Antibacterial Hydrogels for Wound Dressing Applications: Current Status, Progress, Challenges, and Trends
Abstract
:1. Introduction
2. Inherent Antibacterial Hydrogel
2.1. Natural-Polymer-Based Hydrogels
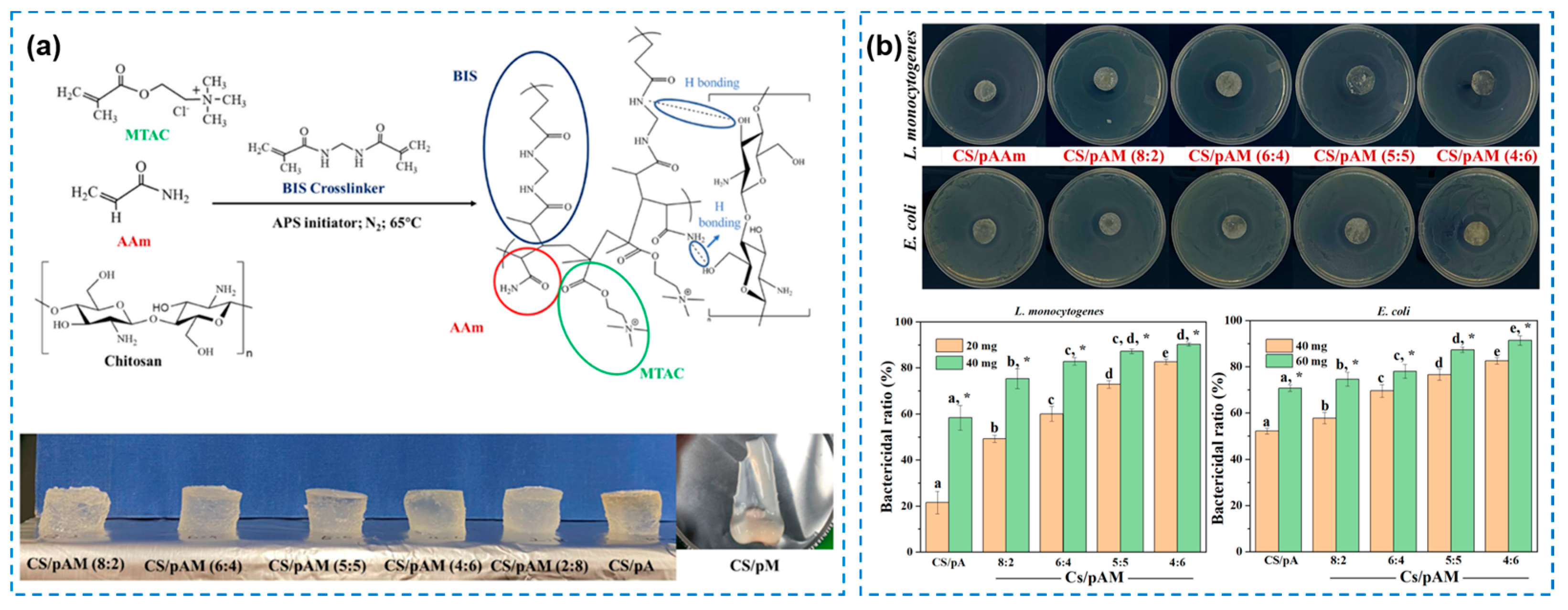
2.2. Synthetic Polymeric or Hybrid Hydrogels
3. Antibacterial Hydrogel with Functional Agents
3.1. Metal-Ion/Metal-Oxide-Nanoparticles-Loaded Hydrogels
| Types of Loading | Types of Metals | Metal Concentration | Antibacterial Ability | Antibacterial Mechanism | Ref. |
|---|---|---|---|---|---|
| Metal ions | Silver | 50 mM | Notable antibacterial activity against S. aureus and Streptococcus mutants (S. mutans) | Ag+ interacts with sulfur-containing proteins in the bacterial cell membrane | [54] |
| Copper | 1 mg/mL | More than 70% against E. coli and MRSA | Cu2+ generates hydroxyl radicals to attack the bacterial membrane | [56] | |
| Zinc | 4.3 mg/mL | E. coli: 99.67%; S. aureus: 96.33% | ROS production, lipopolysaccharide membrane rapture, DNA replication inhibition, and lowering the bacteria’s enzymatic metabolism | [57] | |
| Metal nanoparticles | Silver | Ag content: 41.5 wt% | Qualitative analysis: obvious | Released Ag ions can interface with the enzymes and sulphydryl groups of proteins, and inhibit DNA synthesis of the bacteria | [59] |
| 600 ppm | Pseudomonas aeruginosa: 4.20 ± 0.33 log reduction; MRSA: 4.56 ± 0.26 log reduction | [55] | |||
| Copper | / | S. aureus: 350 μg/mL (MIC) and 1400 μg/mL (MBC); E. coli: 500 μg/mL (MIC) and 2000 μg/mL (MBC) | Cu ions damage bacteria cell wall; the reactive hydroxyl radicals prevent the bacterial reproduction and damage of DNA, lipids, and proteins; electrostatic interactions between positively charged Cu ions and negatively charged bacteria | [60] | |
| Mixture | 0.3 wt% (g/mL) | E. coli: 98.49%; S. aureus: 99.64% | By penetrating the bacterial wall and forming pores on the membrane surface, resulting in cell membrane destruction and leakage of DNA and RNA with cytoplasmic fluid | [58] | |
| 30 wt% of zinc oxide and 5 wt% of hollow silver nanoparticles | Zone of inhabitation of more than 12 mm against S. aureus and 2 mm against Pseudomonas aeruginosa | Combined mechanism | [61] |
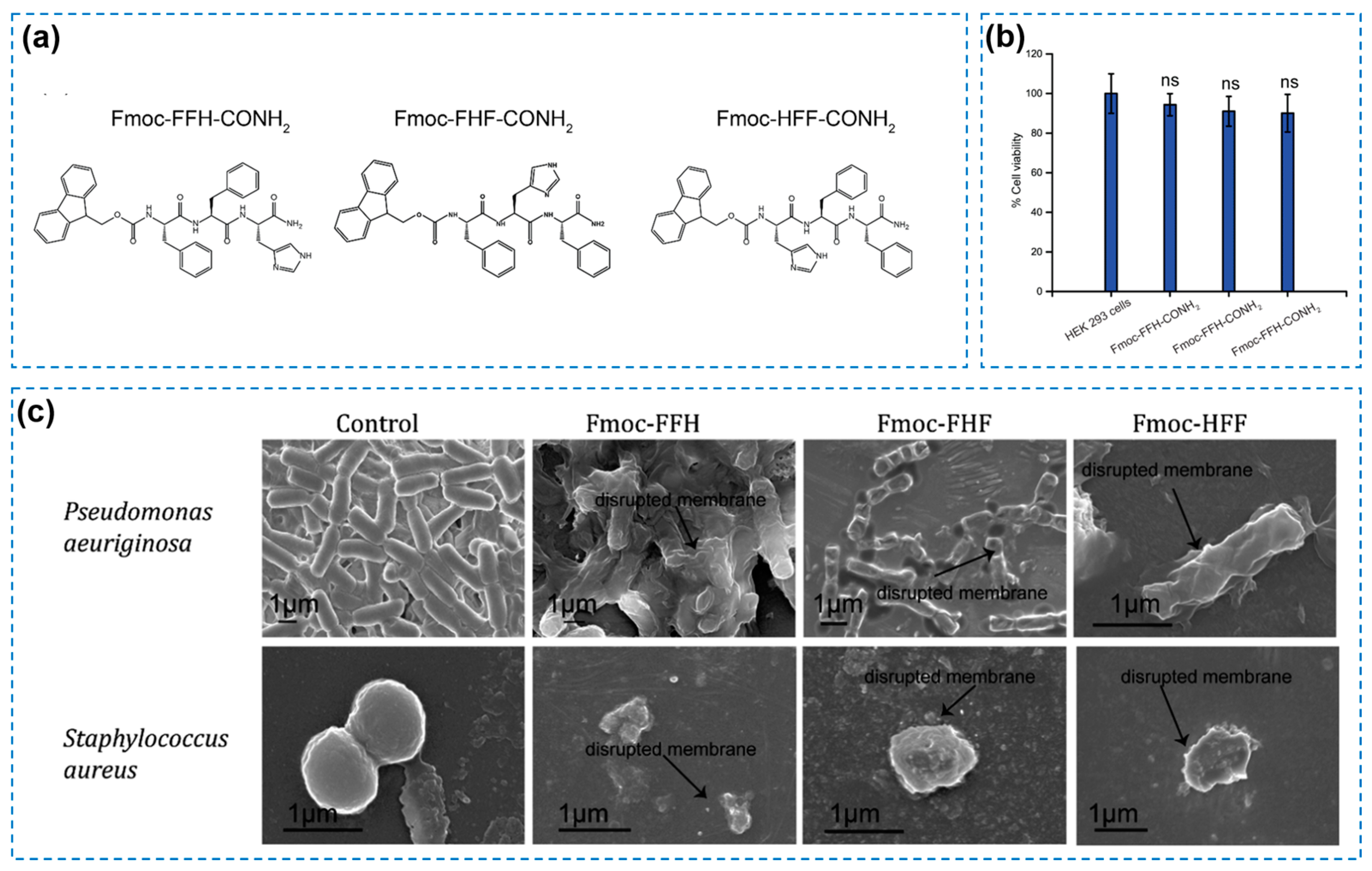
3.2. Antibacterial Hydrogel with Bioactive Agents
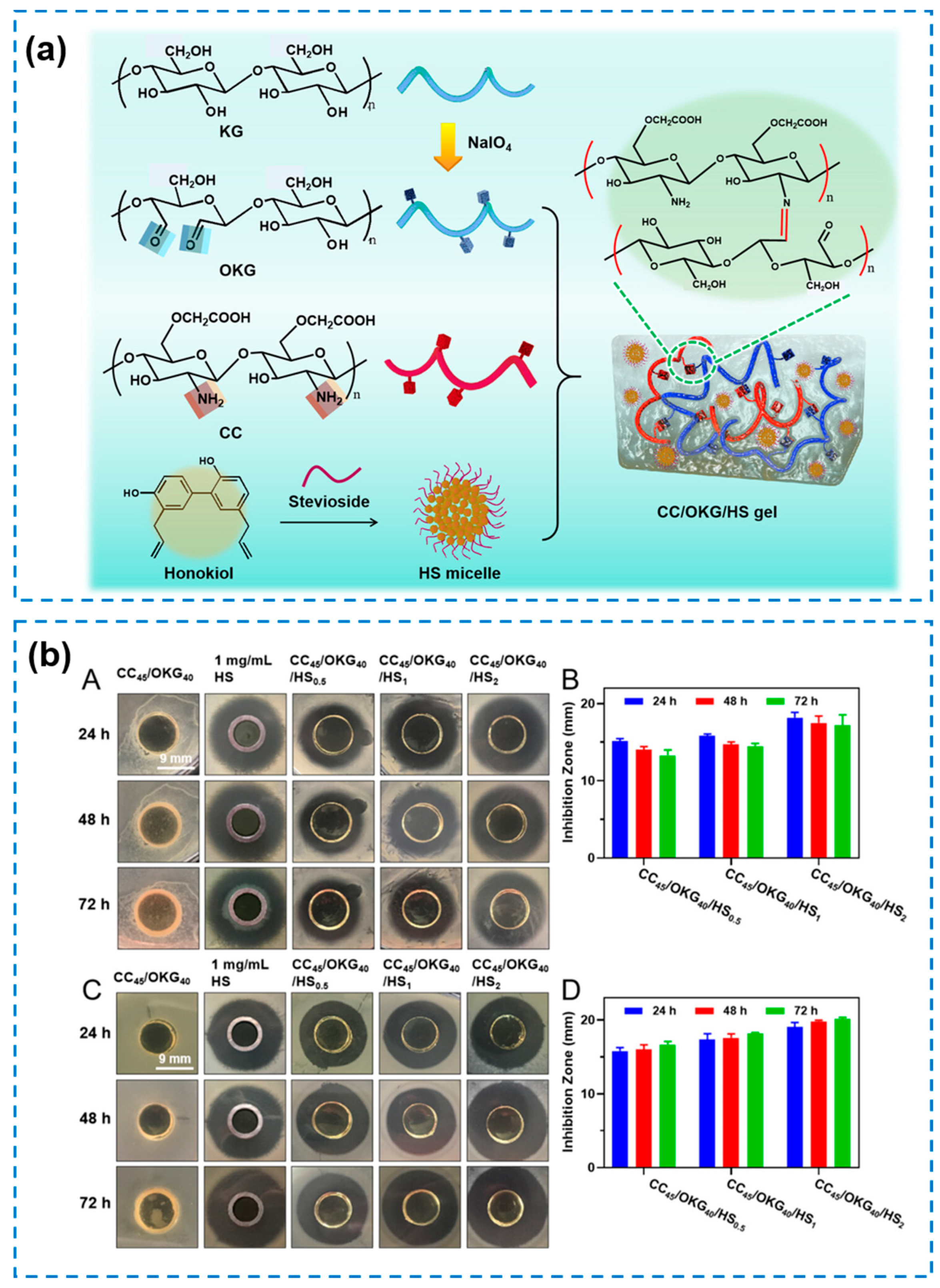
3.3. Composite Hydrogels with Enhanced Antibacterial Property
4. New Therapies for Preparing Antibacterial Hydrogels
4.1. Nanoenzyme-Based Composite Antibacterial Hydrogels
4.2. Photothermal and Photodynamic Antibacterial Hydrogels
4.3. Metal–Organic Framework (MOF)-Based Hydrogels
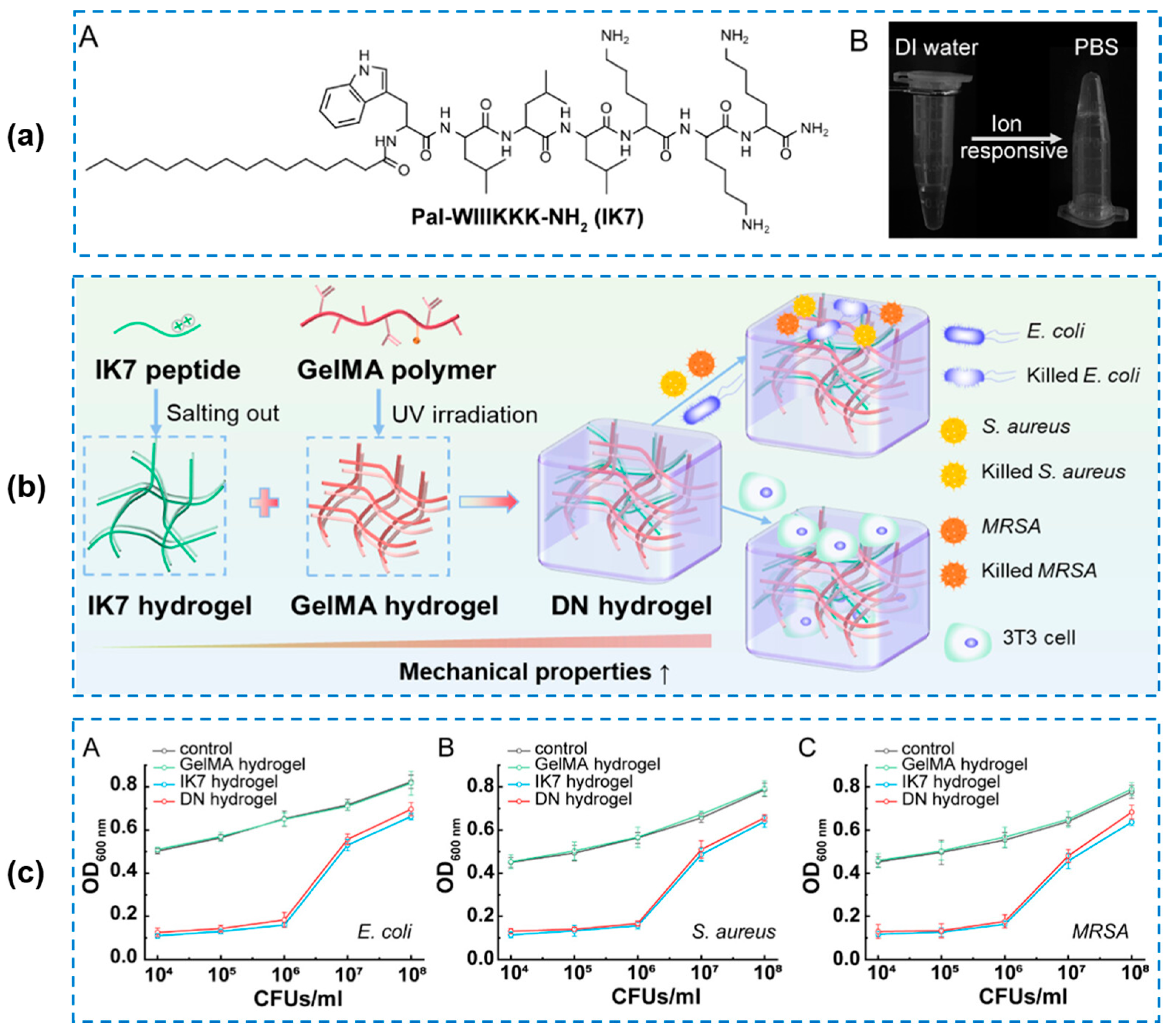
4.4. Other External Stimuli-Responsive Smart Hydrogels
5. Conclusions, Challenges, and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Boehle, K.E.; Gilliand, J.; Wheeldon, C.R.; Holder, A.; Adkins, J.A.; Geiss, B.J.; Ryan, E.P.; Henry, C.S. Utilizing Paper-Based Devices for Antimicrobial-Resistant Bacteria Detection. Angew. Chem.-Int. Edit. 2017, 56, 6886–6890. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Newton, M.A.A.; Rajib, M.; Zhang, Q.; Gao, W.; Lu, Z.; Zheng, Y.; Dai, Z.; Zhu, J. A ZIF-8-encapsulated interpenetrated hydrogel/nanofiber composite patch for chronic wound treatment. J. Mater. Chem. B 2024, 12, 2042. [Google Scholar] [CrossRef]
- Deng, L.W.; Lu, H.D.; Tu, C.X.; Zhou, T.; Cao, W.B.; Gao, C.Y. A tough synthetic hydrogel with excellent post-loading of drugs for promoting the healing of infected wounds in vivo. Biomater. Adv. 2022, 134, 112577. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Su, Y.; Ma, M.; Wang, Y.Q.; Hou, S.; Wang, C.Q.; Sun, L.; Wei, J.S.; Li, M.X. Thiosemicarbazones-Loaded Injectable Hydrogels with Self-Healing and Antibacterial Activity for Wound Healing. ACS Appl. Polym. Mater. 2024, 12, 994. [Google Scholar] [CrossRef]
- Lee, C.-S.; Hwang, H.S. Starch-Based Hydrogels as a Drug Delivery System in Biomedical Applications. Gels 2023, 9, 951. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels 2023, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, 3627. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Cao, Y.; Guo, Z.; Li, F.; Wang, L. Construction of Chitosan-Based Hydrogel Incorporated with Antimonene Nanosheets for Rapid Capture and Elimination of Bacteria. Adv. Funct. Mater. 2020, 30, 3196. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, T.; Chen, S.; Wang, X.; He, J.; Luo, Y. Construction and characterization of chitosan/poly(acrylamide-[2-(methacryloyloxy)ethyl]trimethylammonium chloride) double-network hydrogel with enhanced antibacterial activity. Adv. Compos. Hybrid Mater. 2023, 6, 192. [Google Scholar] [CrossRef]
