-Derived TiO2 Powders. Molecules 2021, 26, 3824. [Google Scholar] [CrossRef] [PubMed]
- Ivanovi´c, M.; Ljiljana, K.; Jelena, G.; Marijana, P.; Ivona, J.-C.; Dušan, B.; Snežana, N. The Effect of the Concentration of Alkaline Activator and Aging Time on the Structure of Metakaolin Based Geopolymer. Sci. Sinter. 2020, 52, 219–229. [Google Scholar] [CrossRef]
- Kirdsiri, K.; Rajaramakrishnab, R.; Damdeea, B.; Kimc, H.J.; Kimc, H.J.; Horphathumd, M.; Kaewkhao, J. Influence of alkaline earth oxides on Eu3+ doped lithium borate glasses for photonic, laser and radiation detection material applications. Solid State Sci. 2019, 89, 57–66. [Google Scholar] [CrossRef]
- Stengl, V.; Bakardjieva, S.; Murafa, N. Preparation and photocatalytic activity of rare earth-doped TiO2 nanoparticles. Mater. Chem. Phys. 2009, 114, 217–226. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Dinkar, A.V.; Shridhar, J.S.; Madhukar, E.N.; Anil, A.; Nitin, K. Sm-Doped TiO2 Nanoparticles with High Photocatalytic Activity for ARS Dye Under Visible Light Synthesized by Ultrasonic Assisted Sol-Gel Method. Orient. J. Chem. 2016, 32, 933–940. [Google Scholar] [CrossRef]
- Nenadovic, S.; Nenadovic, M.; Vukanac, I.; Ðordevic, A.; Dragicevic, S.; Lješevic, M. Vertical distribution of 137Cs in cultivated and undisturbed areas. Nucl. Technol. Radiat. Prot. 2010, 25, 30–36. [Google Scholar] [CrossRef]
- Khade, G.V.; Suwarnkar, M.B.; Gavade, N.L.; Garadkar, K.M. Sol-gel microwave assisted synthesis of Sm-doped TiO2 nanoparticles and their photocatalytic activity for the degradation of Methyl Orange under sunlight. J. Mater. Sci. Mater. Electr. 2016, 27, 6425–6432. [Google Scholar] [CrossRef]
