Rheological and Structural Characterization of Carrageenans during Depolymerization Conducted by a Marine Bacterium Shewanella sp. LE8
Abstract
:1. Introduction
2. Results and Discussion
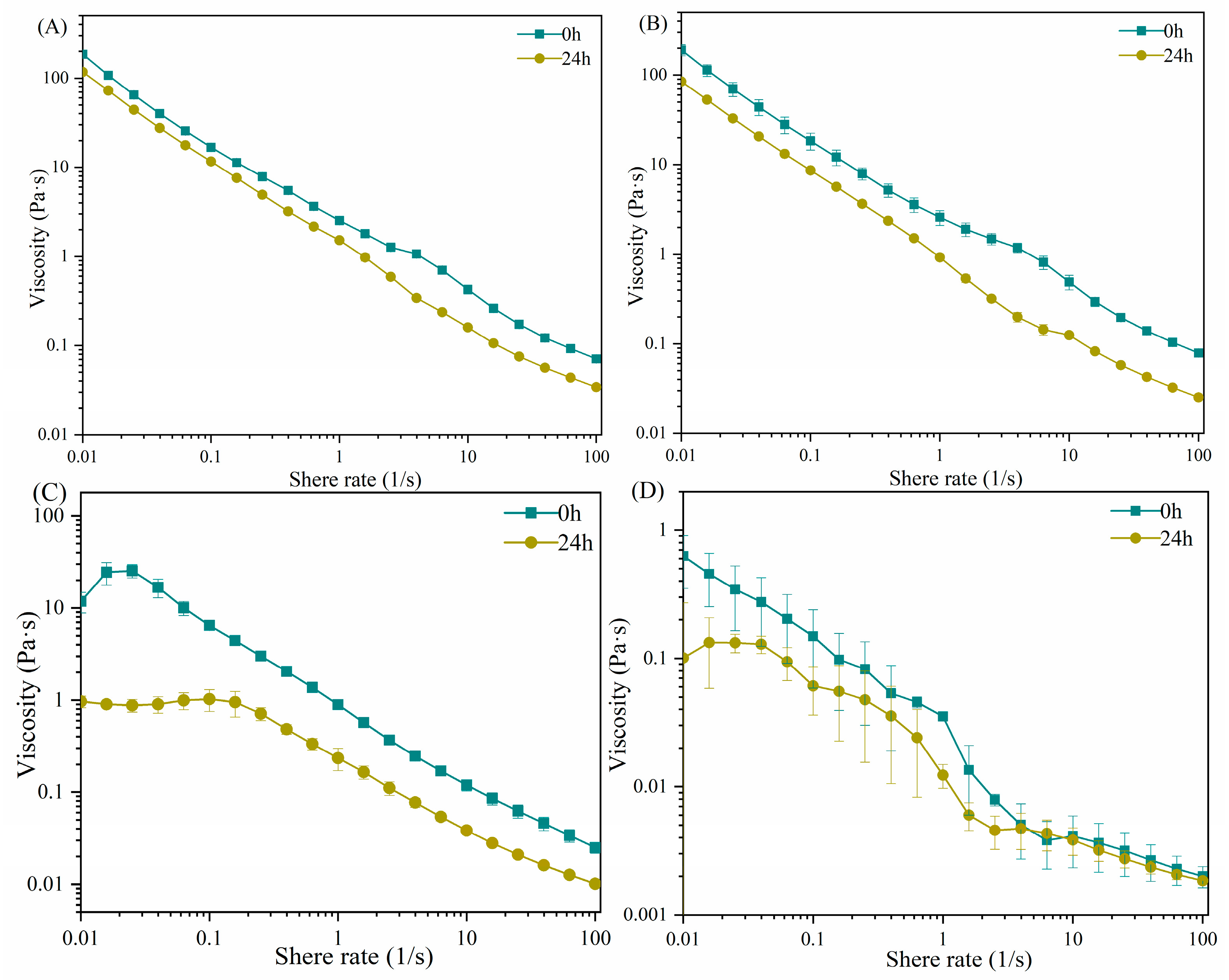
2.1. The Apparent Viscosity of Carrageenans after LE8 Fermentation
2.2. Rheological Characterization of Carrageenans after LE8 Fermentation
2.3. Spectral Changes with Methylene Blue after LE8 Fermentation
2.4. Molecular Weights of Carrageenans after LE8 Fermentation
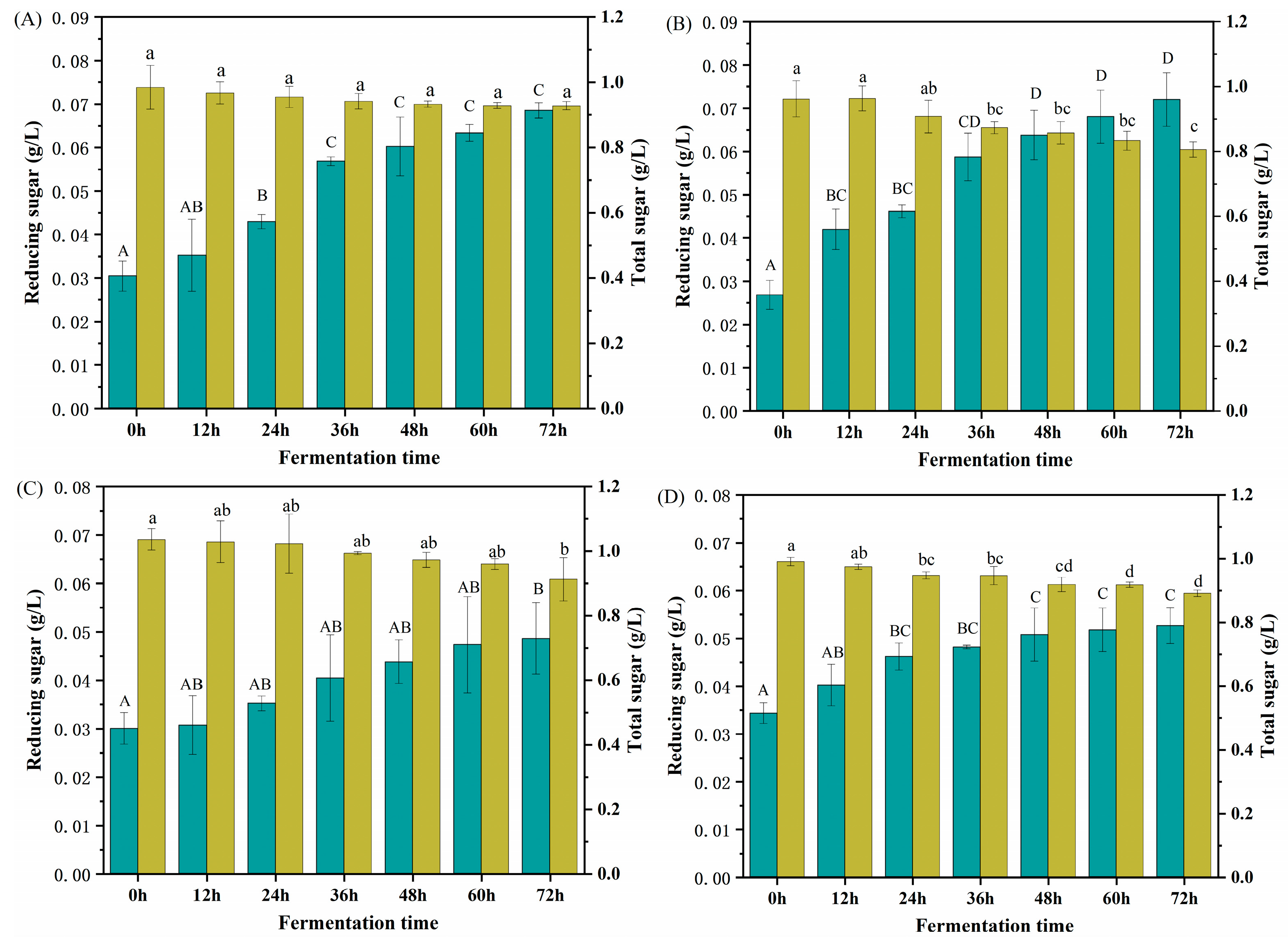
2.5. Changes in Total and Reducing Sugars
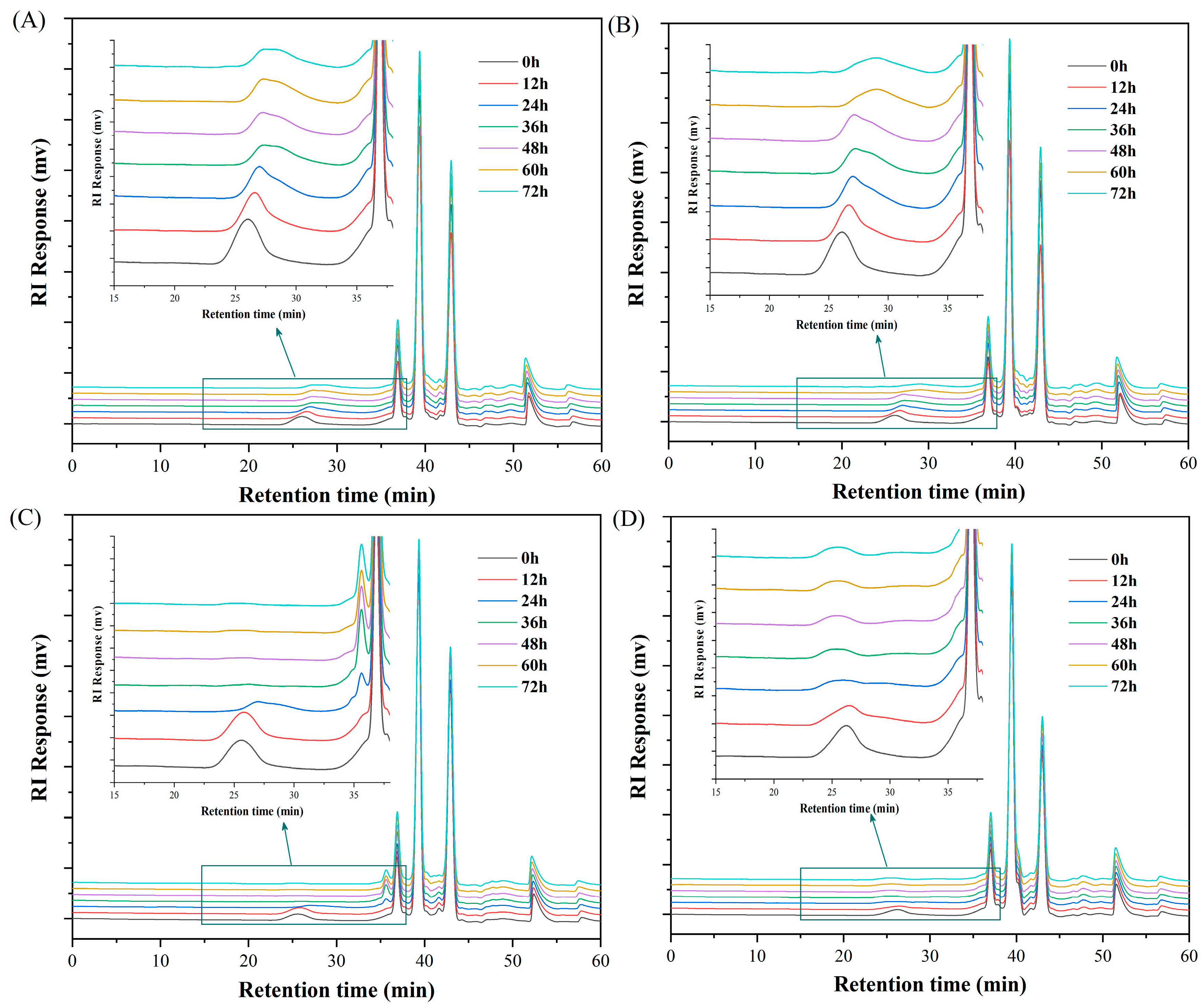
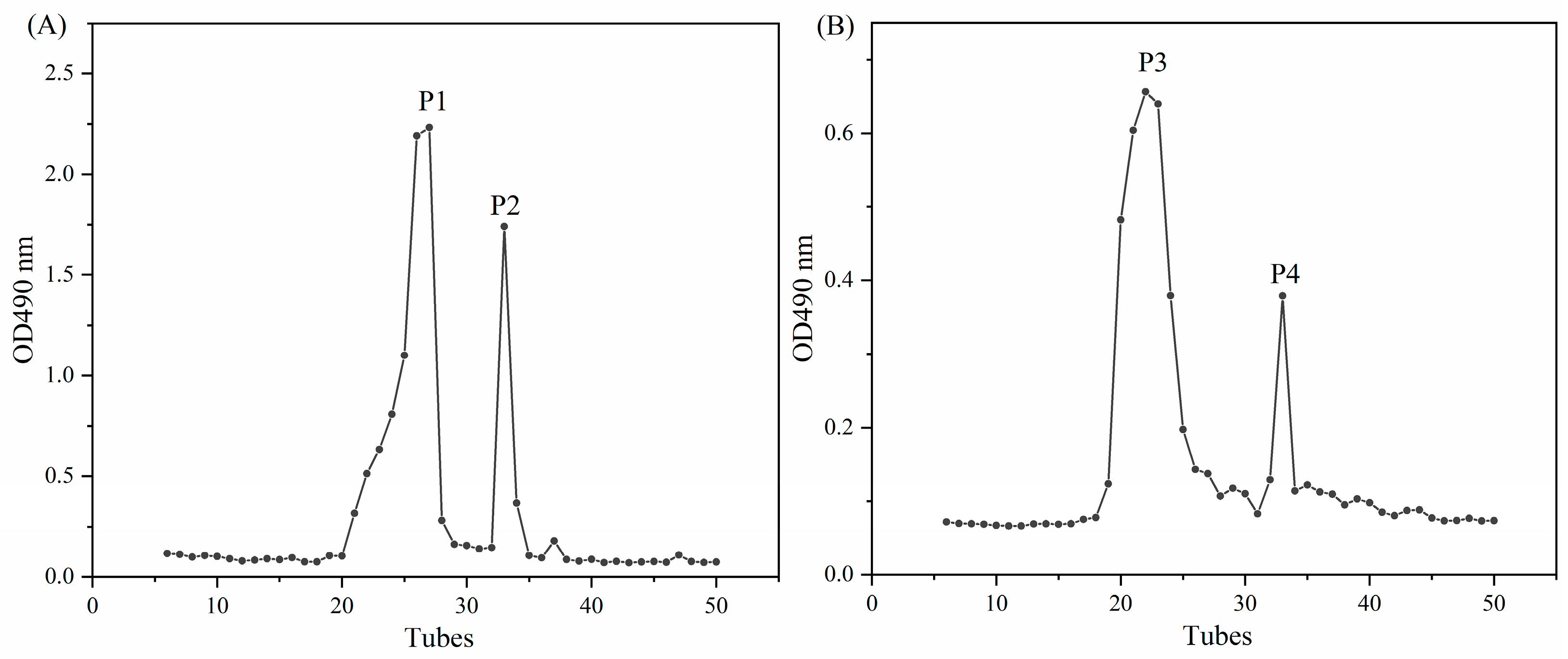
2.6. Purification and Structural Characterization of Fermentation Products
2.6.1. Purification of Carrageenan Fermentation Products
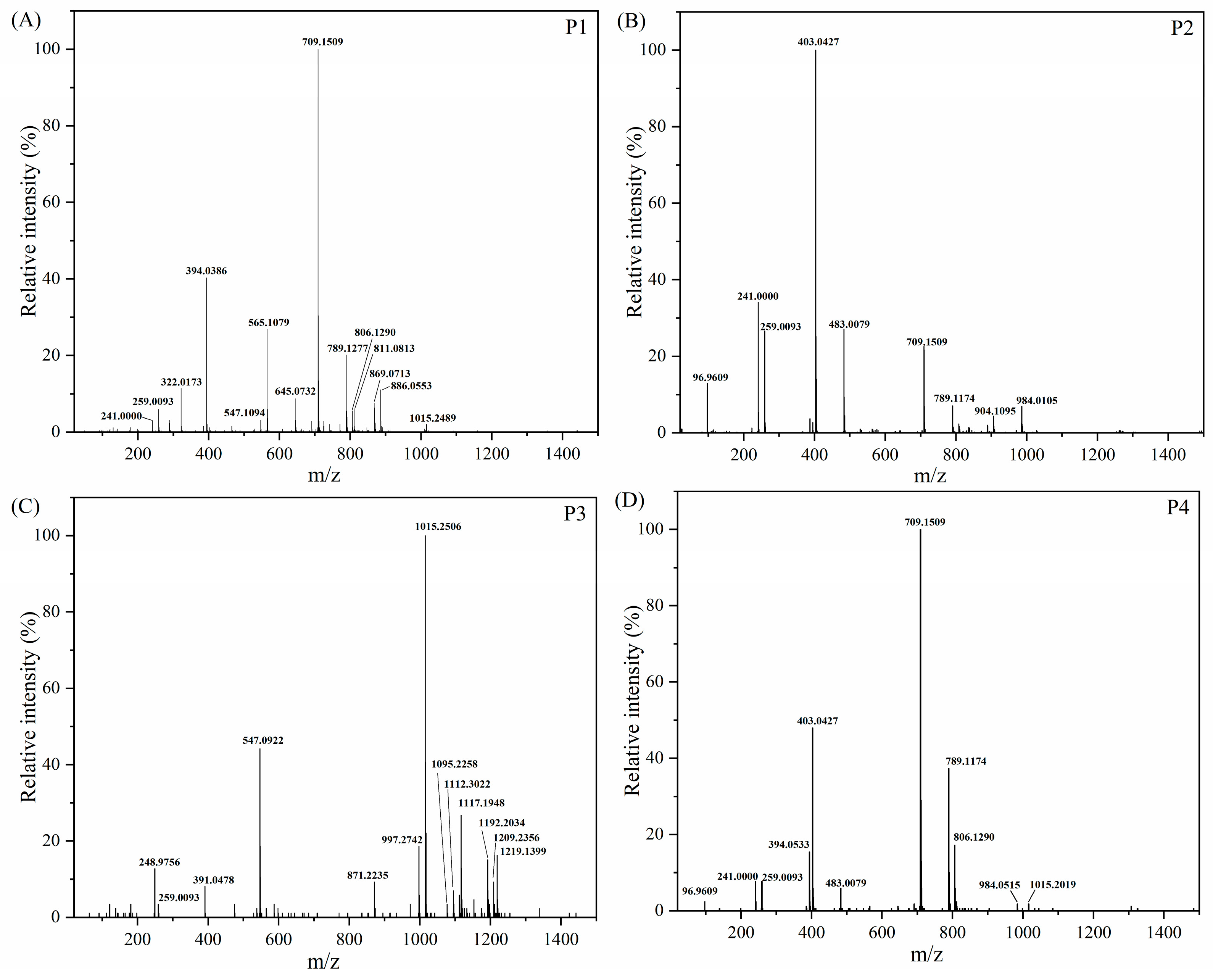
2.6.2. UPLC-TOF MS Analysis of Carrageenan Fermentation Products
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Culture Medium and Growth Conditions
4.3. Dynamic Viscosity and Rheological Analysis
4.4. Methylene Blue Analysis
4.5. Determination of Molecular Weights
4.6. Determination of Total and Reducing Sugars
4.7. Structural Analysis of Carrageenans Fermentation Products
4.7.1. Preparation and Purification of Polysaccharides from Fermentation Products
4.7.2. UPLC-TOF MS Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Gao, L.; Xue, C.; Mao, X. Marine-polysaccharide degrading enzymes: Status and prospects. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2767–2796. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ni, F.; Sun, Y.; Zhu, X.; Yin, H.; Yao, Z.; Du, Y. Insight into carrageenases: Major review of sources, category, property, purification method, structure, and applications. Crit. Rev. Biotechnol. 