- Chakraborty, P.; Oved, H.; Bychenko, D.; Yao, Y.; Tang, Y.; Zilberzwige-Tal, S.; Wei, G.; Dvir, T.; Gazit, E. Nanoengineered Peptide-Based Antimicrobial Conductive Supramolecular Biomaterial for Cardiac Tissue Engineering. Adv. Mater. 2021, 33, 8715. [Google Scholar] [CrossRef]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef]
- Xu, S.; Yan, S.; You, J.; Wu, X. Antibacterial Micelles-Loaded Carboxymethyl Chitosan/Oxidized Konjac Glucomannan Composite Hydrogels for Enhanced Wound Repairing. ACS Appl. Mater. Interfaces 2024, 16, 13563–13572. [Google Scholar] [CrossRef]
- Li, Q.; Ai, R.; Fan, J.; Fu, X.; Zhu, L.; Zhou, Q.; Chen, L.; Ma, W.; Li, Y.; Liu, L. AgNPs-loaded chitosan/sodium alginate hydrogel film by in-situ green reduction with tannins for enhancing antibacterial activity. Mater. Today Commun. 2024, 38, 107927. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Zhong, Y.; Lu, Z.; Wang, D. NIR regulated upconversion nanoparticles@metal-organic framework composite hydrogel dressing with catalase-like performance and enhanced antibacterial efficacy for accelerating wound healing. Int. J. Biol. Macromol. 2023, 235, 123683. [Google Scholar] [CrossRef]
- Ran, P.; Zheng, H.; Cao, W.X.; Jia, X.W.; Zhang, G.Y.; Liu, Y.; Li, X.H. On-Demand Changeable Theranostic Hydrogels and Visual Imaging-Guided Antibacterial Photodynamic Therapy to Promote Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 49375–49388. [Google Scholar] [CrossRef]
- Xie, C.M.; Luo, J.Q.; Luo, Y.J.; Zhou, J.; Guo, X.C.; Lu, X. Electroactive Hydrogels with Photothermal/Photodynamic Effects for Effective Wound Healing Assisted by Polydopamine-Modified Graphene Oxide. ACS Appl. Mater. Interfaces 2023, 15, 42329–42340. [Google Scholar] [CrossRef]
- Qu, H.; Yao, Q.; Chen, T.; Wu, H.; Liu, Y.; Wang, C.; Dong, A. Current status of development and biomedical applications of peptide-based antimicrobial hydrogels. Adv. Colloid Interface Sci. 2024, 325, 103099. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, H.; Wang, X.; Dong, S.; Guo, L.; Zhang, S.; Yang, X.; Liu, C.; Jiang, X.; Kan, M.; et al. Advances in preparation and application of antibacterial hydrogels. J. Nanobiotechnol. 2023, 21, 300. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Xu, H.; Guo, G.; Li, Y.; Zhang, Q. Recent progress of antibacterial hydrogel materials for biomedical applications. J. Mater. Chem. C 2023, 11, 12848–12876. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; He, Y.; Lu, Q.; Chen, R.; Zhao, C.; Dong, D.; Sun, Y.; He, H. Dual light-responsive cellulose nanofibril-based in situ hydrogel for drug-resistant bacteria infected wound healing. Carbohydr. Polym. 2022, 297, 42. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.-C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2022, 9, 35. [Google Scholar] [CrossRef]
- Deng, P.; Yao, L.; Chen, J.; Tang, Z.; Zhou, J. Chitosan-based hydrogels with injectable, self-healing and antibacterial properties for wound healing. Carbohydr. Polym. 2022, 276, 118718. [Google Scholar] [CrossRef]
- Yu, X.S.; Cheng, C.; Peng, X.; Zhang, K.X.; Yu, X.X. A self-healing and injectable oxidized quaternized guar gum/carboxymethyl chitosan hydrogel with efficient hemostatic and antibacterial properties for wound dressing. Colloid Surf. B-Biointerfaces 2022, 209, 112207. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.Y.; Zhao, W.F.; Zhao, C.S. A rapid-triggered approach towards antibacterial hydrogel wound dressing with synergic photothermal and sterilization profiles. Biomater. Adv. 2022, 138, 212873. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Gao, Y.; Zhang, Z.X.; Jia, F.; Gao, G.H. Acetylated Distarch Phosphate-Mediated Tough and Conductive Hydrogel for Antibacterial Wearable Sensors. ACS Appl. Mater. Interfaces 2022, 14, 51420–51428. [Google Scholar] [CrossRef]
- Sun, X.; Yao, M.M.; He, S.S.; Dong, X.R.; Liang, L.; Yao, F.L.; Li, J.J. Antibacterial and UV-Blocking Bioelectronics Based on Transparent, Adhesive, and Strain-Sensitive Multifunctional Hydrogel. Adv. Mater. Technol. 2022, 7, 2101283. [Google Scholar] [CrossRef]
- Kutsevol, N.; Virych, P.; Nadtoka, O.; Virych, P.; Krysa, V. Synthesis of polymeric hydrogels incorporating chlorhexidine as potential antibacterial wound dressings. Mol. Cryst. Liq. Cryst. 2021, 720, 65–71. [Google Scholar] [CrossRef]
- Shen, K.H.; Yeh, Y.Y.; Chiu, T.H.; Wang, R.B.; Yeh, Y.C. Dual Dynamic Covalently Crosslinked Alginate Hydrogels with Tunable Properties and Multiple Stimuli-Responsiveness. ACS Biomater. Sci. Eng. 2022, 13, 571. [Google Scholar] [CrossRef]
