Dual-Temperature/pH-Sensitive Hydrogels with Excellent Strength and Toughness Crosslinked Using Three Crosslinking Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structural Characterization
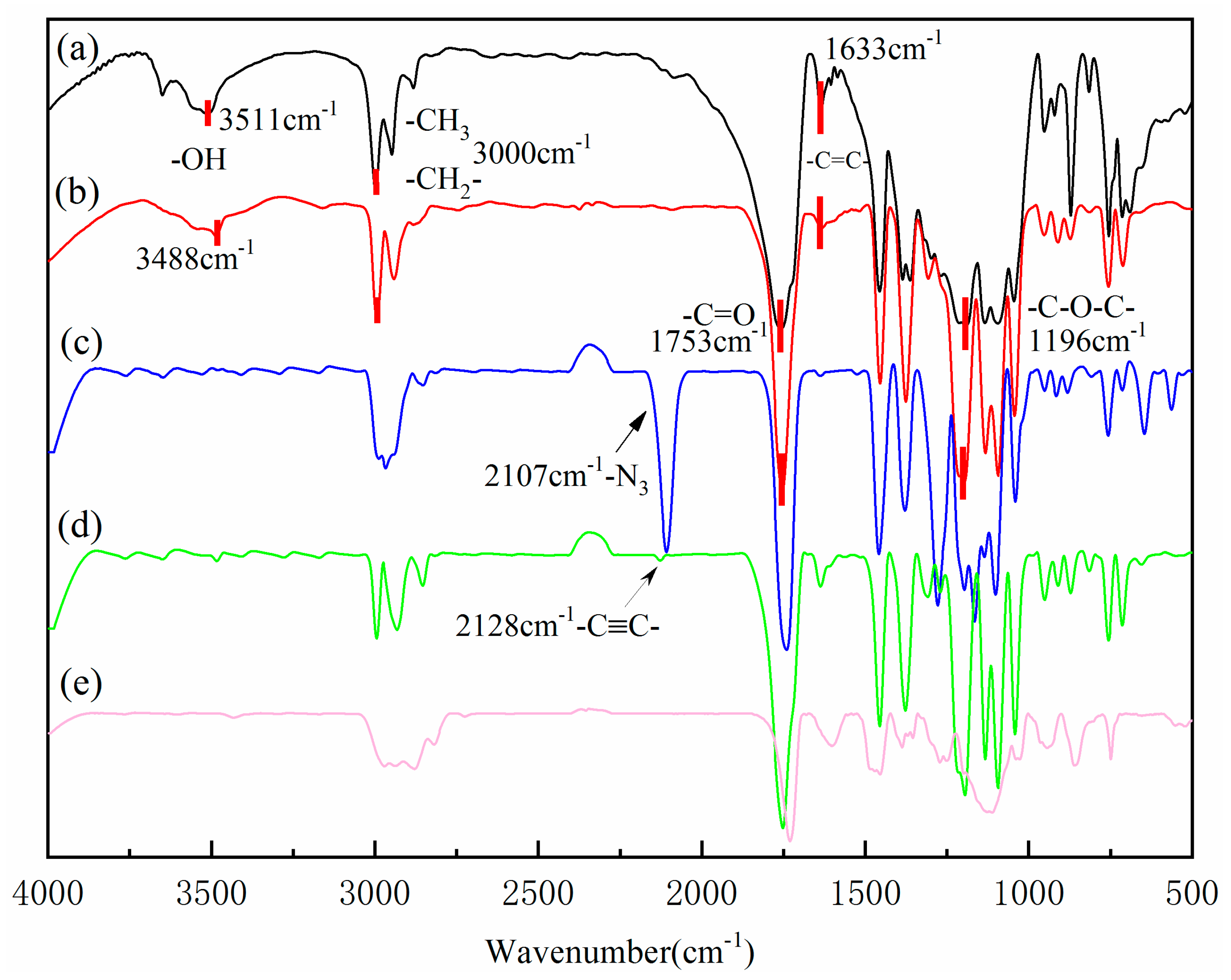
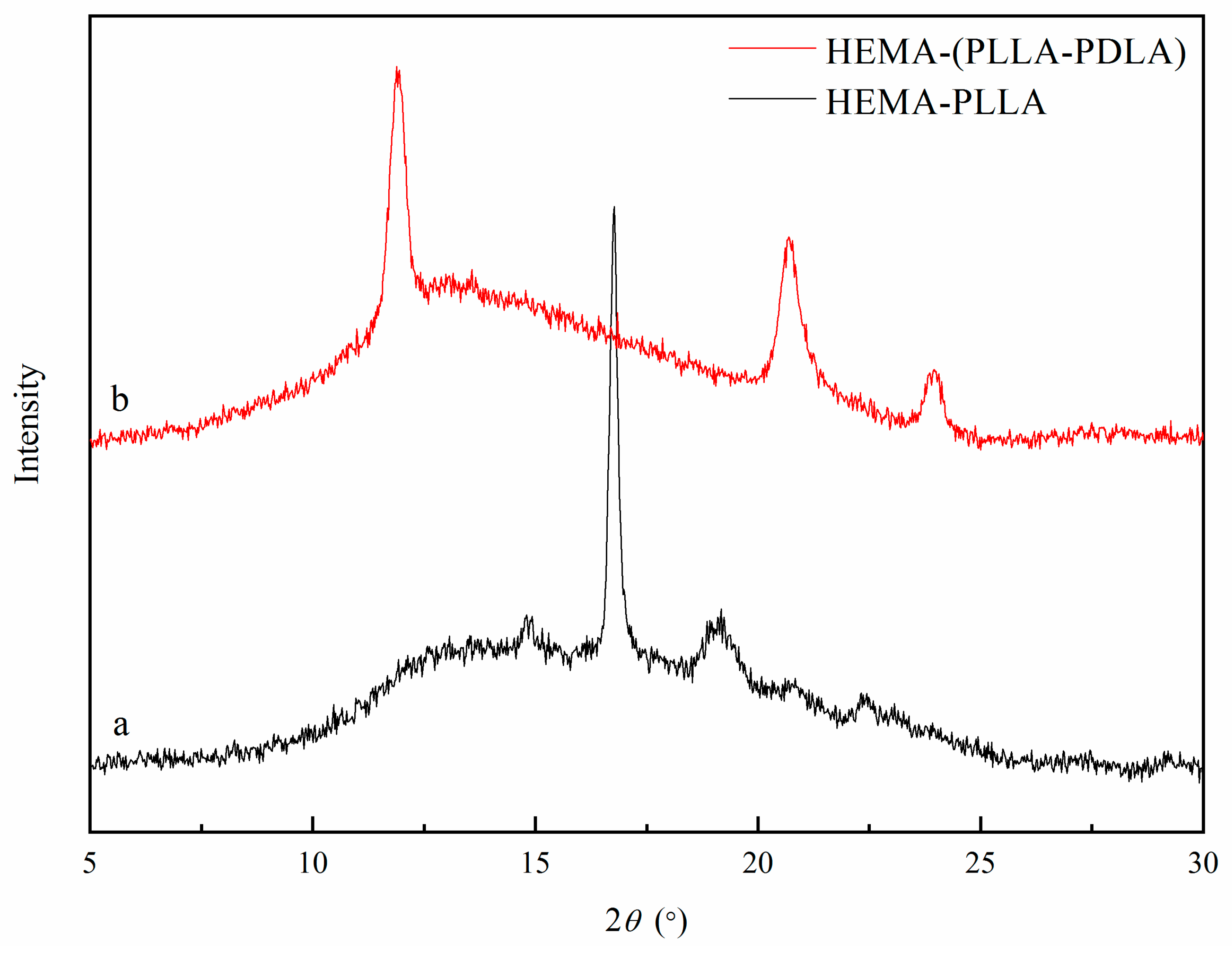
2.2.1. Structural Characterization of HEMA-PLLAn, HEMA-PDLAn and HEMA-(PLLA-PDLA)
2.2.2. Structural Characterization of HEMA-(PLLA-PDLA)-N3 and HEMA-(PLLA-PDLA)-Alkyne
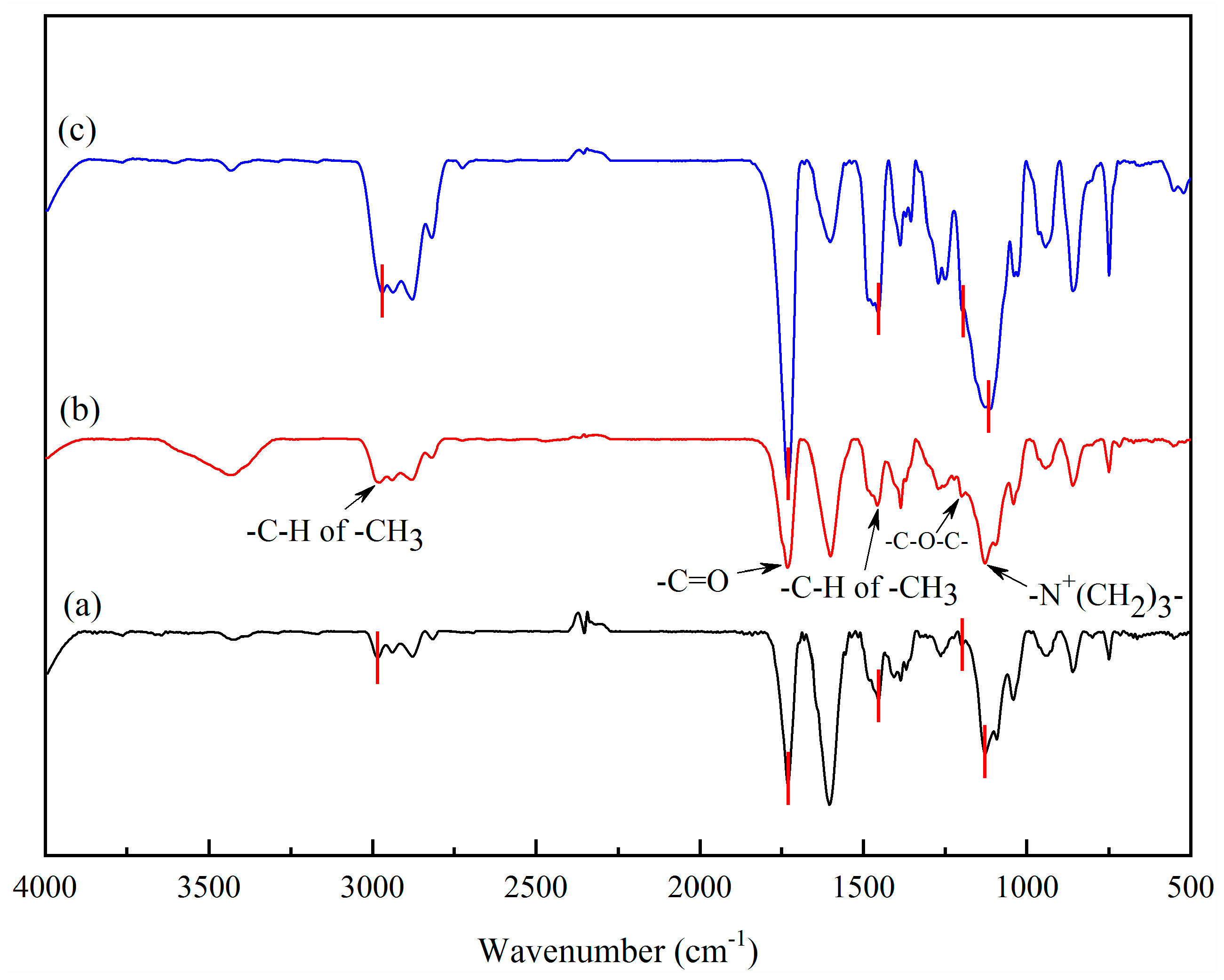
2.2.3. Structural Characterization of Gels
2.3. Morphology Analysis of Gels
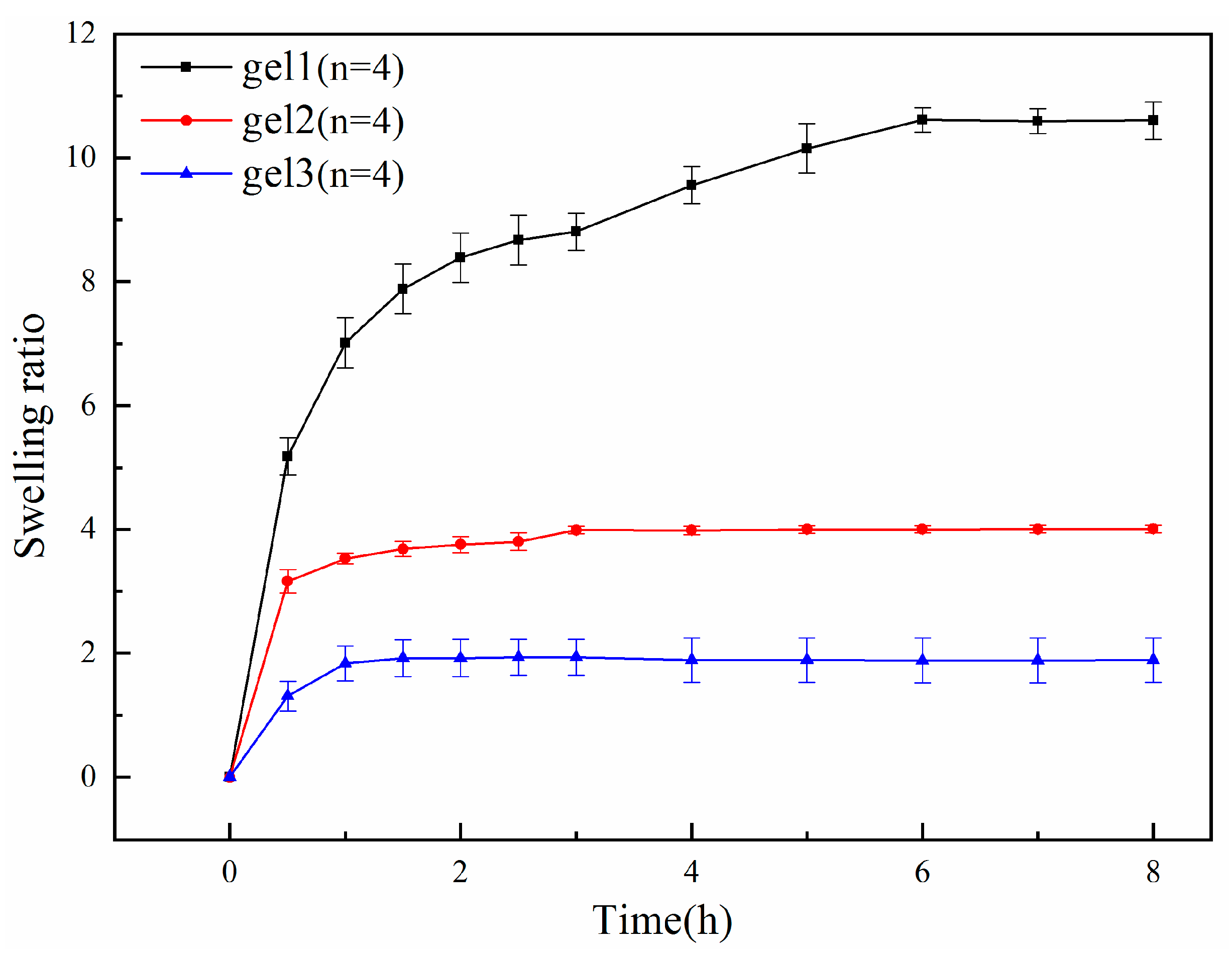
2.4. Temperature Sensitivity Test of Gels
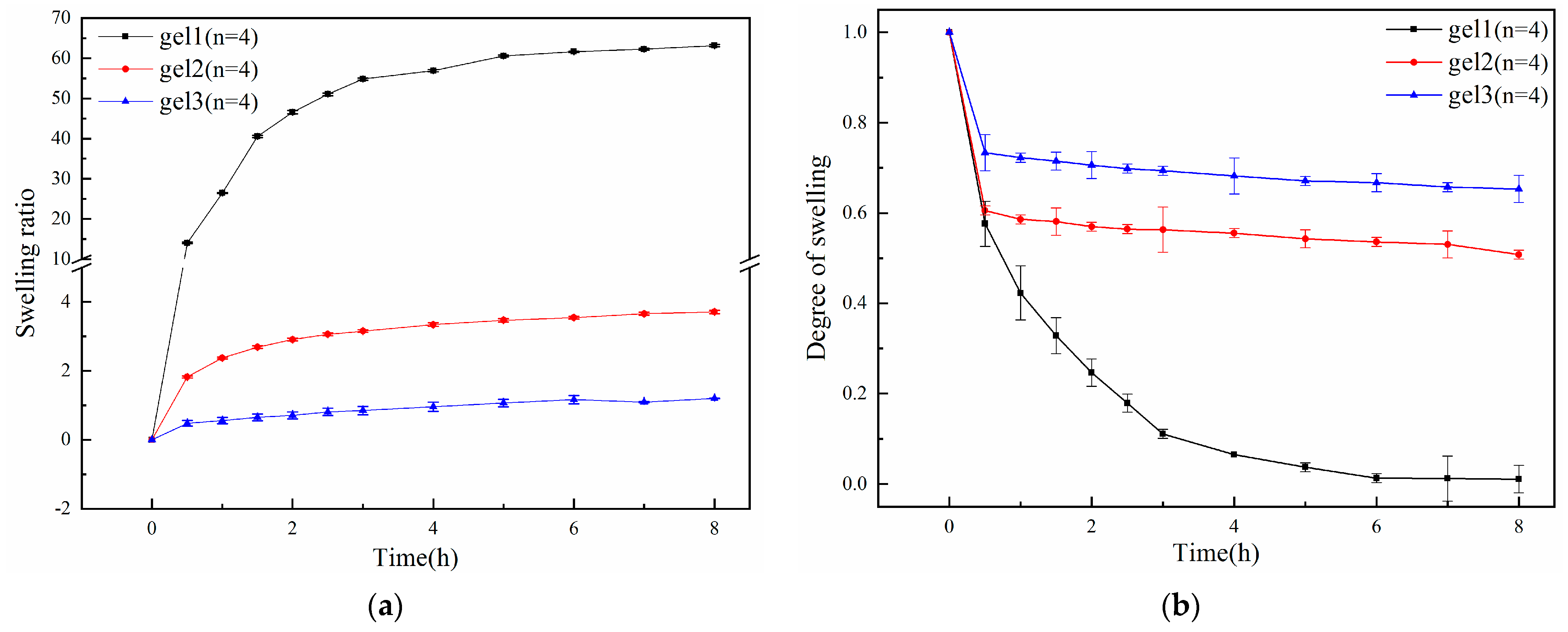
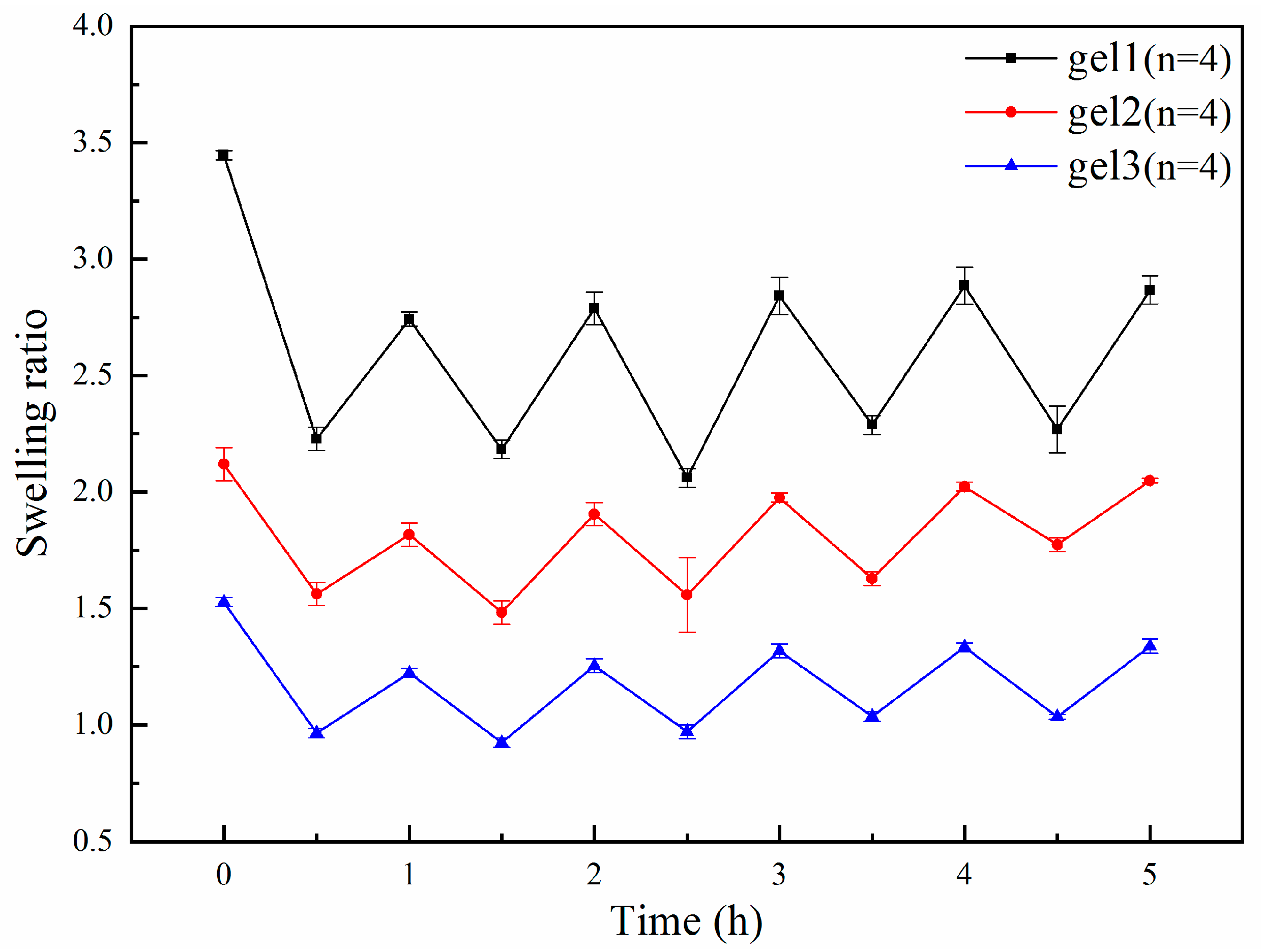
2.5. pH Sensitivity Test of Gels
2.6. Reversibility Test of Gels
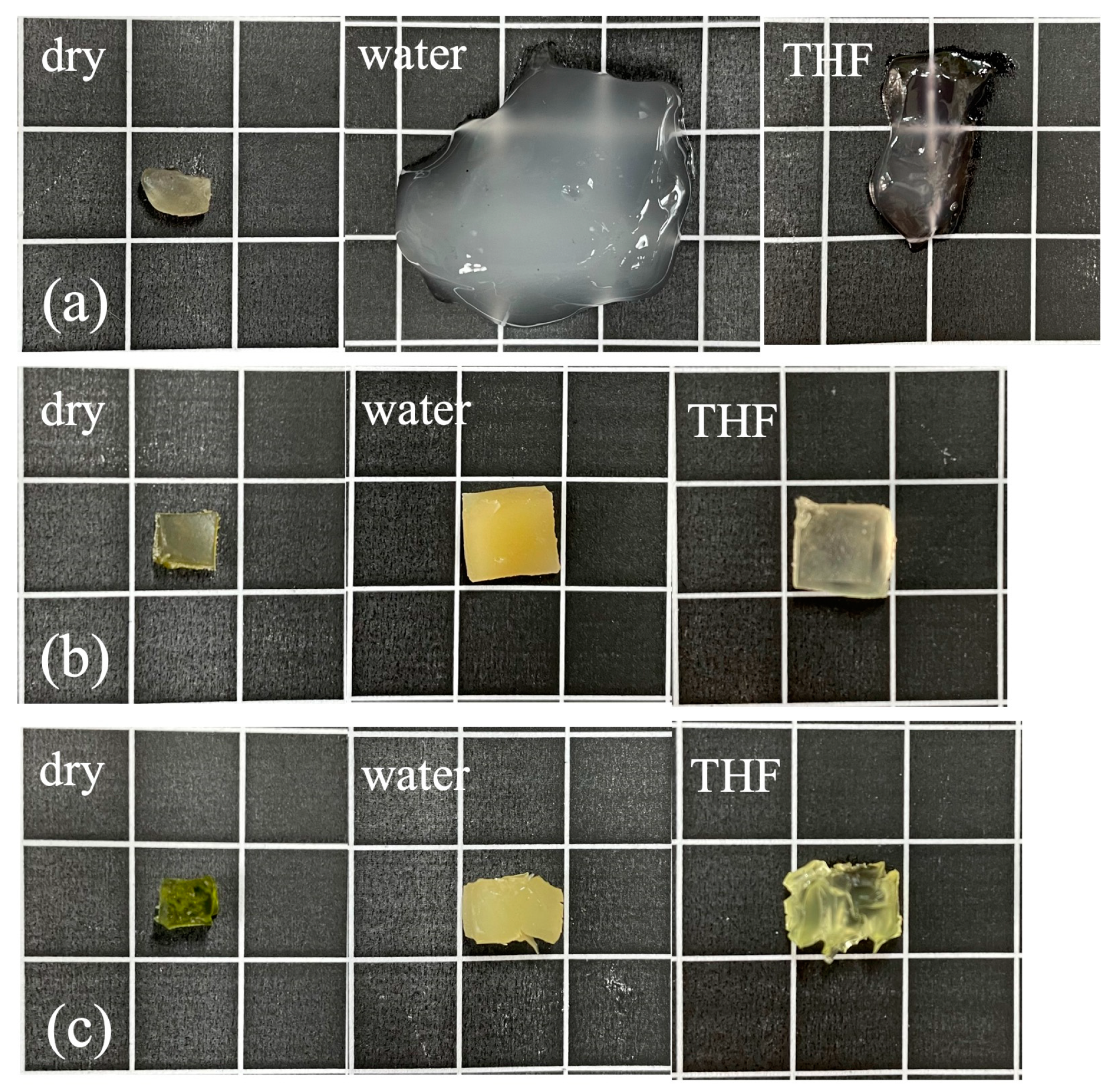
2.7. Amphiphilicity Test of Gels
2.8. Thermal Stability Test of Gels
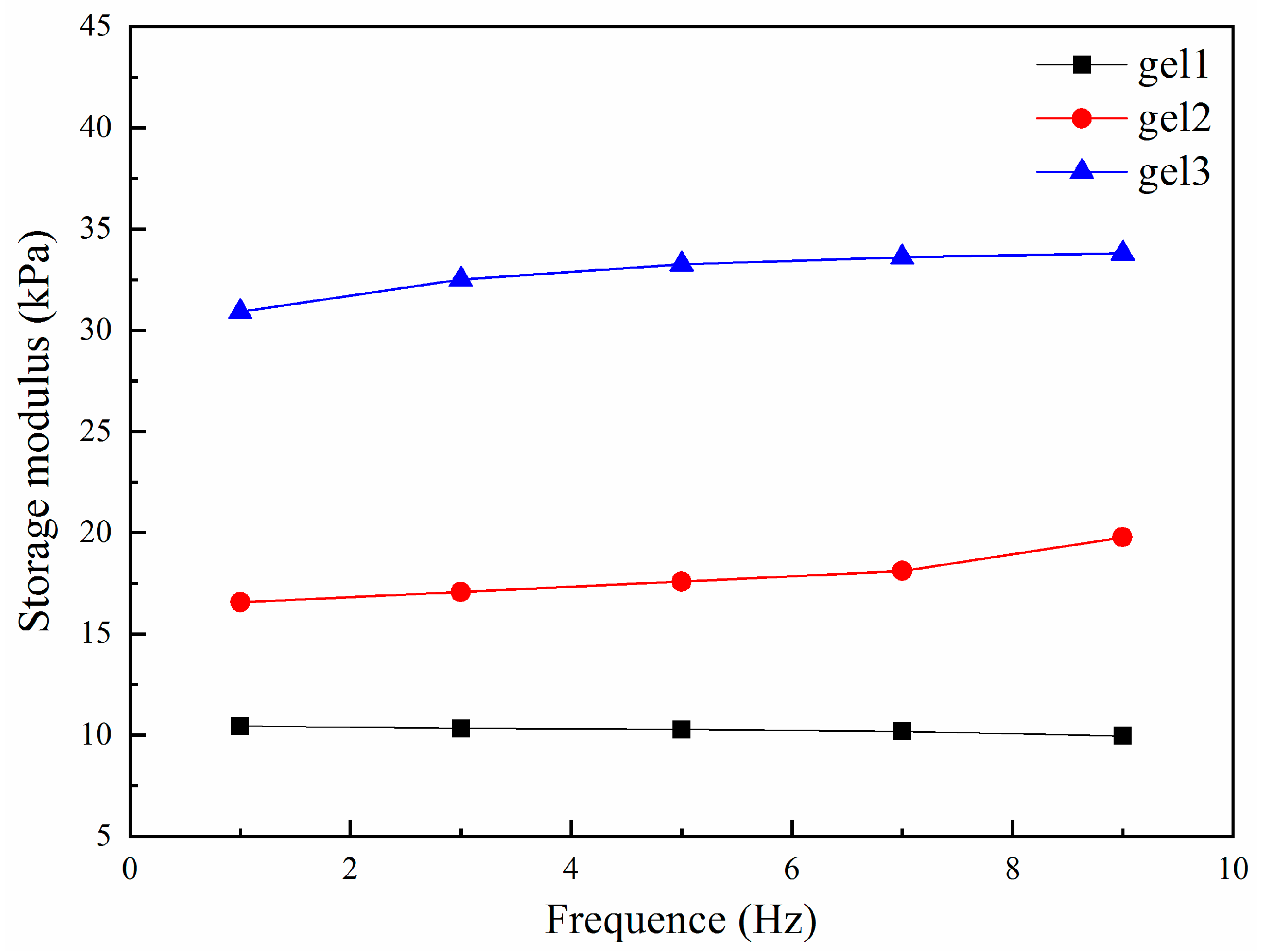
2.9. Mechanical Properties Tests of Gels
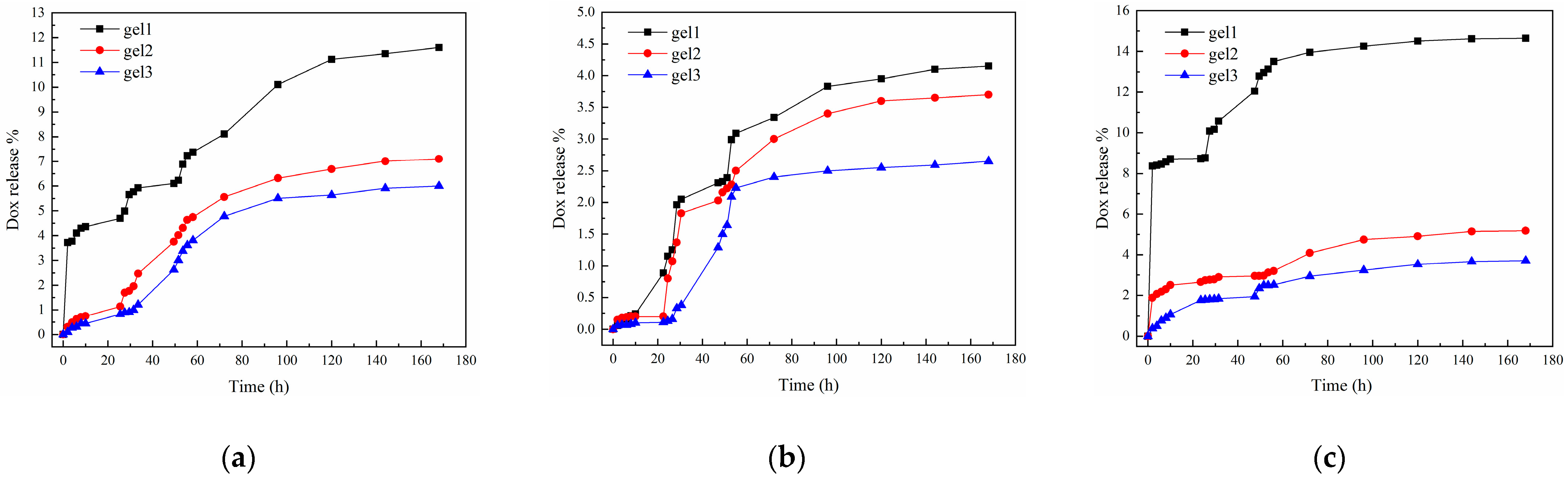
2.10. Sustained Drug Release of the Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.2.1. Synthesis of the Steric Complex HEMA-(PLLA-PDLA)n
4.2.2. Synthesis of the Azide Polymer HEMA-(PLLA-PDLA)-N3
4.2.3. Synthesis of the Alkynylated Polymers HEMA-(PLLA-PDLA)-Alkyne
4.2.4. Synthesis of Physically Crosslinked Hydrogels
4.2.5. Synthesis of Physico-Chemical Double-Crosslinked Hydrogels
4.2.6. Synthesis of Physicochemical Click Triple-Crosslinked Hydrogels
4.3. Structural Characterizations
4.3.1. Nuclear Magnetic Resonance Hydrogen Spectrum (1H NMR)
4.3.2. Infrared Spectrum (FT-IR)
4.3.3. X-ray Diffraction Curves (XRD)
4.4. Character Determination
4.4.1. Swelling Test of Gels
4.4.2. Reversibility Testing of Gels
4.4.3. Amphiphilicity Testing of Gels
4.4.4. Thermal Stability Testing of Gels
4.4.5. Scanning Electron Microscopy (SEM) Analysis of Gels
4.4.6. Mechanical Properties Testing of Gels
4.4.7. Drug-Carrying Capacity Testing of Gels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, E.K.; Yong, S.H.; Lee, E.H.; Kim, E.Y.; Chang, Y.S.; Lee, S.H. New Targeted Therapy for Non-Small Cell Lung Cancer. Tuberc. Respir. Dis. 2023, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Greenwalt, I.; Zaza, N.; Das, S.; Li, B.D. Precision Medicine and Targeted Therapies in Breast Cancer. Surg. Oncol. Clin. N. Am. 2020, 29, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hindy, J.R.; Souaid, T.; Kourie, H.R.; Kattan, J. Targeted therapies in urothelial bladder cancer: A disappointing past preceding a bright future? Future Oncol. 2019, 15, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Wu, L.C.; Bai, X.; Xie, Y.; Wang, A.Q.; Zhang, H.H.; Yang, X.B.; Wan, X.S.; Lu, X.; Sang, X.T.; et al. Combination treatment including targeted therapy for advanced hepatocellular carcinoma. Oncotarget 2016, 7, 71036–71051. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cao, X.N.A.; Ai, B.; Xiao, H.; Huang, Q.F.; Zhang, Z.; Chu, Q.; Zhang, L.; Dai, X.F.; Liao, Y.D. Salvage surgery following downstaging of advanced non-small cell lung cancer by targeted therapy. Thorac. Cancer 2021, 12, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.C.; Dong, X.; Wang, Q.S.; Zhu, D.W.; Mei, L.; Yan, H.S.; Lv, F. A Platelet Intelligent Vehicle with Navigation for Cancer Photothermal-Chemotherapy. ACS Nano 2022, 16, 6359–6371. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Soucy, J.R.; Hebert, J.; Auguste, D.T. Advances in Receptor-Mediated, Tumor-Targeted Drug Delivery. Adv. Ther. 2019, 2, 1800091. [Google Scholar] [CrossRef] [PubMed]
- Madjd, Z.; Gheytanchi, E.; Erfani, E.; Asadi-Lari, M. Application of Stem Cells in Targeted Therapy of Breast Cancer: A Systematic Review. Asian Pac. J. Cancer Prev. 2013, 14, 2789–2800. [Google Scholar] [CrossRef]
- Roth, J.C.; Curiel, D.T.; Pereboeva, L. Cell vehicle targeting strategies. Gene Ther. 2008, 15, 716–729. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.M.; Kim, K.S.; Jung, W.; Kim, D.E. Applications of Cancer Cell-Specific Aptamers in Targeted Delivery of Anticancer Therapeutic Agents. Molecules 2018, 23, 830. [Google Scholar] [CrossRef]
- Gao, Z.B.; Zhang, L.N.; Hu, J.; Sun, Y.J. Mesenchymal stem cells: A potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine 2013, 9, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, J.; Zhao, Y.Y.; Jin, F.; Dong, X.Z.; Zhao, Z.S.; Duan, X.M.; Zheng, M.L. Biocompatible Three-Dimensional Hydrogel Cell Scaffold Fabricated by Sodium Hyaluronate and Chitosan Assisted Two-Photon Polymerization. ACS Appl. Bio Mater. 2019, 2, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, Z.H.; Guan, G.T.; Lu, Z.J.; Yan, S.J.; Du, A.Z.; Wang, L.X.; Li, Q. Polyethylene glycol diacrylate scaffold filled with cell-laden methacrylamide gelatin/alginate hydrogels used for cartilage repair. J. Biomater. Appl. 2022, 36, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Han, H.D.; Nam, D.E.; Seo, D.H.; Kim, T.W.; Shin, B.C.; Choi, H.S. Preparation and biodegradation of thermosensitive chitosan hydrogel as a function of pH and temperature. Macromol. Res. 2004, 12, 507–511. [Google Scholar] [CrossRef]
- Hu, S.; Zhi, Y.F.; Shan, S.Y.; Ni, Y.H. Research progress of smart response composite hydrogels based on nanocellulose. Carbohydr. Polym. 2022, 275, 118741. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Wang, Y.; Li, Q.; Yu, C.; Chu, W.L. Natural Polymer-based Stimuli-responsive Hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Chen, L.; McClements, D.J.; Qiu, C.; Li, C.C.; Zhang, Z.P.; Miao, M.; Tian, Y.Q.; Zhu, K.F.; Jin, Z.Y. Stimulus-responsive hydrogels in food science: A review. Food Hydrocoll. 2022, 124, 107218. [Google Scholar] [CrossRef]