- Shehata, N.; Meehan, K. Potential applications of samarium as a dopant element. In Samarium: Chemical Properties, Occurrence and Potential Applications; Kaitlyn, R., Ed.; Danford Nova Publisher: New York, NY, USA, 2014; pp. 111–136. [Google Scholar]
- Aarnink, W.A.M.; Weishaupt, A.; van Silfhout, A. Angle-resolved X-ray photoelectron spectroscopy (ARXPS) and a modified Levenberg-Marquardt fit procedure: A new combination for modeling thin layers. Appl. Surf. Sci. 1990, 45, 37–48. [Google Scholar] [CrossRef]
- Uwamino, Y.; Ishizuka, T.; Yamatera, H. X-ray photoelectron spectroscopy of rare-earth compounds. J. Electron Spectrosc. Relat. Phenom. 1984, 34, 67–78. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sondebry, C.; Li, Z.; Sogard, E.G. XPS and FT-IR investigation of silicate polymers. J. Mater. 2009, 44, 2079–2088. [Google Scholar] [CrossRef]
- Jhang, J.H.; Boscoboinik, J.A.; Altman, E.I. Ambient pressure X-ray photoelectron spectroscopy study of water formation and adsortption under two-dimesional silica and aluminosilicate layers on Pd (III). J. Chem. Phys. 2020, 152, 084705. [Google Scholar] [CrossRef]
- Revathi, T.; Jeyalakshmi, R. XPS, 29Si, 27Al, 11B MAS -NMR, ATR-IR and FESEM characterization of geopolymer based on borax modified water glass activated Fly ash-GGBS blend. Mater. Res. Express 2019, 6, 085337. [Google Scholar] [CrossRef]
- Xiaohong, Z.; Chengying, B.; Yingjie, Q.; Xiadong, W.; Decland, J.; Hongqiang, L.; Paolo, C. Porous geopolymer composites A review. Compos. Part A 2021, 150, 106629. [Google Scholar]
