Optimized Adipogenic Differentiation and Delivery of Bovine Umbilical Cord Stem Cells for Cultivated Meat
Abstract
:1. Introduction
2. Results and Discussion
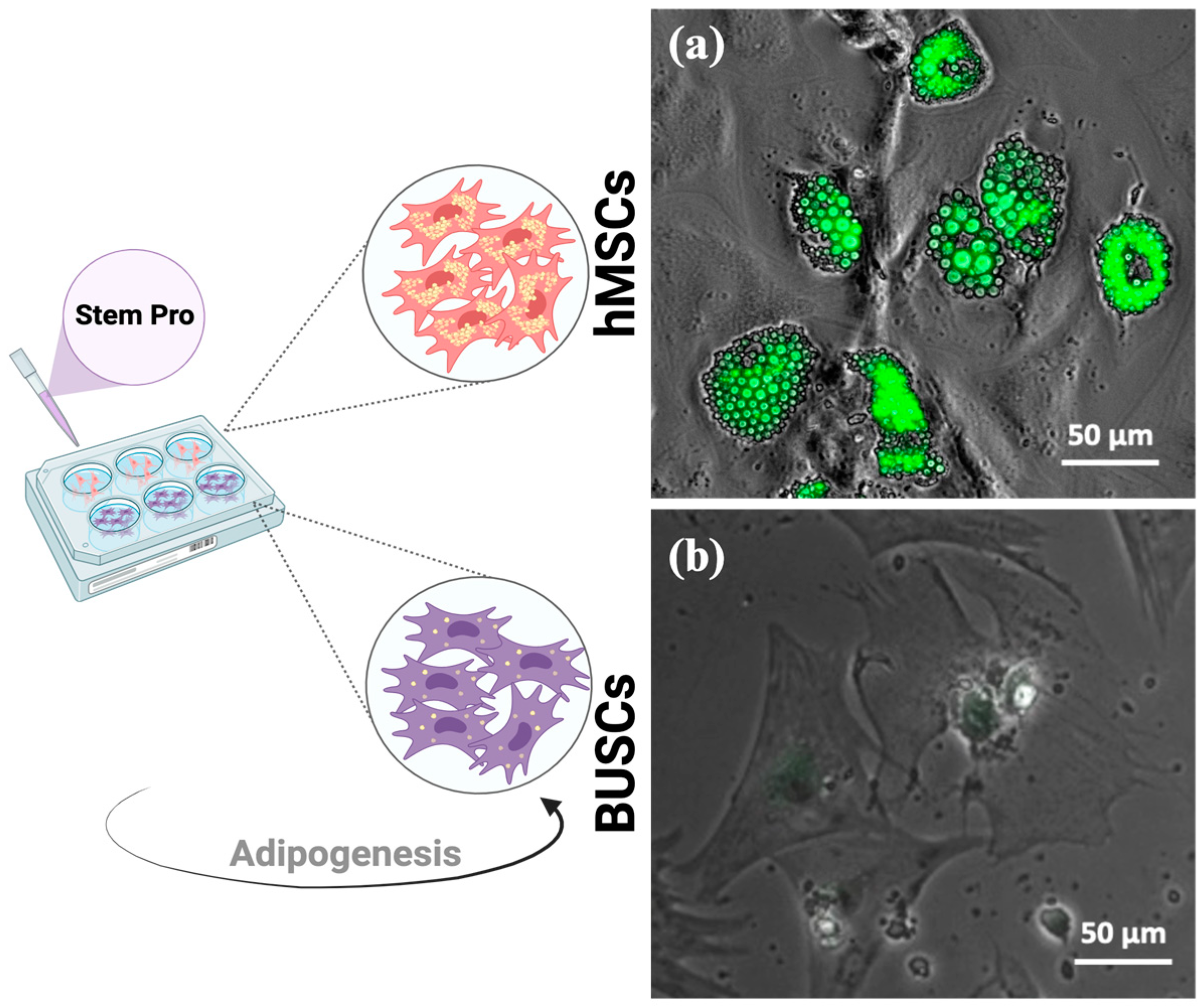
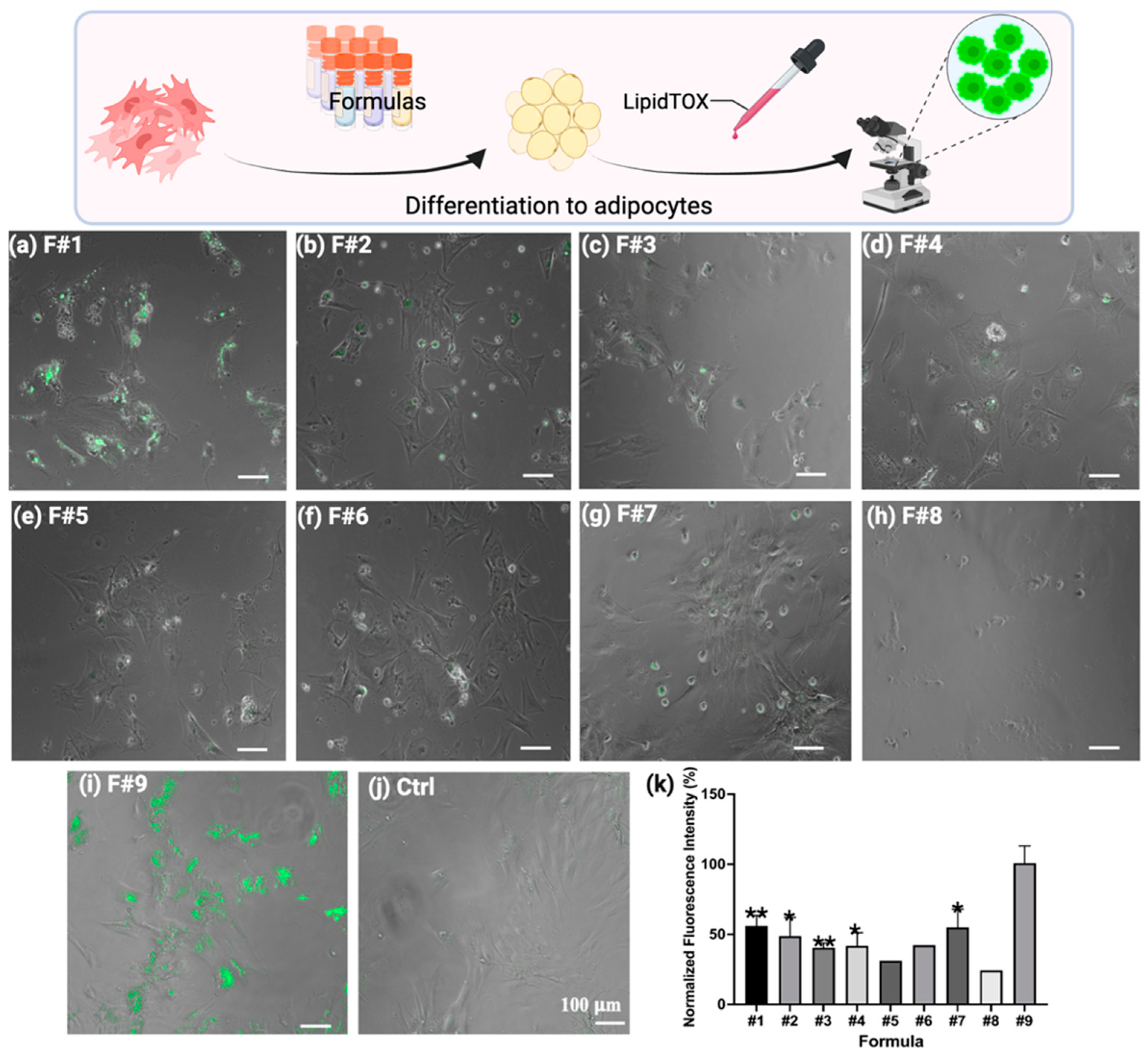
2.1. Determination of an Adipogenic Differentiation Cocktail for BUSCs
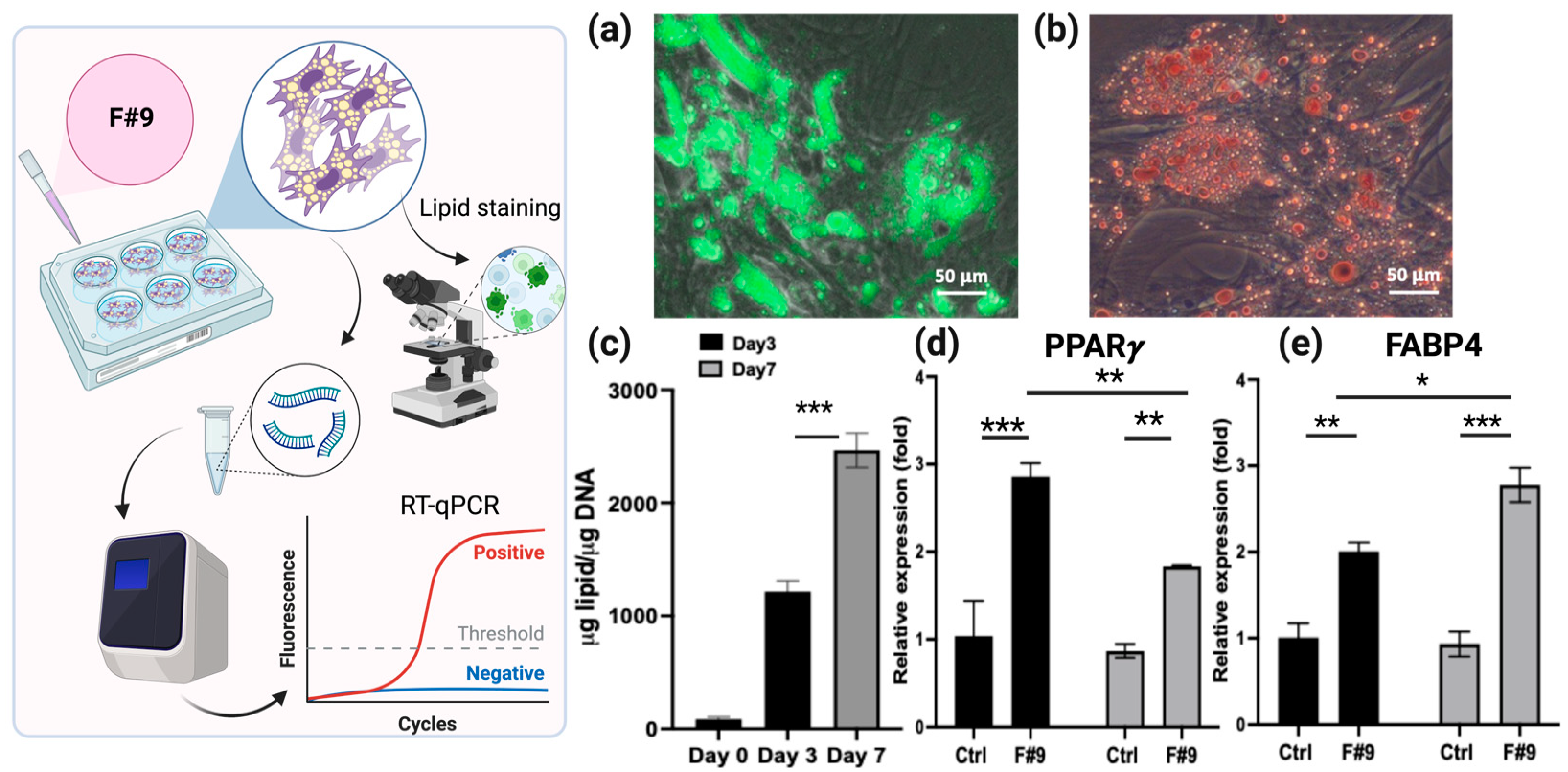
2.2. Determination of Optimized F#9 Concentration for BUSC Adipogenesis
2.3. Evaluation of Pretreatment Effects of Different Adipogenic Inducers on the Adipogenic Differentiation of BUSCs Induced by F#9
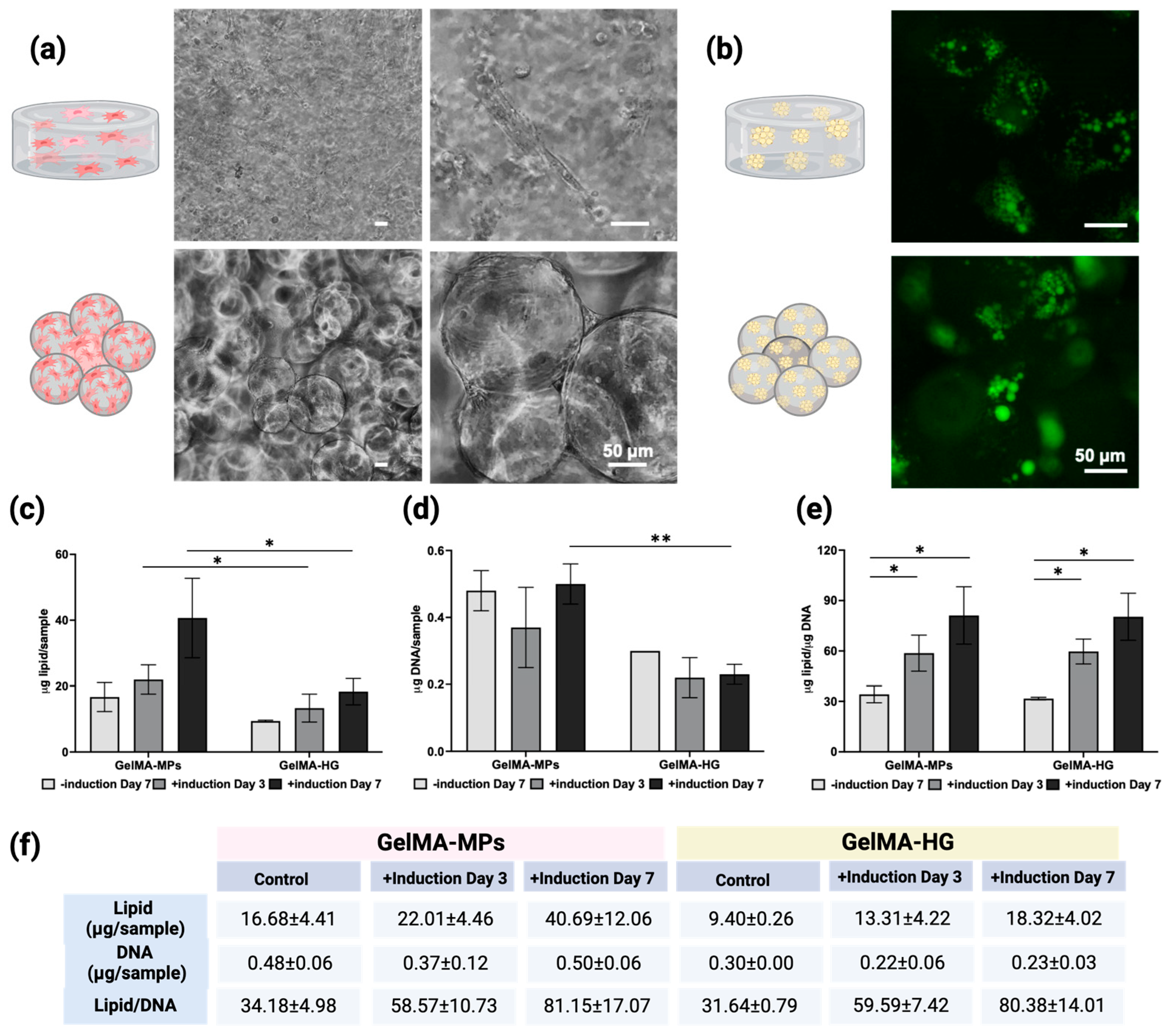
2.4. Delivery of BUSCs on GelMA Microspheres or in GelMA Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Isolation of Bovine Umbilical Cord-Derived Stem Cells
4.2. The Effect of a Commercial Adipogenesis Differentiation Kit on the Adipogenesis of BUSCs
4.3. Different Cocktails for Adipogenic Differentiation of BUSCs
4.4. Quantitative PCR (qPCR) Analyses
4.5. Determination of Optimal F#9 Concentration for the Adipogenesis of BUSCs
4.6. Pretreatment Effect of Different Adipogenic Inducers on the Adipogenic Differentiation of BUSCs in F#9
4.7. Procedure for Gelatin Methacrylation
4.8. Preparation of GelMA Hydrogel Molds and Seeding of BUSCs in GelMA Hydrogels
4.9. Synthesis of GelMA Hydrogel Microparticles (GelMA-MPs) and Seeding of BUSCs
4.10. Adipogenic Differentiation of BUSCs on GelMA-MPs or in GelMA-HG
4.11. Fluorometric Neutral Lipid Quantification Assay
4.12. DNA Quantification