2018, 38, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Groult, H.; Cousin, R.; Chot-Plassot, C.; Maura, M.; Bridiau, N.; Piot, J.M.; Maugard, T.; Fruitier-Arnaudin, I. λ-Carrageenan Oligosaccharides of Distinct Anti-Heparanase and Anticoagulant Activities Inhibit MDA-MB-231 Breast Cancer Cell Migration. Mar. Drugs 2019, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Abad, L.V.; Kudo, H.; Saiki, S.; Nagasawa, N.; Tamada, M.; Katsumura, Y.; Aranilla, C.T.; Relleve, L.S.; De La Rosa, A.M. Radiation degradation studies of carrageenans. Carbohydr. Polym. 2009, 78, 100–106. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Lv, Y.; Li, M. Preparation of κ-carra-oligosaccharides with microwave assisted acid hydrolysis method. J. Ocean Univ. China 2015, 14, 345–349. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Jiang, X.; Guan, H. A κ-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity. J. Appl. Phycol. 2003, 15, 297–303. [Google Scholar] [CrossRef]
- Jouanneau, D.; Boulenguer, P.; Mazoyer, J.; Helbert, W. Enzymatic degradation of hybrid ι-/ν-carrageenan by Alteromonas fortis ι-carrageenase. Carbohydr. Res. 2010, 345, 934–940. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2012, 25, 65–72. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Silva, D.B.D.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, F.; Ning, L.; Yao, Z.; Du, Y. Cloning and biochemical characterization of a novel κ-carrageenase from newly isolated marine bacterium Pedobacter hainanensis NJ-02. Int. J. Biol. Macromol. 2018, 108, 1331–1338. [Google Scholar] [CrossRef]
- Guibet, M.; Colin, S.; Barbeyron, T.; Génicot, S.; Kloareg, B.; Michel, G.; Helbert, W. Degradation of λ-carrageenan by Pseudoalteromonas carrageenovora λ-carrageenase: A new family of glycoside hydrolases unrelated to κ-and ι-carrageenases. Biochem. J. 2007, 404, 105–114. [Google Scholar] [CrossRef]