- Phair, J.W.; Schulz, J.C.; Aldridge, L.P.; Smith, J.D. Small-angle neutron scattering and rheological characterization of aluminosilicate hydrogels. J. Am. Ceram. Soc. 2004, 87, 129–137. [Google Scholar] [CrossRef]
- Mullica, D.F.; Lok, C.K.C.; Perkins, H.O.; Benesh, G.A.; Young, V. X-ray photoemission spectra of Nd(OH)3, Sm(OH)3, Eu(OH)3 and Gd(OH)3. J. Electron Spectros. Relat. Phenom. 1995, 71, 1–20. [Google Scholar] [CrossRef]
- Knežević, S.; Ivanović, M.; Stanković, D.; Kisić, D.; Nenadović, S.; Potočnik, J.; Nenadović, M. Microstructural Analysis of Thermally Treated Geopolymer Incorporated with Neodymium. Nanomaterials 2023, 13, 1663. [Google Scholar] [CrossRef] [PubMed]
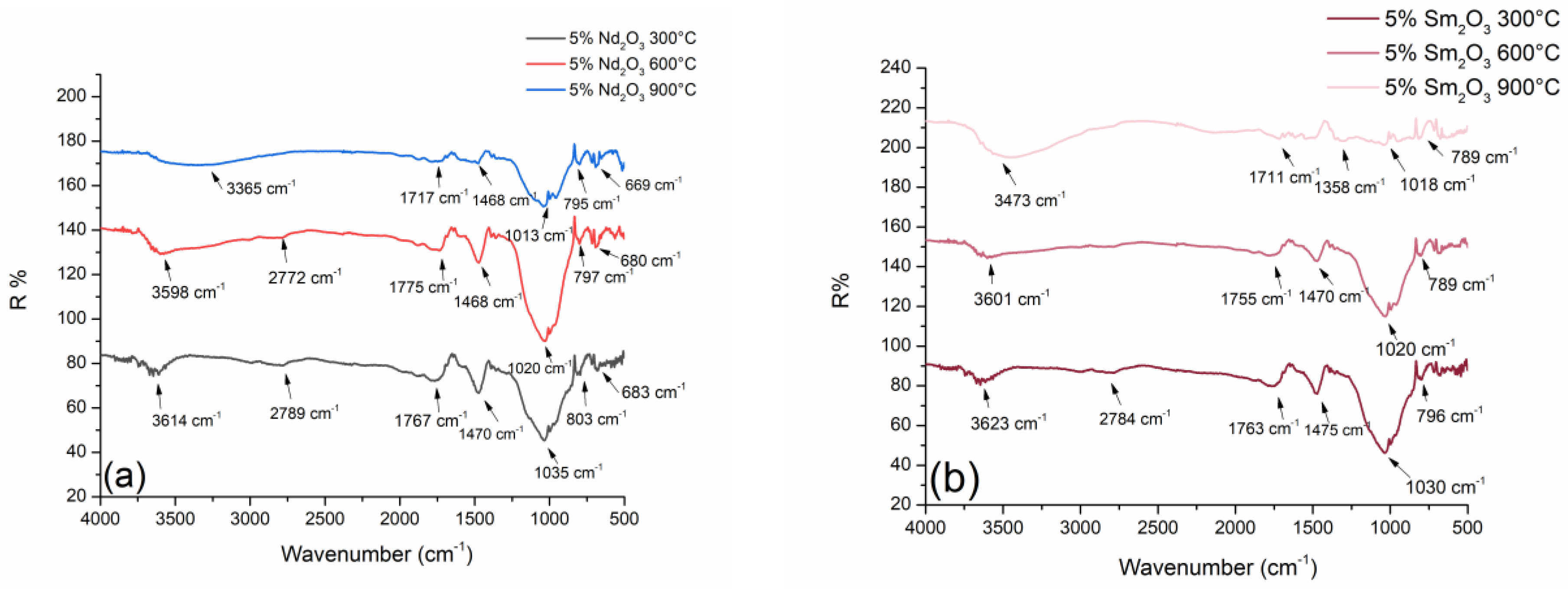

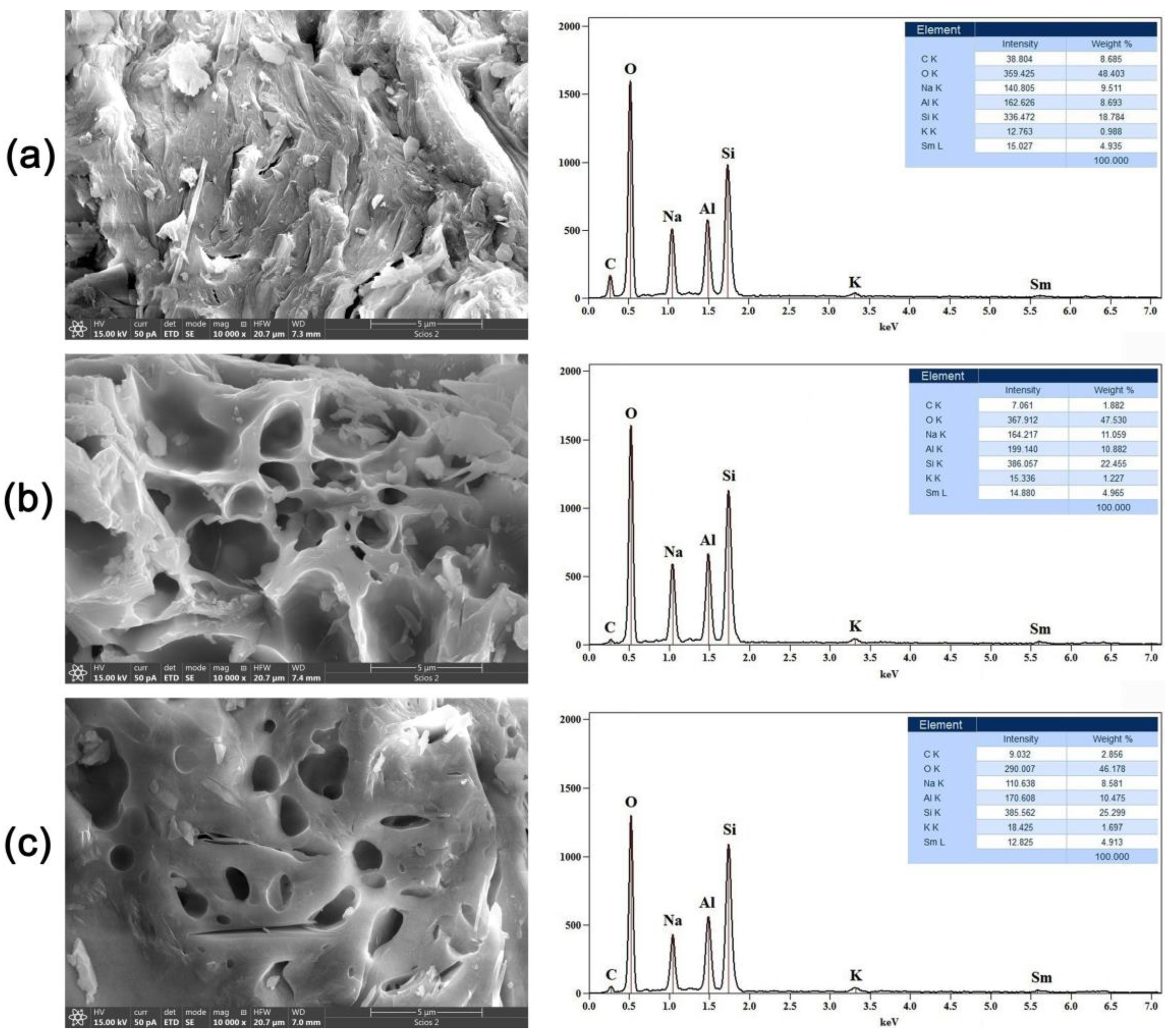

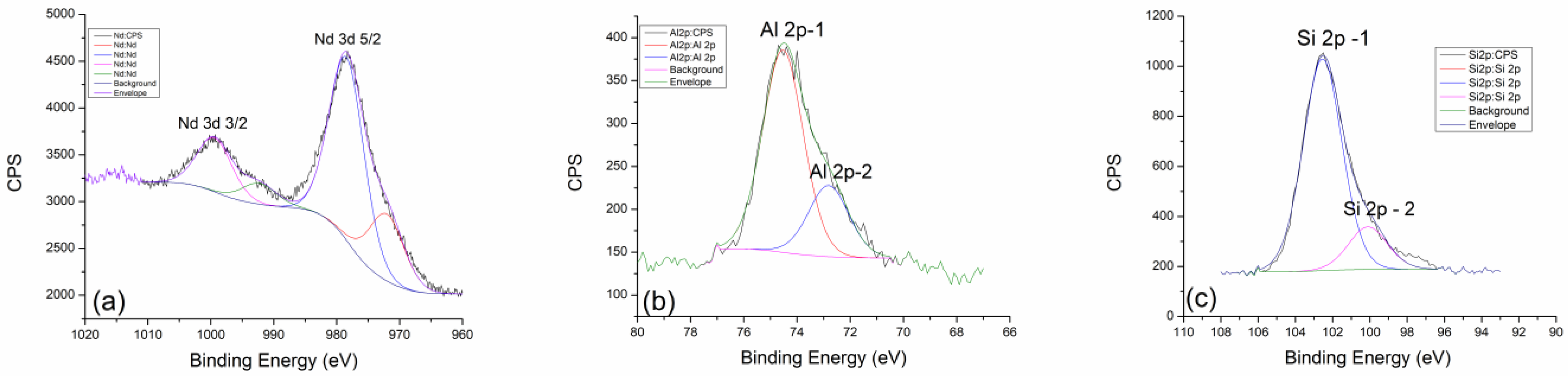
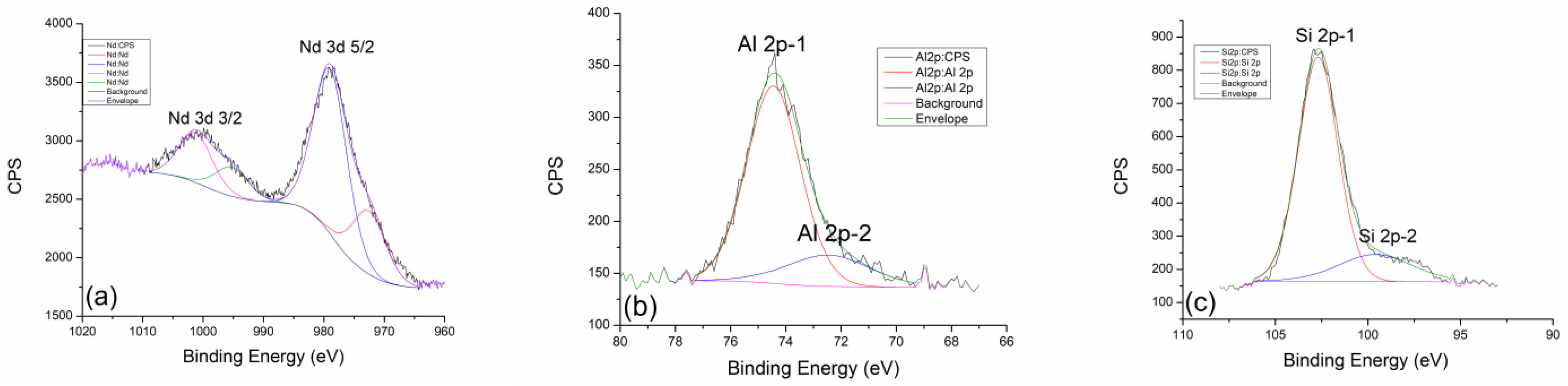



| Values of Wavenumbers (cm−1) | Assignment |
|---|---|
| 3614 | Nd-OH |
| 2798 | adsorption of atmospheric CO2 |
| 1767 | vibration deformation of adsorbed water |
| 1470 | adsorption of atmospheric CO2 |
| 1035 | asymmetric vibration Si-O-Al |
| 796 | symmetrical vibrations Si-O-Al |
| 683 | stretching vibrations Nd-O-Si |
| Values of Wave Numbers (cm−1) | Assignment |
|---|---|
| 3623 | Sm-OH |
| 2784 | adsorption of atmospheric CO2 |
| 1763 | vibration deformation of adsorbed water |
| 1475 | adsorption of atmospheric CO2 |
| 1030 | asymmetric vibration Si-O-Al |
| 796 | stretching vibrations Sm-O |
| 669 | bending vibrations Sm-O-H |
| 561 | stretching vibrations Sm-O-Si |