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramirez-Espinosa, J.J.; Gonzalez-Davalos, L.; Shimada, A.; Pina, E.; Varela-Echavarria, A.; Mora, O. Bovine (Bos taurus) Bone Marrow Mesenchymal Cell Differentiation to Adipogenic and Myogenic Lineages. Cells Tissues Organs 2016, 201, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yin, J.; Zhu, M.J. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Sci. 2010, 86, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhu, M.J.; Dodson, M.V.; Du, M. Developmental programming of fetal skeletal muscle and adipose tissue development. J. Genom. 2013, 1, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed]
- Okamura, L.H.; Cordero, P.; Palomino, J.; Parraguez, V.H.; Torres, C.G.; Peralta, O.A. Myogenic Differentiation Potential of Mesenchymal Stem Cells Derived from Fetal Bovine Bone Marrow. Anim. Biotechnol. 2018, 29, 1–11. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Levenberg, S. Tissue Engineering for Clean Meat Production. Front. Sustain. Food Syst. 2019, 3, 46. [Google Scholar] [CrossRef]
- Ozhava, D.; Bhatia, M.; Freman, J.; Mao, Y. Sustainable Cell Sources for Cultivated Meat. J. Biomed. Res. Environ. Sci. 2022, 3, 1382–1388. [Google Scholar] [CrossRef]
- Fish, K.D.; Rubio, N.R.; Stout, A.J.; Yuen, J.S.K.; Kaplan, D.L. Prospects and challenges for cell-cultured fat as a novel food ingredient. Trends Food Sci. Technol. 2020, 98, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.T.; Singh, S.; Yap, W.S.; Tay, S.H.; Choudhury, D. Cultured meat—A patentometric analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 2738–2748. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhao, X.R.; Li, X.L.; Du, G.C.; Zhou, J.W.; Chen, J. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020, 97, 443–450. [Google Scholar] [CrossRef]
- Sugii, S.; Wong, C.Y.Q.; Lwin, A.K.O.; Chew, L.J.M. Reassessment of adipocyte technology for cellular agriculture of alternative fat. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4146–4163. [Google Scholar] [CrossRef] [PubMed]