- Li, J.; Hu, Q.; Seswita-Zilda, D. Purification and characterization of a thermostable λ-carrageenase from a hot spring bacterium, Bacillus sp. Biotechnol. Lett. 2014, 36, 1669–1674. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, H.; Hamouda, H.I.; Wang, T.; Dong, Y.; Mao, X. Biochemical characterization of a cold-adapted λ-carrageenase OUC-CglA from Maribacter vaceletii: An efficient tool for λ-carrageenan degradation. J. Agric. Food Chem. 2022, 70, 12135–12142. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Hatada, Y. A novel enzyme, lambda-carrageenase, isolated from a deep-sea bacterium. J. Biochem. 2006, 140, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, S.; Zhang, Y.; Xue, C.; Xiao, H.; Chang, Y. Expression and Characterization of a Novel λ-Carrageenase Cgl150A_Wa from Wenyingzhuangia aestuarii. J. Ocean Univ. China 2024, 23, 209–215. [Google Scholar] [CrossRef]
- Hau, H.; Gralnick, J. Ecology and Biotechnology of the Genus Shewanella. Annu. Rev. Microbiol. 2007, 61, 237–258. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Sawabe, T.; Gorshkova, N.M.; Svetashev, V.I.; Mikhailov, V.V.; Nicolau, D.V.; Christen, R. Shewanella japonica sp. nov. Int. J. Syst. Evol. Microbiol. 2001, 51, 1027–1033. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Zhang, S.; Li, J.; Yu, W.; Gong, Q. A new κ-carrageenase CgkS from marine bacterium Shewanella sp. Kz7. J. Ocean Univ. China 2015, 14, 759–763. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Zeng, R.; Wu, J.; Michael, S.V.; Qu, W. The degradation activities for three seaweed polysaccharides of Shewanella sp. WPAGA9 isolated from deep-sea sediments. J. Basic Microbiol. 2021, 61, 406–418. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, Y.; Zhang, Y.; Xu, Y.; Zheng, L.; Zhu, B.; Yao, Z. Carrageenan oligosaccharides: A comprehensive review of preparation, isolation, purification, structure, biological activities and applications. Algal Res. 2022, 61, 102593. [Google Scholar] [CrossRef]