| GP Nd 300 °C | (at%) | GP Nd 600 °C | (at%) | GP Nd 900 °C | (at%) |
|---|---|---|---|---|---|
| Al 2p | 10.06 | Al 2p | 8.77 | Al 2p | 8.43 |
| Si 2p | 20.78 | Si 2p | 21.45 | Si 2p | 22.39 |
| GP Sm 300 °C | (at%) | GP Sm 600 °C | (at%) | GP Sm 900 °C | (at%) |
|---|---|---|---|---|---|
| Al 2p | 9.49 | Al 2p | 8.92 | Al 2p | 9.15 |
| Si 2p | 20.08 | Si 2p | 20.82 | Si 2p | 21.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenadović, M.; Knežević, S.; Ivanović, M.; Nenadović, S.; Kisić, D.; Popović, M.; Potočnik, J. Influence of Thermal Treatment on the Chemical and Structural Properties of Geopolymer Gels Doped with Nd2O3 and Sm2O3. Gels 2024, 10, 468. https://doi.org/10.3390/gels10070468
Nenadović M, Knežević S, Ivanović M, Nenadović S, Kisić D, Popović M, Potočnik J. Influence of Thermal Treatment on the Chemical and Structural Properties of Geopolymer Gels Doped with Nd2O3 and Sm2O3. Gels. 2024; 10(7):468. https://doi.org/10.3390/gels10070468
Chicago/Turabian StyleNenadović, Miloš, Sanja Knežević, Marija Ivanović, Snežana Nenadović, Danilo Kisić, Maja Popović, and Jelena Potočnik. 2024. "Influence of Thermal Treatment on the Chemical and Structural Properties of Geopolymer Gels Doped with Nd2O3 and Sm2O3" Gels 10, no. 7: 468. https://doi.org/10.3390/gels10070468
APA StyleNenadović, M., Knežević, S., Ivanović, M., Nenadović, S., Kisić, D., Popović, M., & Potočnik, J. (2024). Influence of Thermal Treatment on the Chemical and Structural Properties of Geopolymer Gels Doped with Nd2O3 and Sm2O3. Gels, 10(7), 468. https://doi.org/10.3390/gels10070468