- Fraeye, I.; Kratka, M.; Vandenburgh, H.; Thorrez, L. Sensorial and Nutritional Aspects of Cultured Meat in Comparison to Traditional Meat: Much to Be Inferred. Front. Nutr. 2020, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Janderová, L.; McNeil, M.; Murrell, A.N.; Mynatt, R.L.; Smith, S.R. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes. Res. 2003, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ailhaud, G. Adipose cell differentiation in culture. Mol. Cell. Biochem. 1982, 49, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wei, Y.; Chen, N.; Jiang, M.; Wu, J.; Liao, K. DNA synthesis and mitotic clonal expansion is not a required step for 3T3-L1 preadipocyte differentiation into adipocytes. J. Biol. Chem. 2001, 276, 11988–11995. [Google Scholar] [CrossRef] [PubMed]
- Salasznyk, R.M.; Klees, R.F.; Westcott, A.M.; Vandenberg, S.; Bennett, K.; Plopper, G.E. Focusing of gene expression as the basis of stem cell differentiation. Stem Cells Dev. 2005, 14, 608–620. [Google Scholar] [CrossRef]
- Klees, R.F.; Salasznyk, R.M.; Vandenberg, S.; Bennett, K.; Plopper, G.E. Laminin-5 activates extracellular matrix production and osteogenic gene focusing in human mesenchymal stem cells. Matrix Biol. 2007, 26, 106–114. [Google Scholar] [CrossRef]
- Gurriaran-Rodriguez, U.; Al-Massadi, O.; Roca-Rivada, A.; Crujeiras, A.B.; Gallego, R.; Pardo, M.; Seoane, L.M.; Pazos, Y.; Casanueva, F.F.; Camina, J.P. Obestatin as a regulator of adipocyte metabolism and adipogenesis. J. Cell. Mol. Med. 2011, 15, 1927–1940. [Google Scholar] [CrossRef]
- Mehta, F.; Theunissen, R.; Post, M.J. Adipogenesis from Bovine Precursors. Methods Mol. Biol. 2019, 1889, 111–125. [Google Scholar] [CrossRef]