- Yu, Z.K.; Yang, Y.Y.; Shen, C.; Mao, L.H.; Cui, C.C.; Chen, Z.X.; Zhang, Y.H. Thermochromic hydrogels with an adjustable critical response temperature for temperature monitoring and smart windows. J. Mater. Chem. C 2023, 11, 583–592. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Fang, Z.Z.; Hu, Z.R.; Wei, X.T.; Cao, Y.; Han, J.; Li, Y.C. Temperature-Stress Bimodal Sensing Conductive Hydrogel-Liquid Metal by Facile Synthesis for Smart Wearable Sensor. Macromol. Rapid Commun. 2022, 43, 2100543. [Google Scholar] [CrossRef]
- Ma, J.H.; Xu, Y.J.; Zhang, Q.S.; Zha, L.S.; Liang, B.R. Preparation and characterization of pH- and temperature-responsive semi-IPN hydrogels of carboxymethyl chitosan with poly (N-isopropyl acrylamide) crosslinked by clay. Colloid Polym. Sci. 2007, 285, 479–484. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y. Recent advances in multi-temperature-responsive polymeric materials. Polym. J. 2020, 52, 681–689. [Google Scholar] [CrossRef]
- Mun, G.A.; Nurkeeva, Z.S.; Khutoryanskiy, V.V.; Sergaziyev, A.D.; Rosiak, J.M. Radiation synthesis of temperature-responsive hydrogels by copolymerization of 2-(methacryloyloxy)ethyl trimethylammonium chloride with N-isopropylacrylamide. Radiat. Phys. Chem. 2002, 65, 67–70. [Google Scholar] [CrossRef]
- Yao, S.F.; Xiang, L.; Wang, L.Y.; Gong, H.; Chen, F.; Cai, C.Q. pH-responsive DNA hydrogels with ratiometric fluorescence for accurate detection of miRNA-21. Anal. Chim. Acta 2022, 1207, 339795. [Google Scholar] [CrossRef] [PubMed]
- Avais, M.; Chattopadhyay, S. Waterborne pH responsive hydrogels: Synthesis, characterization and selective pH responsive behavior around physiological pH. Polymer 2019, 180, 121701. [Google Scholar] [CrossRef]
- Contin, A.; Frasca, S.; Vivekananthan, J.; Leimkühler, S.; Wollenberger, U.; Plumeré, N.; Schuhmann, W. A pH Responsive Redox Hydrogel for Electrochemical Detection of Redox Silent Biocatalytic Processes. Control of Hydrogel Solvation. Electroanalysis 2015, 27, 938–944. [Google Scholar] [CrossRef]
- Prévoteau, A.; Courjean, O.; Mano, N. Deglycosylation of glucose oxidase to improve biosensors and biofuel cells. Electrochem. Commun. 2010, 12, 213–215. [Google Scholar] [CrossRef]
- Han, W.W.; Liu, Z.X.; Wang, S.C.; Ji, Y.; Zhang, X.L. Construction of a Novel Photoresponsive Supramolecular Fluorescent Hydrogel through Host-Guest Interaction between β-Cyclodextrin and Azobenzene. ChemistrySelect 2020, 5, 2300–2305. [Google Scholar] [CrossRef]