- Mokhtari, H.; Tavakoli, S.; Safarpour, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. Recent Advances in Chemically-Modified and Hybrid Carrageenan-Based Platforms for Drug Delivery, Wound Healing, and Tissue Engineering. Polymers 2021, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.-T.V.; Thomas, S.; Huang, Y.-C.; Kong, Z.-L. Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci. Rep. 2020, 10, 18037. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Tu, Z.-c.; Shangguan, X.; Wang, H.; Sha, X.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef]
- Xu, H.; Fan, Q.; Huang, M.; Cui, L.; Gao, Z.; Liu, L.; Chen, Y.; Jin, J.; Jin, Q.; Wang, X. Combination of carrageenan with sodium alginate, gum arabic, and locust bean gum: Effects on rheological properties and quiescent stabilities of partially crystalline emulsions. Int. J. Biol. Macromol. 2023, 253, 127561. [Google Scholar] [CrossRef] [PubMed]
- Bantang, J.; Bigol, U.; Camacho, D. Gel and Film Composites of Silver Nanoparticles in κ-, ι-, and λ-Carrageenans: One-Pot Synthesis, Characterization, and Bioactivities. BioNanoScience 2021, 11, 53–66. [Google Scholar] [CrossRef]
- Michel, A.S.; Mestdagh, M.M.; Axelos, M.A.V. Physico-chemical properties of carrageenan gels in presence of various cations. Int. J. Biol. Macromol. 1997, 21, 195–200. [Google Scholar] [CrossRef]
- Rodriguez-Canto, W.; Cerqueira, M.A.; Chel-Guerrero, L.; Pastrana, L.M.; Aguilar-Vega, M. Delonix regia galactomannan-based edible films: Effect of molecular weight and k-carrageenan on physicochemical properties. Food Hydrocoll. 2020, 103, 105632. [Google Scholar] [CrossRef]