- Tang, D.F.; Du, J.X.; Zhao, X.J.; Cai, Y.C.; Zou, J.Y.; Qi, J.Q.; Liu, X.; Qin, Z.D.; Tang, W.F. The effect of antimicrobial peptide HX-12C on the properties of chitosan/polyacrylic acid hydrogel. J. Appl. Polym. Sci. 2024, 141, e55265. [Google Scholar] [CrossRef]
- Hao, Y.; Yuan, C.; Deng, J.; Zheng, W.; Ji, Y.; Zhou, Q. Injectable Self-Healing First-Aid Tissue Adhesives with Outstanding Hemostatic and Antibacterial Performances for Trauma Emergency Care. ACS Appl. Mater. Interfaces 2022, 14, 16006–16017. [Google Scholar] [CrossRef]
- Wang, Y.M.; Xiao, D.D.; Quan, L.; Chai, H.B.; Sui, X.F.; Wang, B.J.; Xu, H.; Mao, Z.P. Mussel-inspired adhesive gelatin-polyacrylamide hydrogel wound dressing loaded with tetracycline hydrochloride to enhance complete skin regeneration. Soft Matter 2022, 18, 662–674. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.C.; Xiong, Y.; Zhu, X.W.; Wei, C.Y.; Cao, F.Q.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.F.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Guo, W.; Zhang, F.; Lin, H.M.; Yu, K.; Yang, C.Y.; Qu, F.Y. A multifunctional hydrogel dressing with antibacterial properties for effective wound healing. Dalton Trans. 2022, 51, 6817–6824. [Google Scholar] [CrossRef]
- Park, J.; Kim, T.Y.; Kim, Y.; An, S.; Kim, K.S.; Kang, M.; Kim, S.A.; Kim, J.; Lee, J.; Cho, S.W.; et al. A Mechanically Resilient and Tissue-Conformable Hydrogel with Hemostatic and Antibacterial Capabilities for Wound Care. Adv. Sci. 2023, 10, 3651. [Google Scholar] [CrossRef]
- Aizaz, A.; Nawaz, M.H.; Ismat, M.S.; Zahid, L.; Zahid, S.; Ahmed, S.; Abbas, M.; Vayalpurayil, T.; Rehman, M.A.U. Development and characterization of polyethylene oxide and guar gum-based hydrogel; a detailed in-vitro analysis of degradation and drug release kinetics. Int. J. Biol. Macromol. 2024, 273, 132824. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.Y.; Wang, Y.D.; Liu, L.J.; Dong, H.X.; Zhang, C.H.; Satoh, T. Physically crosslinked PAA/Lys-BPEA hydrogel with rapid self-healing and long-term antibacterial activities. Polymer 2023, 265, 125598. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Demirci, S.; Sahiner, N. Physically and Chemically Crosslinked, Tannic Acid Embedded Linear PEI-Based Hydrogels and Cryogels with Natural Antibacterial and Antioxidant Properties. Biomedicines 2023, 11, 706. [Google Scholar] [CrossRef]
- Yu, R.; Li, Z.; Pan, G.; Guo, B. Antibacterial conductive self-healable supramolecular hydrogel dressing for infected motional wound healing. Sci. China Chem. 2022, 65, 2238–2251. [Google Scholar] [CrossRef]
- Kapanya, A.; Rungrod, A.; Somsunan, R. Effect of Bacterial Cellulose on Silver-loaded Poly(sodium 2-acrylamido-2-methylpropane sulfonate) Hydrogel for Antibacterial Wound Dressing Application. Fiber. Polym. 2022, 23, 3343–3357. [Google Scholar] [CrossRef]
- Liu, Y.L.; Mao, J.; Guo, Z.Y.; Hu, Y.F.; Wang, S. Polyvinyl alcohol/carboxymethyl chitosan hydrogel loaded with silver nanoparticles exhibited antibacterial and self-healing properties. Int. J. Biol. Macromol. 2022, 220, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Li, H.; Xu, L.J.; Yan, J.; Wang, X.Y. Preparation and Properties of Temperature-Sensitive Silver-Loaded Antibacterial Sericin/poly (N-isopropylacrylamide) Hydrogel. J. Macromol. Sci. Part B-Phys. 2023, 20, 803. [Google Scholar] [CrossRef]
- Zhang, M.H.; Chen, S.Y.; Zhong, L.; Wang, B.X.; Wang, H.P.; Hong, F. Zn2+-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 143, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Luo, Y.; Wu, S.H.; Xia, X.; Zhao, H.; Xu, X.; Luo, X.B. Super-Rapid In Situ Formation of a Silver Ion-Induced Supramolecular Hydrogel with Efficient Antibacterial Activity for Root Canal Disinfection. ACS Appl. Mater. Interfaces 2023, 15, 29854–29865. [Google Scholar] [CrossRef]
- Gao, Y.-R.; Zhang, W.-X.; Wei, Y.-N.; Li, Y.; Fei, T.; Shu, Y.; Wang, J.-H. Ionic liquids enable the preparation of a copper-loaded gel with transdermal delivery function for wound dressings. Biomater. Sci. 2022, 10, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, T.; Nourmohammadi, J.; Ghaee, A.; Khodaii, Z. An antibacterial and self-healing hydrogel from aldehyde-carrageenan for wound healing applications. Carbohydr. Polym. 2023, 302, 120371. [Google Scholar] [CrossRef]
- Gou, L.; Xiang, M.; Ni, X. Development of wound therapy in nursing care of infants by using injectable gelatin-cellulose composite hydrogel incorporated with silver nanoparticles. Mater. Lett. 2020, 277, 128340. [Google Scholar] [CrossRef]