- Sumaru, K.; Ohi, K.; Takagi, T.; Kanamori, T.; Shinbo, T. Photoresponsive properties of poly(N-isopropylacrylamide) hydrogel partly modified with spirobenzopyran. Langmuir 2006, 22, 4353–4356. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Song, J.Y. Electroresponsive behavior of 2-hydroxypropyltrimethyl ammonium chloride chitosan/poly(vinyl alcohol) interpenetrating polymer network hydrogel. Polym. Int. 2012, 61, 596–601. [Google Scholar] [CrossRef]
- Tai, Z.X.; Yang, J.; Qi, Y.Y.; Yan, X.B.; Xue, Q.J. Synthesis of a graphene oxide-polyacrylic acid nanocomposite hydrogel and its swelling and electroresponsive properties. RSC Adv. 2013, 3, 12751–12757. [Google Scholar] [CrossRef]
- Wang, W.T.; Fan, X.Q.; Li, F.H.; Qiu, J.J.; Umair, M.M.; Ren, W.C.; Ju, B.Z.; Zhang, S.F.; Tang, B.T. Magnetochromic Photonic Hydrogel for an Alternating Magnetic Field-Responsive Color Display. Adv. Funct. Mater. 2018, 6, 1701093. [Google Scholar] [CrossRef]
- de Cogan, F.; Booth, A.; Gough, J.E.; Webb, S.J. Spatially controlled apoptosis induced by released nickel(II) within a magnetically responsive nanostructured biomaterial. Soft Matter 2013, 9, 2245–2253. [Google Scholar] [CrossRef]
- Zhi, H.; Gao, J.M.; Feng, L. Hydrogel-Based Gas Sensors for NO2 and NH3. ACS Sens. 2020, 5, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Coukouma, A.E.; Smith, N.L.; Asher, S.A. Removable interpenetrating network enables highly-responsive 2-D photonic crystal hydrogel sensors. Analyst 2015, 140, 6517–6521. [Google Scholar] [CrossRef]
- Guo, H.S.; Bai, M.; Zhu, Y.N.; Liu, X.M.; Tian, S.; Long, Y.; Ma, Y.M.; Wen, C.Y.; Li, Q.S.; Yang, J.; et al. Pro-Healing Zwitterionic Skin Sensor Enables Multi-Indicator Distinction and Continuous Real-Time Monitoring. Adv. Funct. Mater. 2021, 31, 2106406. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-stimuli-responsive hydrogels and their medical applications. New J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Liu, G.; Gu, Z.; Ma, Y.; Xue, H. Swelling characterization of poly (vinyl alcohol) hydrogel for prosthetic intervertebral disc nucleus. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2005, 22, 995–998. [Google Scholar] [PubMed]
- Negrini, R.; Fong, W.K.; Boyd, B.J.; Mezzenga, R. pH-responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chem. Commun. 2015, 51, 6671–6674. [Google Scholar] [CrossRef]
- Ingraham, H.A.; Alhadeff, J.A. Characterization of sialytransferase in noncancerous and neoplastic human liver tissue. J. Natl. Cancer Inst. 1978, 61, 1371–1374. [Google Scholar]
- Li, Z.C.; Xia, L.; Li, G.K.; Hu, Y.L. Raman spectroscopic imaging of pH values in cancerous tissue by using polyaniline@gold nanoparticles. Microchim. Acta 2019, 186, 162. [Google Scholar] [CrossRef]
- Mozafari, Z.; Massoumi, B.; Jaymand, M. A Novel Stimuli-Responsive Magnetite Nanocomposite as De Novo Drug Delivery System. Polym. Plast. Technol. Mater. 2019, 58, 405–418. [Google Scholar] [CrossRef]