- Running, C.A.; Falshaw, R.; Janaswamy, S. Trivalent iron induced gelation in lambda-carrageenan. Carbohydr. Polym. 2012, 87, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Nishinari, K. “Weak Gel”-Type Rheological Properties of Aqueous Dispersions of Nonaggregated κ -Carrageenan Helices. J. Agric. Food Chem. 2001, 49, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Bartlová, M.; Ziółkowska, D.; Pospiech, M.; Shyichuk, A.; Tremlová, B. Determination of carrageenan in jellies with new methylene blue dye using spectrophotometry, smartphone-based colorimetry and spectrophotometric titration. Food Sci. Technol. 2020, 41, 81–90. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Michon, C.; Konaté, K.; Cuvelier, G.; Launay, B. Gelatin/carrageenan interactions in coil and ordered conformations followed by a methylene blue spectrophotometric method. Food Hydrocoll. 2002, 16, 613–618. [Google Scholar] [CrossRef]
- Ziółkowska, D.; Kaniewska, A.; Lamkiewicz, J.; Shyichuk, A. Determination of carrageenan by means of photometric titration with Methylene Blue and Toluidine Blue dyes. Carbohydr. Polym. 2017, 165, 1–6. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Sato, T. Macromolecular complexes of the main storage protein of Vicia faba seeds with sulfated polysaccharide. Food Hydrocoll. 2009, 23, 996–1006. [Google Scholar] [CrossRef]
- Soedjak, H.S. Colorimetric Determination of Carrageenans and Other Anionic Hydrocolloids with Methylene Blue. Anal. Chem. 1994, 66, 4514–4518. [Google Scholar] [CrossRef]
- Spada, J.C.; Marczak, L.D.F.; Tessaro, I.C.; Cardozo, N.S.M. Interactions between soy protein from water-soluble soy extract and polysaccharides in solutions with polydextrose. Carbohydr. Polym. 2015, 134, 119–127. [Google Scholar] [CrossRef]
- Shen, J.; Chang, Y.; Dong, S.; Chen, F. Cloning, expression and characterization of a ι-carrageenase from marine bacterium Wenyingzhuangia fucanilytica: A biocatalyst for producing ι-carrageenan oligosaccharides. J. Biotechnology. 2017, 259, 103–109. [Google Scholar] [CrossRef]