- Amiri, N.; Ghaffari, S.; Hassanpour, I.; Chae, T.; Jalili, R.; Kilani, R.T.; Ko, F.; Ghahary, A.; Lange, D. Antibacterial Thermosensitive Silver–Hydrogel Nanocomposite Improves Wound Healing. Gels 2023, 9, 542. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sánchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chen, K.; Qiu, H. Preparation and properties of antibacterial composite hydrogels based on polyvinyl alcohol, chitosan, and nano-metal oxide. Cellulose 2024, 31, 3607–3622. [Google Scholar] [CrossRef]
- Farazin, A.; Mohammadimehr, M.; Naeimi, H. Flexible self-healing nanocomposite based gelatin/tannic acid/acrylic acid reinforced with zinc oxide nanoparticles and hollow silver nanoparticles based on porous silica for rapid wound healing. Int. J. Biol. Macromol. 2023, 241, 124572. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albo, Y.; Pagliaro, M. New Antivirals and Antibacterials Based on Silver Nanoparticles. ChemMedChem 2020, 15, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.G.; Huang, J.J.; Wu, X.W.; Ren, Y.H.; Li, Z.A.; Ren, J.A. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Yu, Y.; Qian, H.L.; Chen, Y.F.; Zou, L.Y.; Zhang, C.M.; Ren, K.F.; Yang, Z.H.; Ji, J. Antibacterial endotracheal tube with silver-containing double-network hydrogel coating. Colloid Interface Sci. Commun. 2023, 55, 724. [Google Scholar] [CrossRef]
- Rastegari, A.; Hasanshakir, F.; Mohammadi, Z.; Saadatpor, F.; Faghihi, H.; Moraffah, F. A chitosan-based hydrogel containing zinc oxide nanoparticles as a carrier for improving antibacterial activity and controlling the release of antibiotics. Micro Nano Lett. 2023, 18, 12172. [Google Scholar] [CrossRef]
- Fahaduddin; Bal, T. Fabrication and evaluation of Dillenia indica-carrageenan blend hybrid superporous hydrogel reinforced with green synthesized MgO nanoparticles as an effective wound dressing material. Int. J. Biol. Macromol. 2024, 265, 130835. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, R.A.; Alminderej, F.M.; Al-Harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Design, Synthesis, and Characterization of Novel Bis-Uracil Chitosan Hydrogels Modified with Zinc Oxide Nanoparticles for Boosting Their Antimicrobial Activity. Polymers 2023, 15, 980. [Google Scholar] [CrossRef]
- Gill, S.Z.; Niazi, M.B.K.; Malik, U.S.; Jahan, Z.; Andleep, S.; Ahmed, T. Development and characterization of SA/PEG hydrogel membranes with Ag/ZnO nanoparticles for enhanced wound dressing. Mater. Chem. Phys. 2024, 317, 129170. [Google Scholar] [CrossRef]
- Yu, Y.C.; Hu, M.H.; Zhuang, H.Z.; Phan, T.H.; Jiang, Y.S.; Jan, J.S. Antibacterial Gelatin Composite Hydrogels Comprised of In Situ Formed Zinc Oxide Nanoparticles. Polymers 2023, 15, 3978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, Z.; Zhang, L.; Lu, S.; Liang, F.; Li, S. Chitosan-Based Thermo-Sensitive Hydrogel Loading Oyster Peptides for Hemostasis Application. Materials 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Dahan, S.; Aibinder, P.; Khalfin, B.; Moran-Gilad, J.; Rapaport, H. Hybrid Hydrogels of FKF-Peptide Assemblies and Gelatin for Sustained Antimicrobial Activity. ACS Biomater. Sci. Eng. 2023, 9, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Pan, S. Co-assembled C13-dipeptide hydrogels by Gallic Acid (CA) and epigallocatechin gallate (EGCG) with antibacterial activity. Food Biosci. 2022, 49, 101962. [Google Scholar] [CrossRef]
- Hu, T.; Xu, Y.; Xu, G.; Pan, S. Sequence-Selected C13-Dipeptide Self-Assembled Hydrogels for Encapsulation of Lemon Essential Oil with Antibacterial Activity. J. Agric. Food Chem. 2022, 70, 7148–7157. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, X.; Li, J. Self-Assembled Peptide Hydrogel for Biomedical Applications. Prog. Chem. 2022, 34, 568–579. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, L.; Yuan, S.; Sun, J.; Li, Z. pH-Responsive Peptide Supramolecular Hydrogels with Antibacterial Activity. Langmuir 2017, 33, 3234–3240. [Google Scholar] [CrossRef]
- Gahane, A.Y.; Ranjan, P.; Singh, V.; Sharma, R.K.; Sinha, N.; Sharma, M.; Chaudhry, R.; Thakur, A.K. Fmoc-phenylalanine displays antibacterial activity against Gram-positive bacteria in gel and solution phases. Soft Matter 2018, 14, 2234–2244. [Google Scholar] [CrossRef]