- Gao, W.W.; Zhang, Y.; Zhang, Q.Z.; Zhang, L.F. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef]
- Xiao, Y.; Gu, Y.H.; Qin, L.; Chen, L.; Chen, X.L.; Cui, W.H.; Li, F.L.; Xiang, N.; He, X.A. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B. Biointerfaces 2021, 200, 111581. [Google Scholar] [CrossRef]
- Li, Z.Q.; Guan, J.J. Thermosensitive hydrogels for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 991–1007. [Google Scholar] [CrossRef]
- Hu, X.H.; Qiu, J.; Tan, H.P.; Li, D.; Ma, X.H. Synthesis and Characterization of Cyclodextrin-containing Hydrogel for Ophthalmic Drugs Delivery. J. Macromol. Sci. Part A 2013, 50, 983–990. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Han, B.H. Supramolecular Hydrogel Based on Graphene Oxides for Controlled Release System. J. Nanosci. Nanotechnol. 2013, 13, 755–760. [Google Scholar] [CrossRef]
- Wu, Y.P.; Tang, Z.Y.; Ma, S.; Du, L.A. The promising application of hydrogel microneedles in medical application. J. Pharm. Pharmacol. 2023, 75, 1011–1020. [Google Scholar] [CrossRef]
- Qiu, H.P.; Guo, H.; Li, D.; Hou, Y.C.; Kuang, T.R.; Ding, J.X. Intravesical Hydrogels as Drug Reservoirs. Trends Biotechnol. 2020, 38, 579–583. [Google Scholar] [CrossRef]
- Kunwar, P.; Jannini, A.V.S.; Long, Z.; Ransbottom, M.J.; Perkins, J.S.; Henderson, J.H.; Hasenwinkel, J.M.; Soman, P. High-Resolution 3D Printing of Stretchable Hydrogel Structures Using Optical Projection Lithography. ACS Appl. Mater. Interfaces 2020, 12, 1640–1649. [Google Scholar] [CrossRef]
- Wang, C.F.; Wu, J.C.; Li, Q.X. Novel luminescent hierarchical porous hydrogels with a three-dimensional interconnected network structure from feather keratin crosslinking reaction. New J. Chem. 2023, 47, 15440–15444. [Google Scholar] [CrossRef]
- Hou, Y.S.; Ma, S.H.; Hao, J.L.; Lin, C.C.; Zhao, J.W.; Sui, X. Construction and Ion Transport-Related Applications of the Hydrogel-Based Membrane with 3D Nanochannels. Polymers 2022, 14, 4037. [Google Scholar] [CrossRef]
- Yu, H.Q.; Feng, Z.G.; Zhang, A.Y.; Sun, L.G.; Qian, L.J. Synthesis and characterization of three-dimensional crosslinked networks based on self-assemly of α-cyclodextrins with thiolated 4-arm PEG using a three-step oxidation. Soft Matter 2006, 2, 343–349. [Google Scholar] [CrossRef]
- Raman, T.S.; Kuehnert, M.; Daikos, O.; Scherzer, T.; Krömmelbein, C.; Mayr, S.G.; Abel, B.; Schulze, A. A study on the material properties of novel PEGDA/gelatin hybrid hydrogels polymerized by electron beam irradiation. Front. Chem. 2023, 10, 1094981. [Google Scholar]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Chen, J.W.; Yang, Z.K.; Shi, D.J.; Zhou, T.Y.; Kaneko, D.; Chen, M.Q. High strength and toughness of double physically cross-linked hydrogels composed of polyvinyl alcohol and calcium alginate. J. Appl. Polym. Sci. 2021, 138, e49987. [Google Scholar] [CrossRef]