- Wolff, A.; Frank, M.; Staehlke, S.; Peters, K. A Comparative Study on the Adipogenic Differentiation of Mesenchymal Stem/Stromal Cells in 2D and 3D Culture. Cells 2022, 11, 1313. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Ferreira, L.P.; Gaspar, V.M.; Mano, J.F. Design of spherically structured 3D in vitro tumor models-Advances and prospects. Acta Biomater. 2018, 75, 11–34. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.J.; Jimenez, A.; Yang, J.Z.; Akpek, A.; Liu, X.; Pi, Q.M.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, e1805510. [Google Scholar] [CrossRef]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Siller, I.G.; Epping, N.M.; Lavrentieva, A.; Scheper, T.; Bahnemann, J. Customizable 3D-Printed (Co-)Cultivation Systems for In Vitro Study of Angiogenesis. Materials 2020, 13, 4290. [Google Scholar] [CrossRef] [PubMed]
- De Dios-Figueroa, G.T.; Aguilera-Marquez, J.D.R.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H. 3D Cell Culture Models in COVID-19 Times: A Review of 3D Technologies to Understand and Accelerate Therapeutic Drug Discovery. Biomedicines 2021, 9, 602. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Bektas, C.K.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- Bektas, C.; Mao, Y. Hydrogel Microparticles for Bone Regeneration. Gels 2023, 10, 28. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Yang, X.; Zhang, Y.; Qiu, X. GelMA-based hydrogel biomaterial scaffold: A versatile platform for regenerative endodontics. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35412. [Google Scholar] [CrossRef]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. [Google Scholar] [CrossRef]