- Prakash, V.; Christian, Y.; Redkar, A.S.; Roy, A.; Anandalakshmi, R.; Ramakrishnan, V. Antibacterial hydrogels of aromatic tripeptides. Soft Matter 2022, 18, 6360–6371. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Lyu, Y.; Tan, M.; Xue, M.; Hou, W.; Yang, C.; Shan, A.; Xiang, W.; Cheng, B. Broad-spectrum hybrid antimicrobial peptides derived from PMAP-23 with potential LPS binding ability. Biochem. Pharmacol. 2023, 210, 115500. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Maity, P.; Halder, A. Charge-Driven Interaction of Antimicrobial Peptide NK-2 with Phospholipid Membranes. ACS Omega 2017, 2, 8859–8867. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, N.; Teng, D.; Mao, R.Y.; Hao, Y.; Ma, X.X.; Wei, L.Y.; Wang, J.H. Antibacterial peptide NZ2114-loaded hydrogel accelerates Staphylococcus aureus-infected wound healing. Appl. Microbiol. Biotechnol. 2022, 106, 3639–3656. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Hou, Y.; Zhang, Y.; Wang, B. Double-Network Hydrogel with Strengthened Mechanical Property for Controllable Release of Antibacterial Peptide. Biomacromolecules 2024, 25, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.Y.; Ahmed, A.; Cong, H.L.; Yu, B.; Shen, Y.Q. Effective Strategies for Developing Potent, Broad-Spectrum Antibacterial and Wound Healing Promotion from Short-Chain Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2023, 15, 32136–32147. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.L.; Chen, J.J.; Feng, W.; Song, Q.; Huo, J.J.; Yu, L.F.; Liu, N.; Wang, T.J.; Li, P.; Huang, W. A multifunctional shape-adaptive and biodegradable hydrogel with hemorrhage control and broad-spectrum antimicrobial activity for wound healing. Biomater. Sci. 2020, 8, 6930–6945. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Hurren, C.; Lu, Z.T.; Wang, D. pH-sensitive alginate hydrogel for synergistic anti-infection. Int. J. Biol. Macromol. 2022, 222, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.B.; Yang, C.; Cheng, C.X.; Shi, C.X.; Sun, M.D.; Hu, H.Z.; Shi, T.F.; Chen, X.N.; He, X.; Zheng, X.; et al. Bioactive Injectable Hydrogel Dressings for Bacteria-Infected Diabetic Wound Healing: A “Pull-Push” Approach. ACS Appl. Mater. Interfaces 2022, 14, 26404–26417. [Google Scholar] [CrossRef]
- Hong, Y.X.; Xi, Y.J.; Zhang, J.X.; Wang, D.D.; Zhang, H.L.; Yan, N.; He, S.S.; Du, J.Z. Polymersome-hydrogel composites with combined quick and long-term antibacterial activities. J. Mat. Chem. B 2018, 6, 6311–6321. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zhang, X.; Cao, Q.; Wu, S.Q.; Wang, X.Q.; Peng, N.; Zeng, D.L.; Liao, J.F.; Xu, H. Facile fabrication of chitin/ZnO composite hydrogels for infected wound healing. Biomater. Sci. 2022, 10, 5888–5899. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, J.; Sun, Y.G.; Wang, H.L.; Du, X.S.; Cheng, X.; Du, Z.L.; Wang, H.B. Skin-inspired nanofibrillated cellulose-reinforced hydrogels with high mechanical strength, long-term antibacterial, and self-recovery ability for wearable strain/pressure sensors. Carbohydr. Polym. 2021, 261, 117894. [Google Scholar] [CrossRef]
- Li, P.; Feng, Z.P.; Yu, Z.Y.; Chen, Y.; Li, P.W.; Yang, Z.M.; Li, S.D.; Jin, S.H. Preparation of chitosan-Cu2+/NH3 physical hydrogel and its properties. Int. J. Biol. Macromol. 2019, 133, 67–75. [Google Scholar] [CrossRef]
- Meng, W.L.; Lin, Z.; Cheng, X.; Gou, S.Q.; Wang, R.; Bu, P.Z.; Li, Y.; Mi, B.B.; Yu, Y.S.; Feng, Q.; et al. Thiourea-Cation Chelation Based Hydrogel and its Application as Antibacterial Dressing for the Repair of Diabetic Wound. Adv. Funct. Mater. 2024, 14, 314202. [Google Scholar] [CrossRef]
- Qu, J.; Li, J.; Zhu, W.; Xu, Y.; Zhou, S.; Yang, Y.; Qian, X. Hybrid nanocomposite multinetwork hydrogel containing magnesium hydroxide nanoparticles with enhanced antibacterial activity for wound dressing applications. Polymer 2022, 251, 124902. [Google Scholar] [CrossRef]
- Yang, Z.F.; Huang, R.K.; Zheng, B.N.; Guo, W.T.; Li, C.K.; He, W.Y.; Wei, Y.G.; Du, Y.; Wang, H.M.; Wu, D.C.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 3627. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Li, F.; Xiao, Y.; Zhang, Y.; Bu, T.; Jia, P.; Zhe, T.; Wang, L. Multifunctional Magnetic Copper Ferrite Nanoparticles as Fenton-like Reaction and Near-Infrared Photothermal Agents for Synergetic Antibacterial Therapy. ACS Appl. Mater. Interfaces 2019, 11, 31649–31660. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Q.; Zha, Z.; Zhu, D.; Zheng, L.; Shi, L.; Wei, X.; Lian, L.; Wu, K.; Cheng, L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact. Mater. 2021, 6, 4389–4401. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, R.; Zhang, X.; Ren, Y.; Du, T.; Ni, Y.; Yan, H.; Zhang, L.; Sun, J.; Zhang, W.; et al. A photothermal and self-induced Fenton dual-modal antibacterial platform for synergistic enhanced bacterial elimination. Appl. Catal. B-Environ. 2021, 295, 120315. [Google Scholar] [CrossRef]