- Lin, H.; Yin, C.L.; Mo, A.C.; Hong, G. Applications of Hydrogel with Special Physical Properties in Bone and Cartilage Regeneration. Materials 2021, 14, 235. [Google Scholar] [CrossRef]
- Zhao, F.; Qin, X.P.; Feng, S.Y. Microstructure, mechanical and swelling properties of microgel composite hydrogels with high microgel content and a microgel cluster crosslinker. RSC Adv. 2015, 5, 45113–45121. [Google Scholar] [CrossRef]
- Bian, S.Q.; Hao, L.Z.; Qiu, X.; Wu, J.; Chang, H.; Kuang, G.M.; Zhang, S.; Hu, X.H.; Dai, Y.K.; Zhou, Z.Y.; et al. An Injectable Rapid-Adhesion and Anti-Swelling Adhesive Hydrogel for Hemostasis and Wound Sealing. Adv. Funct. Mater. 2022, 32, 2207741. [Google Scholar] [CrossRef]
- Dou, X.Y.; Wang, H.F.; Yang, F.; Shen, H.; Wang, X.; Wu, D.C. One-Step Soaking Strategy toward Anti-Swelling Hydrogels with a Stiff “Armor”. Adv. Sci. 2023, 10, 2206242. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Dai, Y.Y.; Wang, Q.; Xiang, P.; Li, Y.H.; Gao, G.H. Amylopectin based hydrogel strain sensor with good biocompatibility, high toughness and stable anti-swelling in multiple liquid media. Eur. Polym. J. 2022, 164, 110981. [Google Scholar] [CrossRef]
- Zheng, D.Y.; Zhang, C.Y.; Chen, Z.W.; Zhu, P.Z.; Gao, C.X. Tough and anti-swelling γ-polyglutamic acid/polyvinyl alcohol hydrogels for wearable sensors. J. Appl. Polym. Sci. 2023, 140, e53792. [Google Scholar] [CrossRef]
- Zhan, Y.W.; Fu, W.J.; Xing, Y.C.; Ma, X.M.; Chen, C.Y. Advances in versatile anti-swelling polymer hydrogels. Mater. Sci. Eng. C 2021, 127, 112208. [Google Scholar] [CrossRef]
- Wu, J.; Shi, X.Y.; Wang, Z.D.; Song, F.; Gao, W.; Liu, S.X. Stereocomplex Poly(Lactic Acid) Amphiphilic Conetwork Gel with Temperature and pH Dual Sensitivity. Polymers 2019, 11, 1940. [Google Scholar] [CrossRef]
- Yang, W.Y.; Wang, J.Q.; Jia, L.J.; Li, J.Y.; Liu, S.X. Stereo-Complex and Click-Chemical Bicrosslinked Amphiphilic Network Gels with Temperature/pH Response. Gels 2023, 9, 647. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, J.C.; Zhong, Y.; Li, K.; Zhang, L.N.; Cai, J. High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Rebers, L.; Reichsöllner, R.; Regett, S.; Tovar, G.E.M.; Borchers, K.; Baudis, S.; Southan, A. Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Sci. Rep. 2021, 11, 3256. [Google Scholar] [CrossRef]
- Bonami, L.; Van Camp, W.; Van Rijckegem, D.; Du Prez, F.E. Facile Access to an Efficient Solid-Supported Click Catalyst System Based on Poly(ethyleneimine). Macromol. Rapid Commun. 2009, 30, 34–38. [Google Scholar] [CrossRef]