- Hackett, C.H.; Fortier, L.A. Embryonic stem cells and iPS cells: Sources and characteristics. Vet. Clin. N. Am. Equine Pract. 2011, 27, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.; Bister, A.; Dohmen, R.G.J.; Gouveia, M.A.; Hueber, R.; Melzener, L.; Messmer, T.; Papadopoulos, J.; Pimenta, J.; Raina, D.; et al. Advances and Challenges in Cell Biology for Cultured Meat. Annu. Rev. Anim. Biosci. 2024, 12, 345–368. [Google Scholar] [CrossRef]
- Xiong, H.; Bai, C.Y.; Wu, S.; Gao, Y.H.; Lu, T.F.; Hu, Q.Y.; Guan, W.J.; Ma, Y.H. Biological characterization of mesenchymal stem cells from bovine umbilical cord. Anim. Cells Syst. 2014, 18, 59–67. [Google Scholar] [CrossRef]
- Lee, K.; Jackson, A.; John, N.; Zhang, R.; Ozhava, D.; Bhatia, M.; Mao, Y. Bovine Fibroblast-Derived Extracellular Matrix Promotes the Growth and Preserves the Stemness of Bovine Stromal Cells during In Vitro Expansion. J. Funct. Biomater. 2023, 14, 218. [Google Scholar] [CrossRef]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kim, K.H. Dexamethasone-induced selenoprotein S degradation is required for adipogenesis. J. Lipid Res. 2013, 54, 2069–2082. [Google Scholar] [CrossRef]
- Kim, S.P.; Ha, J.M.; Yun, S.J.; Kim, E.K.; Chung, S.W.; Hong, K.W.; Kim, C.D.; Bae, S.S. Transcriptional activation of peroxisome proliferator-activated receptor-gamma requires activation of both protein kinase A and Akt during adipocyte differentiation. Biochem. Biophys. Res. Commun. 2010, 399, 55–59. [Google Scholar] [CrossRef]
- Yuan, C.; Chakraborty, S.; Chitta, K.K.; Subramanian, S.; Lim, T.E.; Han, W.; Bhanu Prakash, K.N.; Sugii, S. Fast Adipogenesis Tracking System (FATS)—A robust, high-throughput, automation-ready adipogenesis quantification technique. Stem Cell Res. Ther. 2019, 10, 38. [Google Scholar] [CrossRef]