- Wei, Z.W.; Wang, L.Y.; Tang, C.Q.; Chen, S.Q.; Wang, Z.J.; Wang, Y.L.; Bao, J.X.; Xie, Y.; Zhao, W.F.; Su, B.H.; et al. Metal-Phenolic Networks Nanoplatform to Mimic Antioxidant Defense System for Broad-Spectrum Radical Eliminating and Endotoxemia Treatment. Adv. Funct. Mater. 2020, 30, 2234. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, S.Q.; Zheng, H.; Shen, H.D.; Zhou, L.; Yang, Z.T.; Li, Q.Y.; Zhang, Q.Y.; Zhang, H.P. Ceria Nanoenzyme-Based Hydrogel with Antiglycative and Antioxidative Performance for Infected Diabetic Wound Healing. Small Methods 2022, 6, 949. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; He, C.Y.; Dong, W.T.; Yang, X.K.; Wu, Y.; Liu, J.L.; Kong, Q.Q.; He, J.; Yan, B. A self-healing injectable hydrogel integrated with enzymatic and nonenzymatic antioxidants as artificial antioxidant defense system for diabetic wound healing. Mater. Des. 2024, 237, 112620. [Google Scholar] [CrossRef]
- Tu, C.X.; Lu, H.D.; Zhou, T.; Zhang, W.Y.; Deng, L.W.; Cao, W.B.; Yang, Z.J.; Wang, Z.L.; Wu, X.Y.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef]
- He, S.; Li, Z.H.; Wang, L.; Yao, N.N.; Wen, H.D.; Yuan, H.G.; Zhang, J.T.; Li, Z.Q.; Shen, C.A. A nanoenzyme-modified hydrogel targets macrophage reprogramming-angiogenesis crosstalk to boost diabetic wound repair. Bioact. Mater. 2024, 35, 17–30. [Google Scholar] [CrossRef]
- Li, Z.G.; Fan, X.T.; Luo, Z.; Loh, X.J.; Ma, Y.D.; Ye, E.Y.; Wu, Y.L.; He, C.B.; Li, Z.B. Nanoenzyme-chitosan hydrogel complex with cascade catalytic and self-reinforced antibacterial performance for accelerated healing of diabetic wounds. Nanoscale 2022, 14, 14970–14983. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Wang, L.; Zhang, Q. Nanoenzyme-Reinforced Injectable Hydrogel for Healing Diabetic Wounds Infected with Multidrug Resistant Bacteria. Nano Lett. 2020, 20, 5149–5158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Q.; Qi, X.; Li, Y.; Ma, H.; Grinholc, M.; Nakonieczna, J.; Yu, B.; Wang, X.; Zhang, L. Iron-blocking antibacterial therapy with cationic heme-mimetic gallium porphyrin photosensitizer for combating antibiotic resistance and enhancing photodynamic antibacterial activity. Chem. Eng. J. 2023, 451, 138261. [Google Scholar] [CrossRef]
- Du, C.; Gao, D.; Gao, M.; Yuan, H.; Liu, X.; Wang, B.; Xing, C. Property Regulation of Conjugated Oligoelectrolytes with Polyisocyanide to Achieve Efficient Photodynamic Antibacterial Biomimetic Hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 27955–27962. [Google Scholar] [CrossRef]
- Liu, S.; Wang, B.; Yu, Y.; Liu, Y.; Zhuang, Z.; Zhao, Z.; Feng, G.; Qin, A.; Tang, B.Z. Cationization-Enhanced Type I and Type II ROS Generation for Photodynamic Treatment of Drug-Resistant Bacteria. ACS Nano 2022, 16, 9130–9141. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Feng, Y.P.; Liu, Z.X.; Chen, Q.Y.; Shen, Y.P.; Feng, F.; Liu, L.Z.; Zhong, M.Q.; Zhai, Y.; Bockstaller, M.; et al. Degradable GO-Nanocomposite hydrogels with synergistic photothermal and antibacterial response. Polymer 2021, 230, 124018. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, B.Q.; Jiang, M.; Yuan, L.L.; Jiang, X.Y.; Li, S.L.; Cai, R.; Chen, J.L.; Jiang, X.; He, Y.; et al. Facile and eco-friendly fabrication of biocompatible hydrogel containing CuS@Ser NPs with mechanical flexibility and photothermal antibacterial activity to promote infected wound healing. J. Nanobiotechnol. 2023, 21, 266. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.P.; Guo, B.L.; Yin, Z.H.; Zhu, D.; Han, Y. Injectable dry cryogels with excellent blood-sucking expansion and blood clotting to cease hemorrhage for lethal deep-wounds, coagulopathy and tissue regeneration. Chem. Eng. J. 2021, 403, 126329. [Google Scholar] [CrossRef]
- Mao, C.Y.; Xiang, Y.M.; Liu, X.M.; Cui, Z.D.; Yang, X.J.; Yeung, K.W.K.; Pan, H.B.; Wang, X.B.; Chu, P.K.; Wu, S.L. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.Y.; Xiang, Y.M.; Liu, X.M.; Cui, Z.D.; Yang, X.J.; Li, Z.Y.; Zhu, S.L.; Zheng, Y.F.; Yeung, K.W.K.; Wu, S.L. Repeatable Photodynamic Therapy with Triggered Signaling Pathways of Fibroblast Cell Proliferation and Differentiation to Promote Bacteria-Accompanied Wound Healing. ACS Nano 2018, 12, 1747–1759. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Zhang, Y.W.; Liu, X.Z.; Ma, X.F.; Qin, X.T.; Jia, S.R.; Zhong, C. Aggregation-induced emission-active amino acid/berberine hydrogels with enhanced photodynamic antibacterial and anti-biofilm activity. Chem. Eng. J. 2021, 413, 127542. [Google Scholar] [CrossRef]
- Dong, A.Q.; Xiao, W.R.; Yuan, W.Z.; Zuo, K.Q. Self-Healable and Injectable Nanocomposite Hydrogel Loading Iron-Doped Carbon Dots for Synergistic Antibacterial Peptide-Photothermal-Photodynamic Antibacterial Therapy. ACS Appl. Polym. Mater. 2023, 5, 9564–9573. [Google Scholar] [CrossRef]