- Vieira, F.T.; Corrêa, C.G.R.; Peralta, A.R.; Peralta-Muniz-Moreira, F.R.; Bracht, A.; Peralta, M.R. An Overview of Structural Aspects and Health Beneficial Effects of Antioxidant Oligosaccharides. Curr. Pharm. Design. 2020, 26, 1759–1777. [Google Scholar] [CrossRef] [PubMed]
- Duedahl-Olesen, L.; Haastrup Pedersen, L.; Lambertsen Larsen, K. Suitability and limitations of methods for characterisation of activity of malto-oligosaccharide-forming amylases. Carbohydr. Res. 2000, 329, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Iriti, M. Sephadex® LH-20, Isolation, and Purification of Flavonoids from Plant Species: A Comprehensive Review. Molecules 2020, 25, 4146. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, Y.; Yin, X.; Nan, Y.; Shi, Y.; Chen, X.; Liang, H.; Zhang, J.; Ma, B. Isolation and structural elucidation of prebiotic oligosaccharides from Ziziphi Spinosae Semen. Carbohydr. Res. 2023, 534, 108948. [Google Scholar] [CrossRef]
- Michel, G.; Chantalat, L.; Fanchon, E.; Henrissat, B.; Kloareg, B.; Dideberg, O. The ι-carrageenase of alteromonas fortis: A β-helix fold-containing enzyme for the degradation of a highly polyanionic polysaccharide. J. Biol. Chem. 2001, 276, 40202–40209. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Yu, Y.; Liu, Z.; Tian, L.; Mou, H. An effective method for the preparation of carrageenan oligosaccharides directly from Eucheuma cottonii using cellulase and recombinant κ-carrageenase. Algal Res. 2016, 15, 93–99. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Kube, M.; Bauer, M.; Teeling, H.; Lombardot, T.; Ludwig, W.; Gade, D.; Beck, A.; Borzym, K.; Heitmann, K. Complete genome sequence of the marine planctomycete Pirellula sp. strain 1. Proc. Natl. Acad. Sci. USA 2003, 100, 8298–8303. [Google Scholar] [CrossRef] [PubMed]