- Lee, S.H.; Cha, S.H.; Kim, C.L.; Lillehoj, H.S.; Song, J.Y.; Lee, K.W. Enhanced adipogenic differentiation of bovine bone marrow-derived mesenchymal stem cells. J. Appl. Anim. Res. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Thompson, B.R.; Lobo, S.; Bernlohr, D.A. Fatty acid flux in adipocytes: The in’s and out’s of fat cell lipid trafficking. Mol. Cell. Endocrinol. 2010, 318, 24–33. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Regulation of Adipocyte Gene-Expression and Differentiation by Peroxisome Proliferator Activated Receptor-Gamma. Curr. Opin. Genet. Dev. 1995, 5, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Malodobra-Mazur, M.; Cierzniak, A.; Dobosz, T. Oleic acid influences the adipogenesis of 3T3-L1 cells via DNA Methylation and may predispose to obesity and obesity-related disorders. Lipids Health Dis. 2019, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Sugii, S.; Wong, C.Y.Q.; Lwin, A.K.O.; Chew, L.J.M. Alternative fat: Redefining adipocytes for biomanufacturing cultivated meat. Trends Biotechnol. 2023, 41, 686–700. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Tsukerman, A.; Safina, D.; Maor-Shoshani, A.; Lavon, N.; Levenberg, S. Co-culture approaches for cultivated meat production. Nat. Rev. Bioeng. 2023, 1, 817–831. [Google Scholar] [CrossRef]
- Shah, N.; Hallur, P.M.; Ganesh, R.A.; Sonpatki, P.; Naik, D.; Chandrachari, K.P.; Puchalski, R.B.; Chaubey, A. Gelatin methacrylate hydrogels culture model for glioblastoma cells enriches for mesenchymal-like state and models interactions with immune cells. Sci. Rep. 2021, 11, 17727. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Noorani, B.; Xu, C. Effects of Encapsulated Cells on the Physical-Mechanical Properties and Microstructure of Gelatin Methacrylate Hydrogels. Int. J. Mol. Sci. 2019, 20, 5061. [Google Scholar] [CrossRef] [PubMed]