- Tan, B.H.; Hussain, H.; Lin, T.T.; Chua, Y.C.; Leong, Y.W.; Tjiu, W.W.; Wong, P.K.; He, C.B. Stable Dispersions of Hybrid Nanoparticles Induced by Stereocomplexation between Enantiomeric Poly(lactide) Star Polymers. Langmuir 2011, 27, 10538–10547. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Sang, Z.H.; Huang, Y.F.; Ru, J.F.; Zhong, G.J.; Ji, X.; Wang, R.Y.; Li, Z.M. Enhanced Heat Deflection Resistance via Shear Flow-Induced Stereocomplex Crystallization of Polylactide Systems. ACS Sustain. Chem. Eng. 2017, 5, 1692–1703. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.M.; Yang, F. The effects of varying poly(ethylene glycol) hydrogel crosslinking density and the crosslinking mechanism on protein accumulation in three-dimensional hydrogels. Acta Biomater. 2014, 10, 4167–4174. [Google Scholar] [CrossRef]
- Manganiello, M.J.; Cheng, C.; Convertine, A.J.; Bryers, J.D.; Stayton, P.S. Diblock copolymers with tunable pH transitions for gene delivery. Biomaterials 2012, 33, 2301–2309. [Google Scholar] [CrossRef]
- Fan, X.S.; Wang, M.A.; Yuan, D.; He, C.B. Amphiphilic Conetworks and Gels Physically Cross-Linked via Stereocomplexation of Polylactide. Langmuir 2013, 29, 14307–14313. [Google Scholar] [CrossRef]
- Dabbaghi, A.; Rahmani, S. Synthesis and characterization of biodegradable multicomponent amphiphilic conetworks with tunable swelling through combination of ring-opening polymerization and “click” chemistry method as a controlled release formulation for 2,4-dichlorophenoxyacetic acid herbicide. Polym. Adv. Technol. 2019, 30, 368–380. [Google Scholar]
- Hao, Y.P.; Chen, J.; Wang, F.; Liu, Y.; Ai, X.; Tian, H.C. Influence of Crosslinking on Rheological Properties, Crystallization Behavior and Thermal Stability of Poly(lactic acid). Fibers Polym. 2022, 23, 1763–1769. [Google Scholar] [CrossRef]
- Xiong, S.Y.; Sun, W.; Chen, R.; Yuan, Z.Q.; Cheng, X.J. Fluorescent dialdehyde-BODIPY chitosan hydrogel and its highly sensing ability to Cu2+ ion. Carbohydr. Polym. 2021, 273, 118590. [Google Scholar] [CrossRef]
- Xu, J.W.; Wang, Q.; Yu, J.Y.; Yan, S.Z.; Qi, B.K. Effect of Fe(III) on soybean protein-gallic acid hydrogel: Structure, functional properties, and digestive properties. Food Hydrocoll. 2024, 156, 110309. [Google Scholar] [CrossRef]
- Monteiro, G.A.A.; de Sousa, R.G.; da Silva, W.M.; Gastelois, P.L.; Macedo, W.A.D.; de Sousa, E.M.B. Microwave radiation-assisted covalent functionalization of boron nitride nanotubes and their grafting with cationic thermo and pH-sensitive hydrogel. Appl. Nanosci. 2021, 11, 505–520. [Google Scholar] [CrossRef]
- Jafari, M.; Hasanzadeh, M. Cell-specific frequency as a new hallmark to early detection of cancer and efficient therapy: Recording of cancer voice as a new horizon. Biomed. Pharmacother. 2020, 122, 109770. [Google Scholar] [CrossRef]