- Reddy, Y.N.; De, A.A.A.; Paul, S.; Pujari, A.K.; Bhaumik, J. In Situ Nanoarchitectonics of a MOF Hydrogel: A Self-Adhesive and pH-Responsive Smart Platform for Phototherapeutic Delivery. Biomacromolecules 2023, 24, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qiu, W.W.; Yao, C.J.; Wang, C.; Wu, D.Q.; Pradeep, S.; Yu, J.Y.; Dai, Z.J. Water-stable zirconium-based metal-organic frameworks armed polyvinyl alcohol nanofibrous membrane with enhanced antibacterial therapy for wound healing. J. Colloid Interface Sci. 2021, 603, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Li, W.; Li, J.; Han, H.; Song, Z. Responsive metal-organic framework nanocarrier delivery system: An effective solution against bacterial infection. Coord. Chem. Rev. 2023, 496, 215431. [Google Scholar] [CrossRef]
- Yao, X.X.; Zhu, G.S.; Zhu, P.G.; Ma, J.; Chen, W.W.; Liu, Z.; Kong, T.T. Omniphobic ZIF-8@Hydrogel Membrane by Microfluidic-Emulsion-Templating Method for Wound Healing. Adv. Funct. Mater. 2020, 30, 1909389. [Google Scholar] [CrossRef]
- Li, N.; Liu, W.; Zheng, X.Y.; Wang, Q.; Shen, L.X.; Hui, J.F.; Fan, D.D. Antimicrobial hydrogel with multiple pH-responsiveness for infected burn wound healing. Nano Res. 2023, 16, 11139–11148. [Google Scholar] [CrossRef]
- Xiao, J.S.; Chen, S.Y.; Yi, J.; Zhang, H.F.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Han, I.; Lee, S.; Kim, Y.; Lee, D.N. Novel Metal-Organic Framework-Based Photocrosslinked Hydrogel System for Efficient Antibacterial Applications. ACS Appl. Mater. Interfaces 2020, 12, 20234–20242. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Kim, H.C.; Huh, S.; Kim, Y.; Lee, D.N. Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands. Dalton Trans. 2019, 48, 8084–8093. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Lee, S.; Kim, Y.; Choi, J.; Kim, S.; Kim, S.J.; Hong, H.J.; Hwang, Y.; Mori, M.; Lee, D.N. Construction of a bioactive copper-based metal organic framework-embedded dual-crosslinked alginate hydrogel for antimicrobial applications. Int. J. Biol. Macromol. 2023, 242, 124840. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Liu, K.; Jiang, T.; Ren, S.; Kang, Y.; Li, W.Q.; Yao, H.B.; Yang, X.F.; Dai, H.L.; Chen, Z.B. Injectable and self-healing chitosan-based hydrogel with MOF-loaded α-lipoic acid promotes diabetic wound healing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 131, 112519. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Y.; Song, J.; Li, S.H.; Sun, H.Z.; Wang, J.J.; Su, F.; Li, S.M. Chitosan-based GOx@Co-MOF composite hydrogel: A promising strategy for enhanced antibacterial and wound healing effects. Int. J. Biol. Macromol. 2024, 270, 132120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Chen, Y.W.; Yang, J.J.; Hao, P.Y.; Ren, L.J.; Zhou, H.Y. Bicomponent hydrogels assisted templating synthesis of hierarchically porous ZIF-8 for efficient antibacterial applications. J. Mol. Struct. 2023, 1277, 134824. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Long, L.Y.; Hu, C.; Kong, Q.Q.; Wang, Y.B. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef]
- Wang, C.R.; Jiang, X.; Kim, H.J.; Zhang, S.M.; Zhou, X.W.; Chen, Y.; Ling, H.A.; Xue, Y.M.; Chen, Z.W.; Qu, M.Y.; et al. Flexible patch with printable and antibacterial conductive hydrogel electrodes for accelerated wound healing. Biomaterials 2022, 285, 121479. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.L.; Wang, W.; Gao, Y.; Wang, W.Y.; Zhang, R.W.; Wu, D.J.; Yu, L.D.; Chen, Y. Nanosonosensitizers-engineered injectable thermogel for augmented chemo-sonodynamic therapy of melanoma and infected wound healing. Mater. Today Bio 2023, 20, 621. [Google Scholar] [CrossRef] [PubMed]


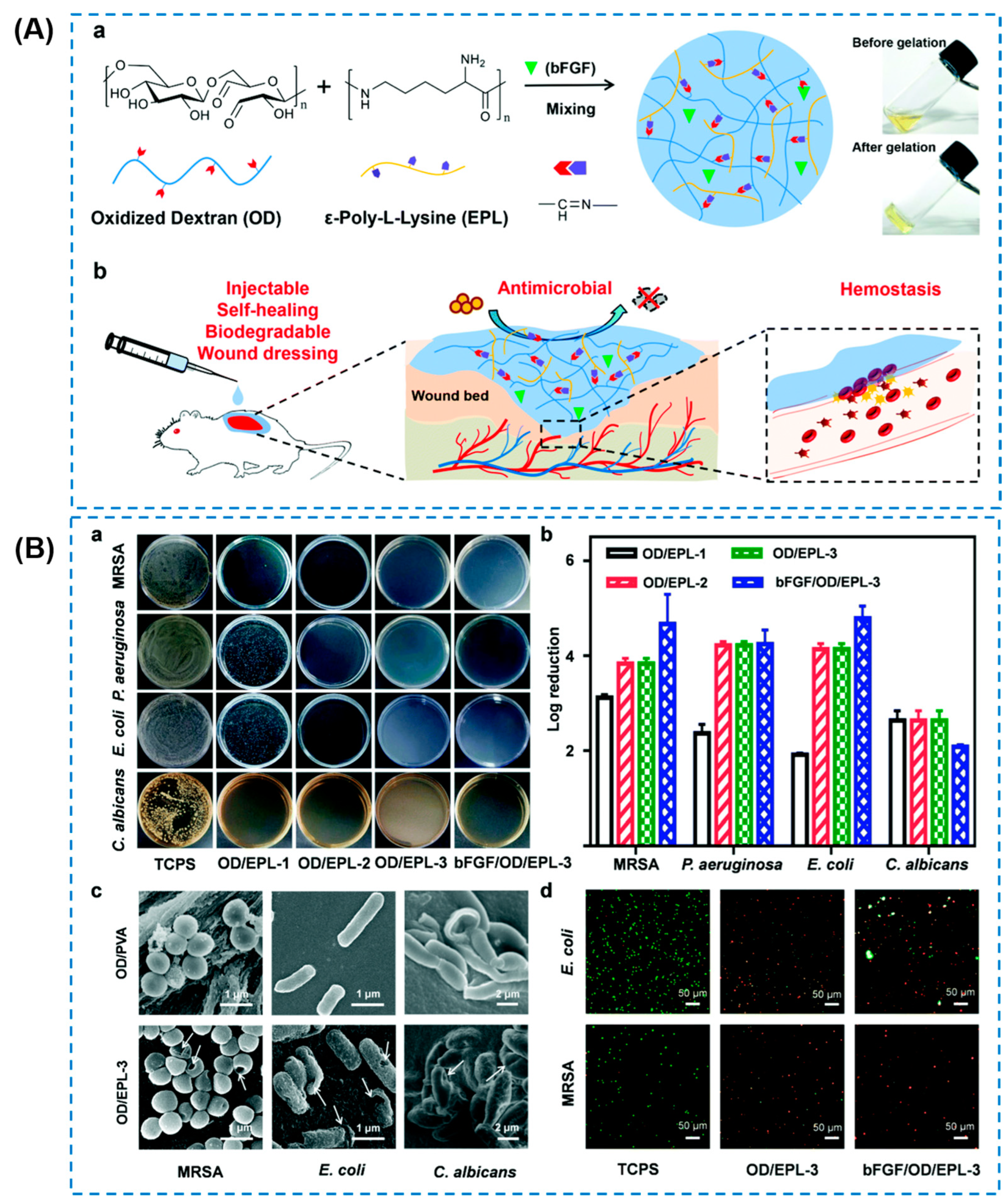
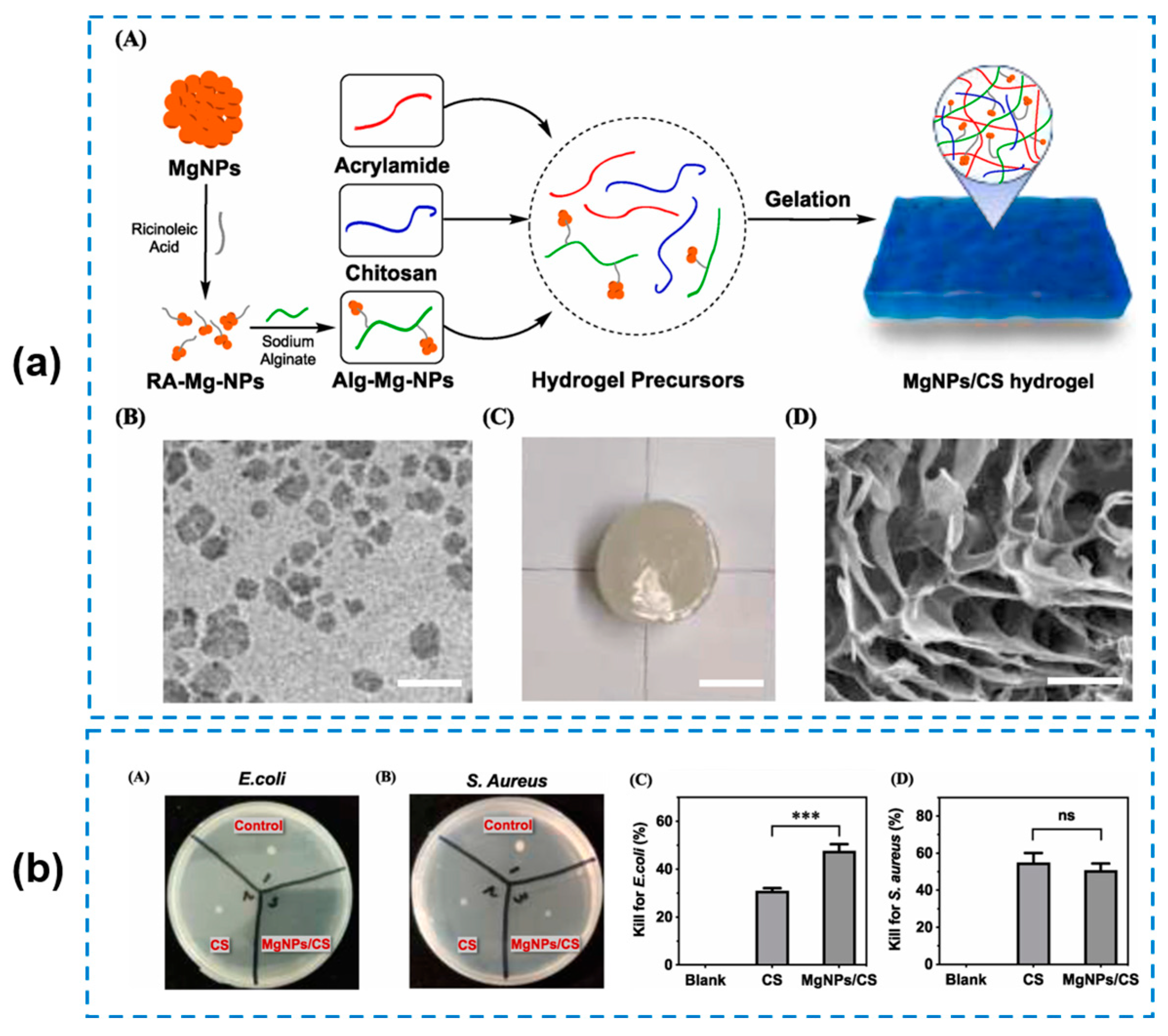


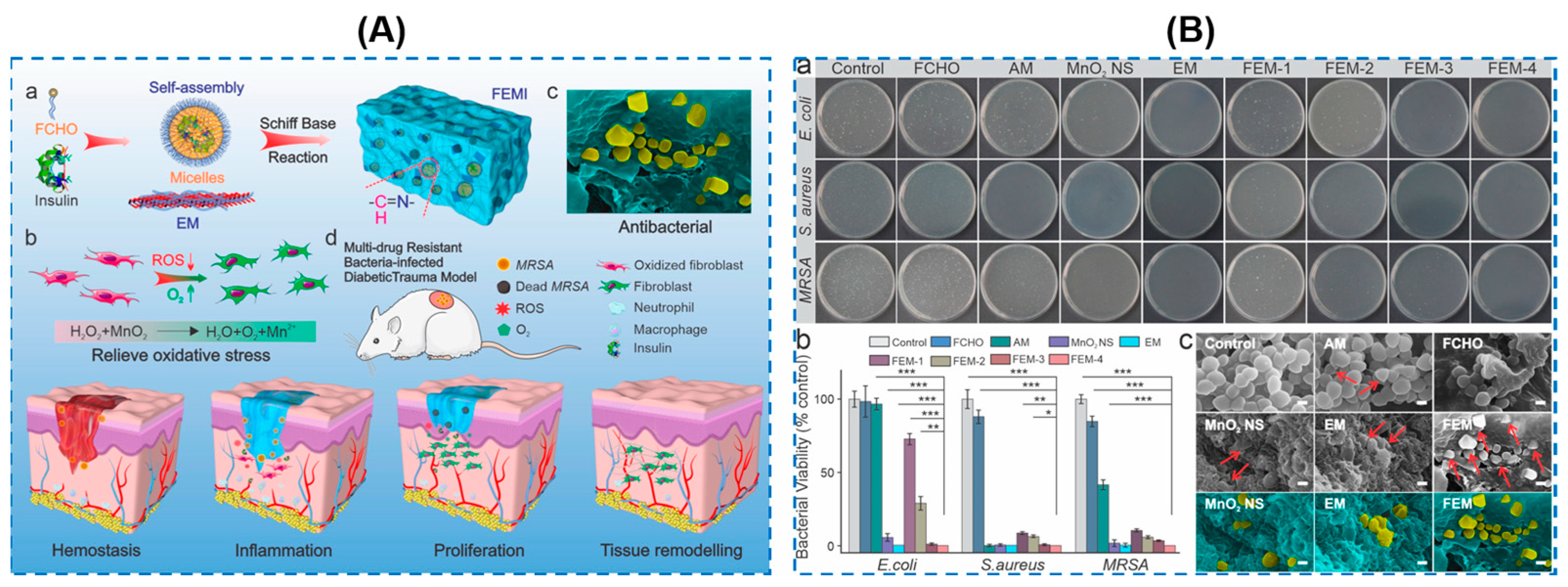

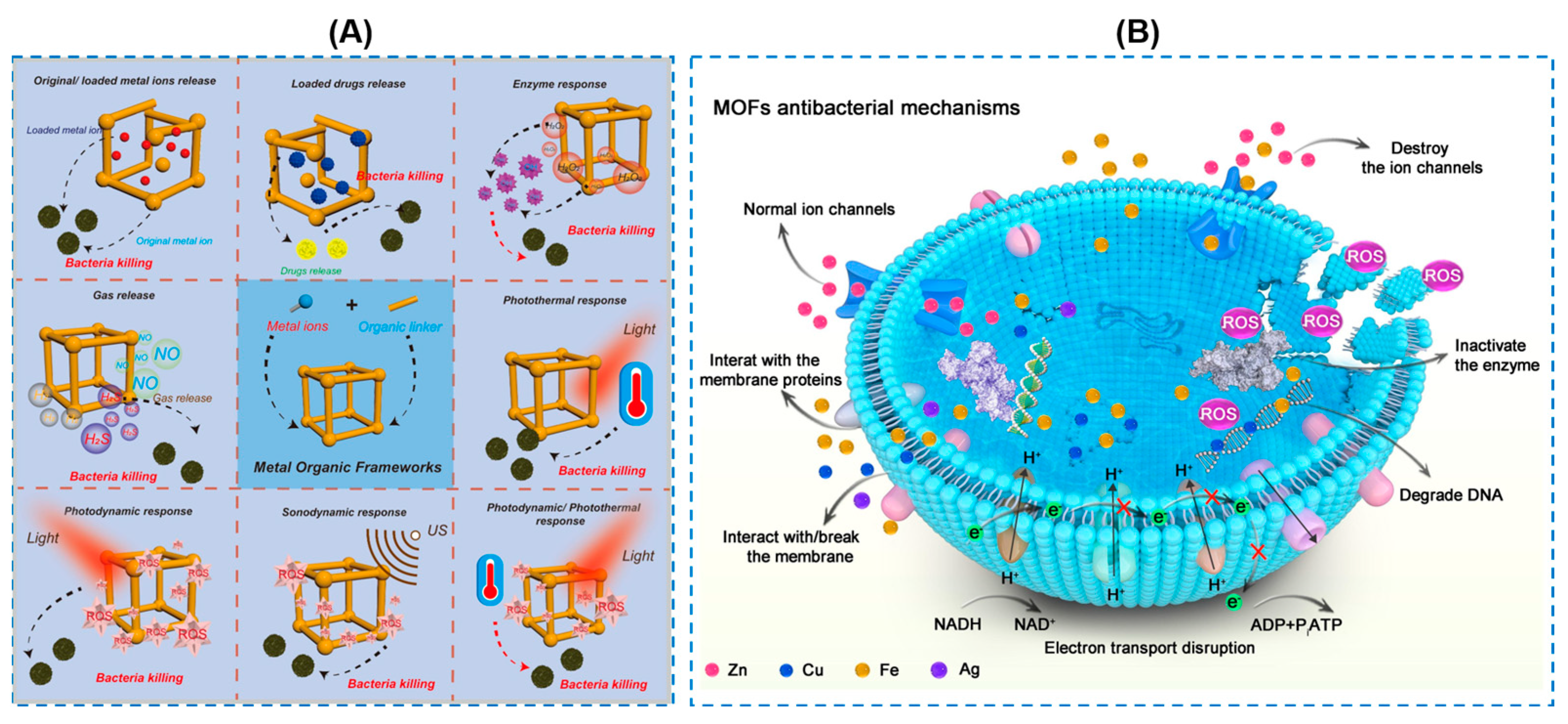
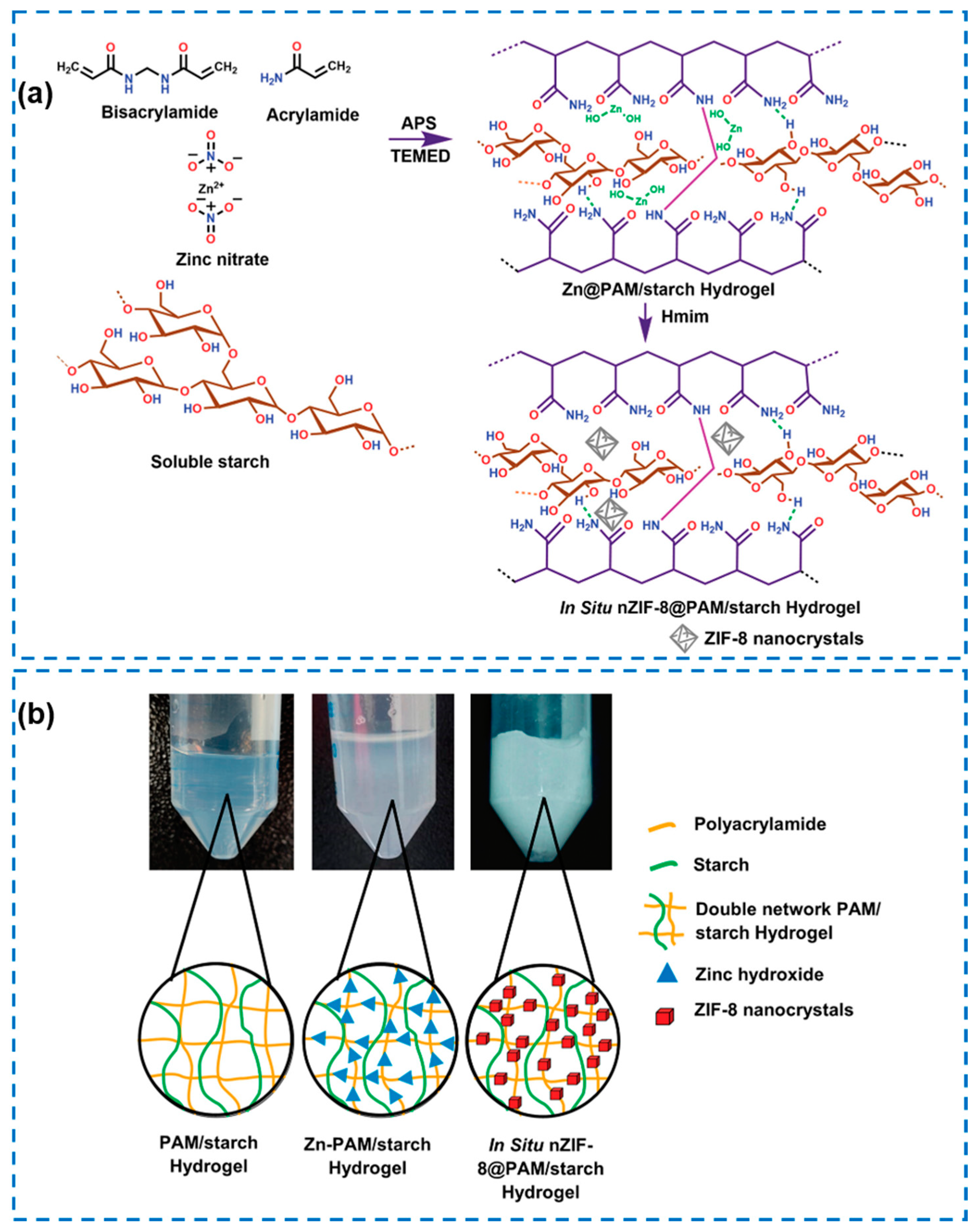
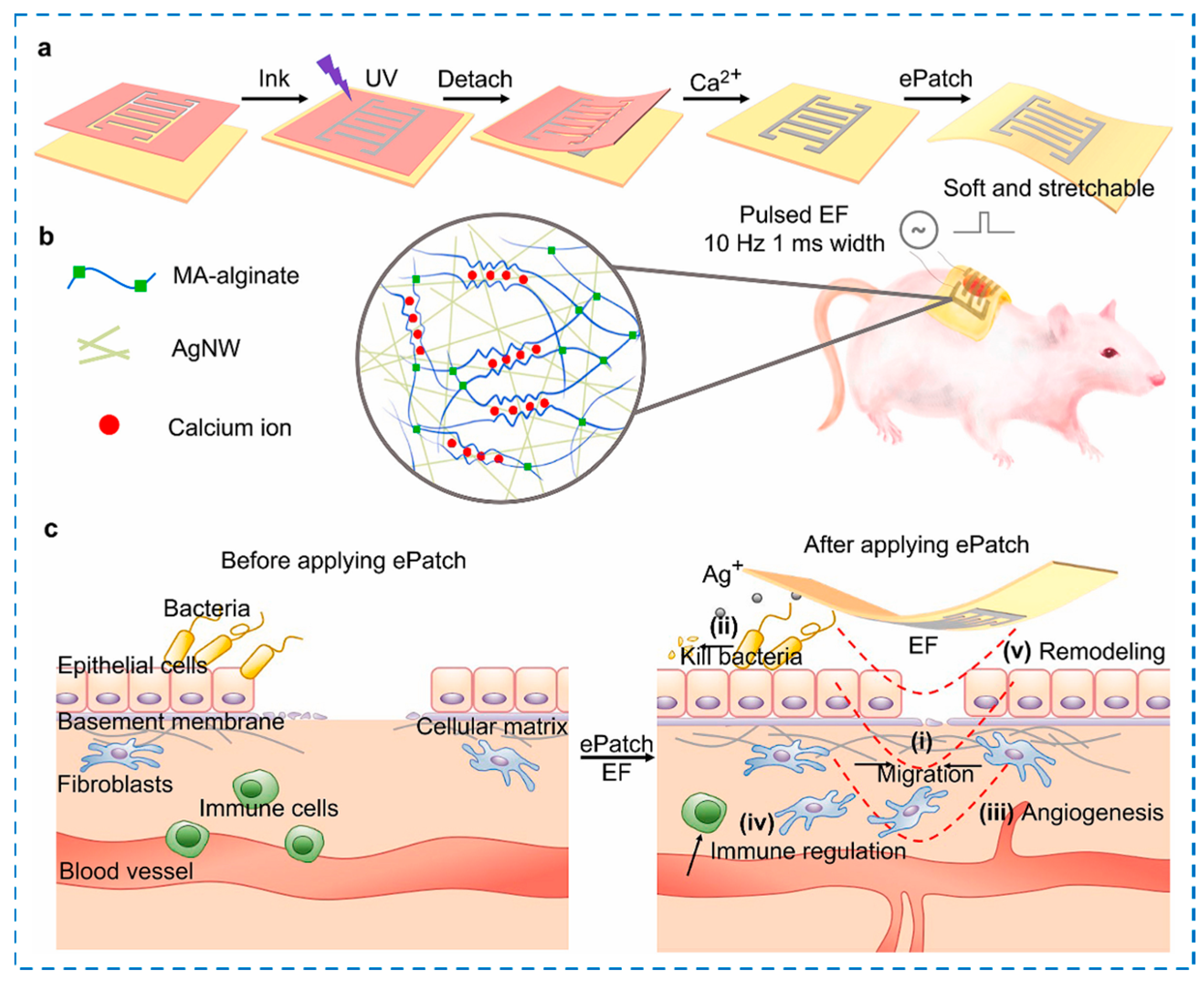
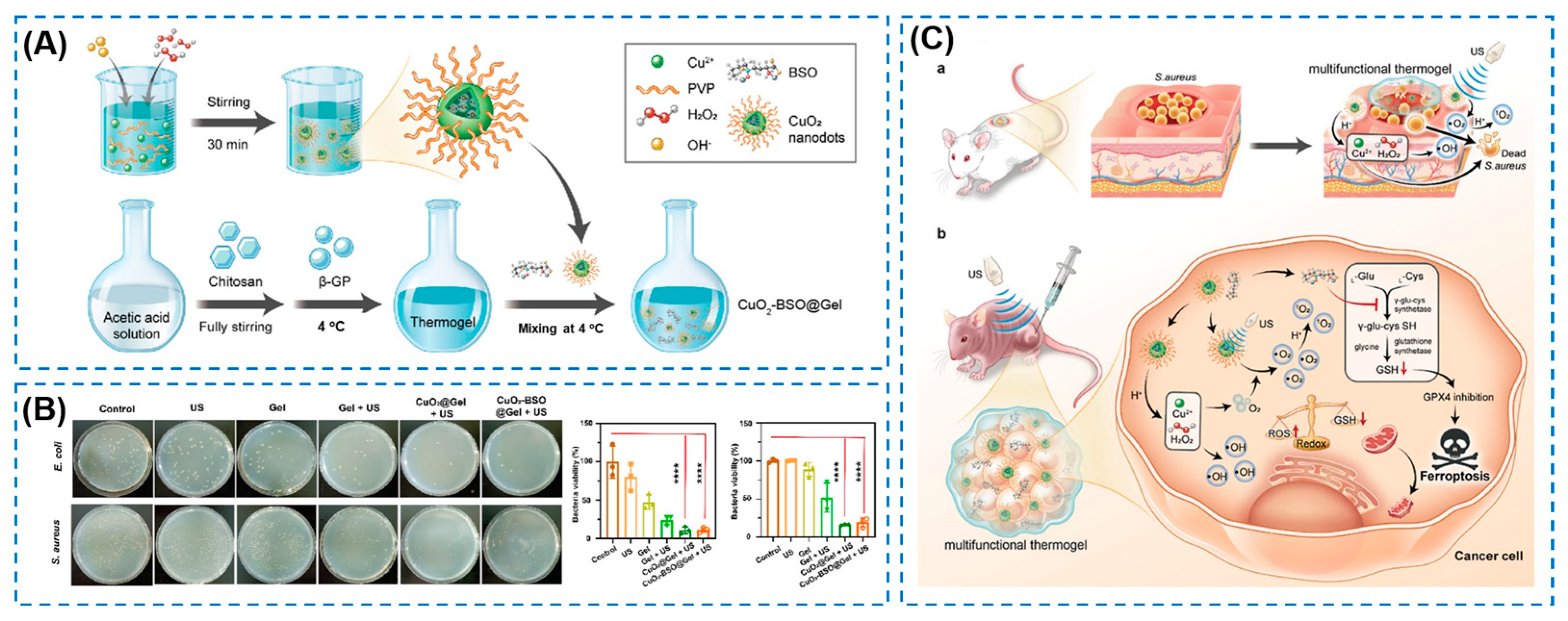
| Components | Antibacterial Mechanism | Antibacterial Ability | Other Performance | Ref. |
|---|---|---|---|---|
| PVA/PAA/TA | TA interrupts the biological activity of bacteria | Obvious inhibition zones for S. aureus (11.5 mm) and E. coli (9.83 mm) | Self-healable, elastic, highly toughness, tissue-adhesive, hemostatic | [37] |
| polyethylene oxide (PEO)/guar gum (GG)/rosemary (RM)/citric acid (CA) | RM attacks the cell membrane of bacteria, subsequently diminishing bacterial cell growth | Obvious inhibition zones for S. aureus (9 mm) and E. coli (2 mm) | Moisture adsorption, hydrophily, and cell attachment and proliferation | [38] |