- Génicot, S.; Groisillier, A.; Rogniaux, H.; Meslet-Cladiere, L.; Barbeyron, T.; Helbert, W. Discovery of a novel iota carrageenan sulfatase isolated from the marine bacterium Pseudoalteromonas carrageenovora. Front. Chem. 2014, 2, 67. [Google Scholar] [CrossRef]
- Saluri, K.; Tuvikene, R. Anticoagulant and antioxidant activity of lambda- and theta-carrageenans of different molecular weights. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100243. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Palla, C.S.; Dethe, D.H.; Joshi, Y.M. Rheological investigation of the network structure in mixed gels of Kappa and Iota Carrageenan. Food Hydrocoll. 2024, 146, 109298. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Shen, M.; Chen, Y.; Yu, Q.; Xie, J. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci. Technol. 2022, 123, 198–209. [Google Scholar] [CrossRef]
- Song, C.; You, Y.; Wen, C.; Fu, Y.; Yang, J.; Zhao, J.; Song, S. Characterization and Gel Properties of Low-Molecular-Weight Carrageenans Prepared by Photocatalytic Degradation. Polymers 2023, 15, 602. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Borthakur, A.; Dudeja, P.K.; Tobacman, J.K. Carrageenan Induces Cell Cycle Arrest in Human Intestinal Epithelial Cells in Vitro123. J. Nutr. 2008, 138, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Yan, X.J.; Wang, F.; Xu, W.F.; Zhang, L. Assessment of the oxidative cellular toxicity of a κ-carrageenan oxidative degradation product towards Caco-2 cells. Food Res. Int. 2010, 43, 2390–2401. [Google Scholar] [CrossRef]