- Kilic Bektas, C.; Zhang, W.; Mao, Y.; Wu, X.; Kohn, J.; Yelick, P.C. Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids. Bioengineering 2022, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Ozhava, D.; Bektas, C.; Lee, K.; Jackson, A.; Mao, Y. Human Mesenchymal Stem Cells on Size-Sorted Gelatin Hydrogel Microparticles Show Enhanced In Vitro Wound Healing Activities. Gels 2024, 10, 97. [Google Scholar] [CrossRef]
- Sebo, Z.L.; Rodeheffer, M.S. Prepubertal androgen signaling is required to establish male fat distribution. Stem Cell Rep. 2022, 17, 1081–1088. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zheng, Y.; VanderGheynst, J.S. Rapid quantitative analysis of lipids using a colorimetric method in a microplate format. Lipids 2011, 46, 95–103. [Google Scholar] [CrossRef]
- Johnson, K.R.; Ellis, G.; Toothill, C. The sulfophosphovanillin reaction for serum lipids: A reappraisal. Clin. Chem. 1977, 23, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.C.; Ferrari, H.F.; Garcia, A.F.; Novais, J.B.; Silva-Frade, C.; Ferrarezi, M.C.; Andrade, A.L.; Gameiro, R. Isolation and characterization of Wharton’s jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnol. 2012, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [PubMed]



| Formula | Medium | Inducers |
|---|---|---|
| #1 | DMEM + 10% FBS | 50 μM Indomethacin 0.5 μM Dexamethasone |
| #2 | F1 | 20 μM Rosiglitazone |
| #3 | F2 | 10 μg/mL Insulin |
| #4 | DMEM + 10% FBS | 0.5 mM IBMX 100 μM Indomethacin 1.0 μM Dexamethasone 20 μM Rosiglitazone |
| #5 | Stem Pro | Commercial |
| #6 | F1 | 10 μg/mL Insulin |