| PAA/L-lysine derived branched polyetheramides (Lys-BPEA) | Lys-BPEA rupture of the bacteria cell membrane | The inhibition rate against E. coli and S. aureus exceeded 80%; long-term stable antibacterial activity | Good mechanical strength, self-healing, and no obvious hemolytic behavior | [39] |
| Types of Nanoenzymes | Other Components | Antibacterial Property | Other Performance as Wound Dressings | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Fe3O4 | TA | No data | Blood compatibility, antioxidative ability, excellent therapeutic efficacy, promote wound healing | Catalyzing the decomposition of H2O2 to generate nontoxic products in neutral environment or to generate hydroxyl radical in acidic environment | [89] |
| CeO2 nanorods | Oxide dextran/ε-polylysine (EPL) | Broad-spectrum antibacterial activity (almost 100%) against E. coli, S. aureus, and MRSA | Self-healing behavior, good adhesiveness, hemostatic ability and promoted MRSA-infected diabetic wound healing | Affecting bacteria plasmalemma and physiological metabolism; releasing antibacterial EPL | [90] |
| PDA@MnO2 | Polydopamine (PDA)/thioctic acid/TA | Notable antibacterial activity against E. coli and S. aureus | Injectable, self-healing, adhesive, biocompatible, antioxidant, anti-inflammatory, and promoted the chronic diabetic wound healing | Scavenging various types of reactive nitrogen and oxygen species, and generating O2 by degrading H2O2 | [91] |
| MnO2 nanosheets | Poly(ethylene glycol) methyl ether methacrylate (PEGMA)/glycidyl methacrylate (GMA)/acrylamide (AAm) | Broad-spectrum antibacterial activity against MRSA, E. coli and Pseudomonas aeruginosa (as high as 99.9%) | ROS-scavenging, O2 generation, anti-oxidative, accelerated the infected diabetic skin wound healing | Decreasing the level of ROS, suppressing the inflammation and neutrophil infiltration, and promoting the polarization of macrophages into M2-type | [92] |
| Therapy Type | Functional Agents | Concentration | Antibacterial Ability | Antibacterial Mechanism | Ref. | |
|---|---|---|---|---|---|---|
| PTT | Graphene oxide (GO) | 3–5 wt% | E. coli and S. aureus: 99.9% | The use of photothermal reagents to convert light energy into heat energy | [99] | |
| CuS nanoparticles (CuSNPs) | 2 mM | Qualitative analysis: obvious | [100] | |||
| Protocatechualdehyde (PA) | 4.5 mg/mL | E. coli: 89.2%; S. aureus: 87.0% (1 min); E. coli and S. aureus: 100% (10 min) | [101] | |||
| PDT | Ag/Ag@AgCl | / | E. coli: 95.95%; S. aureus: 98.49% | Photosensitizers (PSs) combined with light and oxygen can generate ROS | [102] | |
| Black Phosphorus (BP) | / | E. col: 98.90%; S. aureus: 99.51% | [103] | |||
| Berberine Chloride (BBR) | 312.5 μM | E. coli and S. aureus: 625.0 μM (MIC); E. col: 79.1%; S. aureus: 100% | [104] | |||
| PTT/PDT | iron-doped carbon dots (Fe-CDs) | / | E. coli and S. aureus: more than 99% | Fe-CDs can act as photothermal therapeutic agents and photodynamic therapeutic agents to generate heat (approximately 50 °C) and rapidly catalyze the decomposition of hydrogen peroxide to produce hydroxyl radicals | [105] | |
| Types of MOF | Metal Ions | Antibacterial Ability | Ref. |
|---|---|---|---|
| Cu-MOFs | Cu2+ | 99.9% against S. mutans and MRSA; 78.7% against C. albicans | [115] |
| K-MOF | K+ | Qualitative analysis: obvious | [116] |
| Co-MOF | Co2+ | Higher long-lasting antibacterial effect | [117] |
| ZIF-8 | Zn2+ | Qualitative analysis: obvious | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Cheng, H.; Zhang, Z.; Chen, K.; Zhang, Q.; Zhang, C.; Gao, W.; Zheng, Y. Antibacterial Hydrogels for Wound Dressing Applications: Current Status, Progress, Challenges, and Trends. Gels 2024, 10, 495. https://doi.org/10.3390/gels10080495
Zhu J, Cheng H, Zhang Z, Chen K, Zhang Q, Zhang C, Gao W, Zheng Y. Antibacterial Hydrogels for Wound Dressing Applications: Current Status, Progress, Challenges, and Trends. Gels. 2024; 10(8):495. https://doi.org/10.3390/gels10080495
Chicago/Turabian StyleZhu, Jie, Hongju Cheng, Zixian Zhang, Kaikai Chen, Qinchen Zhang, Chen Zhang, Weihong Gao, and Yuansheng Zheng. 2024. "Antibacterial Hydrogels for Wound Dressing Applications: Current Status, Progress, Challenges, and Trends" Gels 10, no. 8: 495. https://doi.org/10.3390/gels10080495
APA StyleZhu, J., Cheng, H., Zhang, Z., Chen, K., Zhang, Q., Zhang, C., Gao, W., & Zheng, Y. (2024). Antibacterial Hydrogels for Wound Dressing Applications: Current Status, Progress, Challenges, and Trends. Gels, 10(8), 495. https://doi.org/10.3390/gels10080495