- Li, S.; He, N.; Han, Q.; Li, X.; Jung, S.; Suk Lee, B.; Kumar Mongre, R.; Wang, Z.-P.; Wang, L.; Lee, M.-S. Production of a thermo-tolerant κ-carrageenase via a food-grade host and anti-oxidant activity of its enzymatic hydrolysate. Food Chem. 2021, 339, 128027. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, W.; Hu, C.; Lin, C.; You, L.; Mao, J. Comparative Analysis of Gracilaria chouae Polysaccharides Derived from Different Geographical Regions: Focusing on Their Chemical Composition, Rheological Properties, and Gel Characteristics. Gels 2024, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, B.; Chi-Keung Cheung, P.; Wang, J.; Zheng, X.; You, L. Depolymerized non-digestible sulfated algal polysaccharides produced by hydrothermal treatment with enhanced bacterial fermentation characteristics. Food Hydrocoll. 2022, 130, 107687. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Qin, H.; Huang, M.; Liu, S.; Chang, R.; Xi, B.; Mao, J.; Zhang, S. Obtaining non-digestible polysaccharides from distillers’ grains of Chinese baijiu after extrusion with enhanced antioxidation capability. Int. J. Biol. Macromol. 2023, 243, 124799. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
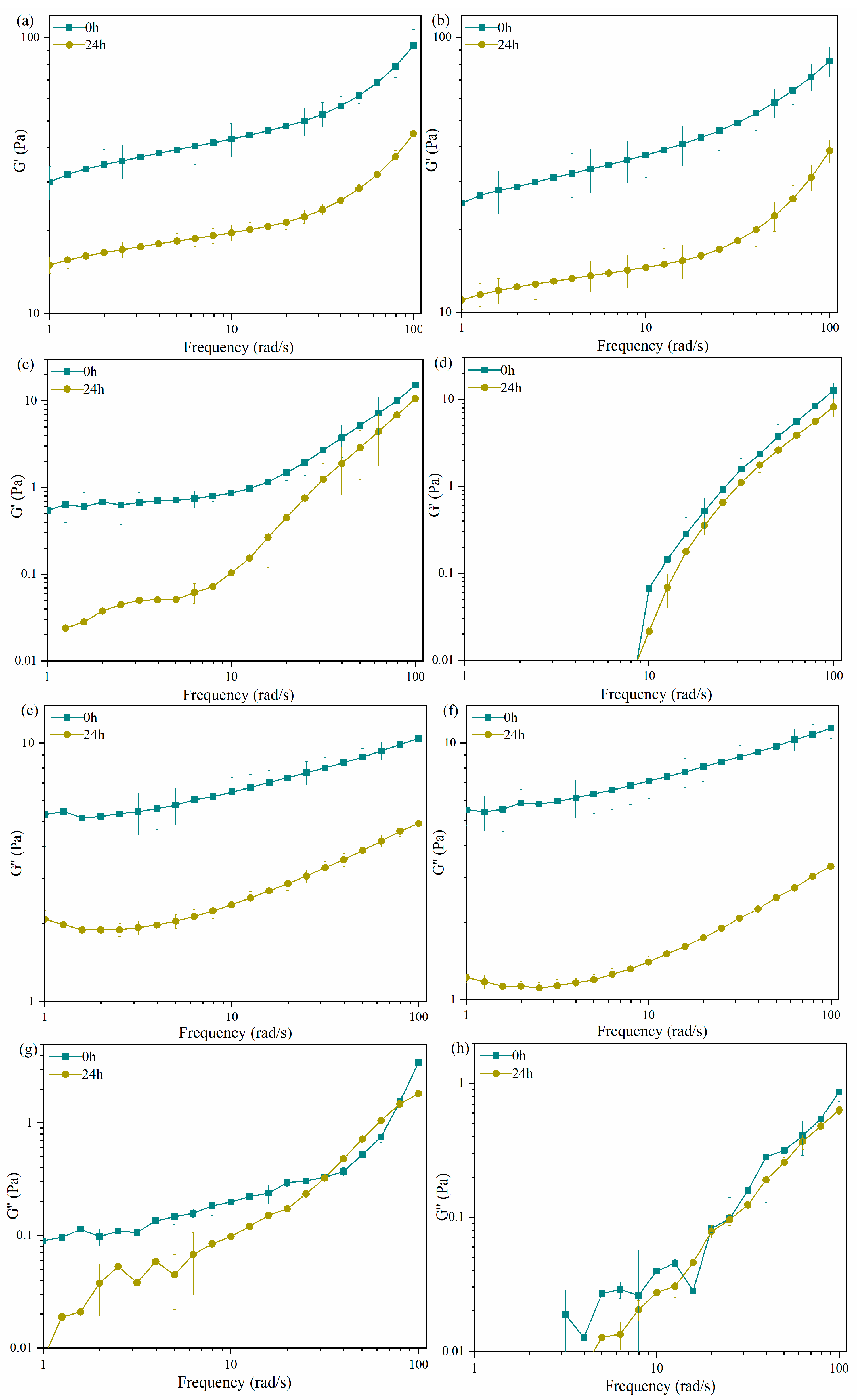

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, C.; Liu, Y.; Han, G.; Lin, C.; Chen, X.; Mao, J. Rheological and Structural Characterization of Carrageenans during Depolymerization Conducted by a Marine Bacterium Shewanella sp. LE8. Gels 2024, 10, 502. https://doi.org/10.3390/gels10080502
Li X, Li C, Liu Y, Han G, Lin C, Chen X, Mao J. Rheological and Structural Characterization of Carrageenans during Depolymerization Conducted by a Marine Bacterium Shewanella sp. LE8. Gels. 2024; 10(8):502. https://doi.org/10.3390/gels10080502
Chicago/Turabian StyleLi, Xiong, Chuyi Li, Yizhou Liu, Gang Han, Congyu Lin, Xiaoli Chen, and Jian Mao. 2024. "Rheological and Structural Characterization of Carrageenans during Depolymerization Conducted by a Marine Bacterium Shewanella sp. LE8" Gels 10, no. 8: 502. https://doi.org/10.3390/gels10080502