- Guo, W.X.; Long, Z.J.; Zhang, Z.; Hu, L.X. Antitumor efficacy of poly(brassylic acid-pentadecandioic acid) copolymer. Mat. Sci. Eng. C 2007, 27, 51–56. [Google Scholar] [CrossRef]








| Samples | HEMA-(PLLA-PDLA) (mmol) | EGDMA (%) | HEMA-(PLLA-PDLA)-Alkyne (w/g) | HEMA-(PLLA-PDLA)-N3 (w/g) | Crosslinking Types |
|---|---|---|---|---|---|
| gel1 | 0.2 | 0 | 0 | 0 | Physical crosslinking |
| gel2 | 0.2 | 0.6 | 0 | 0 | Physical-chemical dual crosslinking |
| gel3 | 0 | 0.6 | 0.2 | 0.16 | Physical-chemical-clicking triple crosslinking |
| Time (h) | 0 | 0.5 | 1 | 1.5 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | ||||||||||||
| gel1 | 0 | 16.94 | 31.07 | 39.88 | 47.39 | 57 | 61.1 | 63.12 | 64.42 | 61.4 | 60.94 | |
| gel2 | 0 | 4.99 | 5.38 | 5.42 | 5.41 | 5.56 | 5.53 | 5.56 | 5.59 | 5.4 | 5 | |
| gel3 | 0 | 1.99 | 2.65 | 2.98 | 3.15 | 3.29 | 3.32 | 3.32 | 3.18 | 3.16 | 3.14 | |
| Time (h) | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | |||||||||||||
| gel1 | 0 | 14.06 | 26.44 | 40.52 | 46.56 | 51.07 | 54.81 | 56.89 | 60.54 | 61.59 | 62.23 | 63.14 | |
| gel2 | 0 | 1.82 | 2.37 | 2.69 | 2.91 | 3.06 | 3.15 | 3.34 | 3.47 | 3.54 | 3.66 | 3.71 | |
| gel3 | 0 | 0.48 | 0.56 | 0.65 | 0.71 | 0.81 | 0.85 | 0.96 | 1.07 | 1.16 | 1.09 | 1.20 | |
| Frequence (Hz) | 1 | 3 | 5 | 7 | 9 | |
|---|---|---|---|---|---|---|
| Samples | ||||||
| gel1 | 10.44 | 10.33 | 10.28 | 10.19 | 9.96 | |
| gel2 | 16.57 | 17.07 | 17.58 | 18.11 | 19.77 | |
| gel3 | 30.92 | 32.52 | 33.26 | 33.61 | 33.81 | |
| Reaction Conditions | pH = 5.3, 37 °C | pH = 7.4, 37 °C | pH = 7.4, 40 °C | |
|---|---|---|---|---|
| Samples | ||||
| gel1 | 11.60 | 4.15 | 14.65 | |
| gel2 | 7.10 | 3.70 | 5.18 | |
| gel3 | 6.00 | 2.65 | 3.71 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, W.; Li, Y.; Ma, X.; Xie, Y.; Zhou, G.; Liu, S. Dual-Temperature/pH-Sensitive Hydrogels with Excellent Strength and Toughness Crosslinked Using Three Crosslinking Methods. Gels 2024, 10, 480. https://doi.org/10.3390/gels10070480
Wang J, Yang W, Li Y, Ma X, Xie Y, Zhou G, Liu S. Dual-Temperature/pH-Sensitive Hydrogels with Excellent Strength and Toughness Crosslinked Using Three Crosslinking Methods. Gels. 2024; 10(7):480. https://doi.org/10.3390/gels10070480
Chicago/Turabian StyleWang, Jiaqi, Wanying Yang, Yutong Li, Xuerong Ma, Yuxin Xie, Guangyan Zhou, and Shouxin Liu. 2024. "Dual-Temperature/pH-Sensitive Hydrogels with Excellent Strength and Toughness Crosslinked Using Three Crosslinking Methods" Gels 10, no. 7: 480. https://doi.org/10.3390/gels10070480
APA StyleWang, J., Yang, W., Li, Y., Ma, X., Xie, Y., Zhou, G., & Liu, S. (2024). Dual-Temperature/pH-Sensitive Hydrogels with Excellent Strength and Toughness Crosslinked Using Three Crosslinking Methods. Gels, 10(7), 480. https://doi.org/10.3390/gels10070480