| #7 1 | F4 | DMEM + 10% FBS + 10 μg/mL Insulin |
| #8 | Stem Pro | 0.5 mM IBMX 100 μM Indomethacin 1.0 μM Dexamethasone 20 μM Rosiglitazone 10 μg/mL Insulin |
| #9 | DMEM + 10% FBS | 50 μM Myristoleic Acid 50 μM Pristanic Acid 50 μM Phytanic Acid 50 μM Erucic Acid 50 μM Elaidic Acid 50 μM Oleic acid 50 μM Palmitoleic Acid |
| Ind. | Inducers |
|---|---|
| #1 | 0.5 mM IBMX + 0.5 μM Dexamethasone |
| #2 | 0.5 mM IBMX + 0.5 μM Dexamethasone + 50 μM Indomethacin |
| #3 | 0.5 mM IBMX + 0.5 μM Dexamethasone + 50 μM Indomethacin + 10 μg/mL Insulin |
| #4 | 0.5 mM IBMX + 1.0 μM Dexamethasone + 50 μM Indomethacin |
| #5 | 0.5 mM IBMX + 1.0 μM Dexamethasone + 10 μg/mL Insulin |
| #6 | DMEM + 10%FBS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozhava, D.; Lee, K.; Bektas, C.; Jackson, A.; Patel, K.; Mao, Y. Optimized Adipogenic Differentiation and Delivery of Bovine Umbilical Cord Stem Cells for Cultivated Meat. Gels 2024, 10, 488. https://doi.org/10.3390/gels10080488
Ozhava D, Lee K, Bektas C, Jackson A, Patel K, Mao Y. Optimized Adipogenic Differentiation and Delivery of Bovine Umbilical Cord Stem Cells for Cultivated Meat. Gels. 2024; 10(8):488. https://doi.org/10.3390/gels10080488
Chicago/Turabian StyleOzhava, Derya, Kathleen Lee, Cemile Bektas, Anisha Jackson, Krishi Patel, and Yong Mao. 2024. "Optimized Adipogenic Differentiation and Delivery of Bovine Umbilical Cord Stem Cells for Cultivated Meat" Gels 10, no. 8: 488. https://doi.org/10.3390/gels10080488
APA StyleOzhava, D., Lee, K., Bektas, C., Jackson, A., Patel, K., & Mao, Y. (2024). Optimized Adipogenic Differentiation and Delivery of Bovine Umbilical Cord Stem Cells for Cultivated Meat. Gels, 10(8), 488. https://doi.org/10.3390/gels10080488







