High-Performance Supercapacitors Using Compact Carbon Hydrogels Derived from Polybenzoxazine
Abstract
1. Introduction
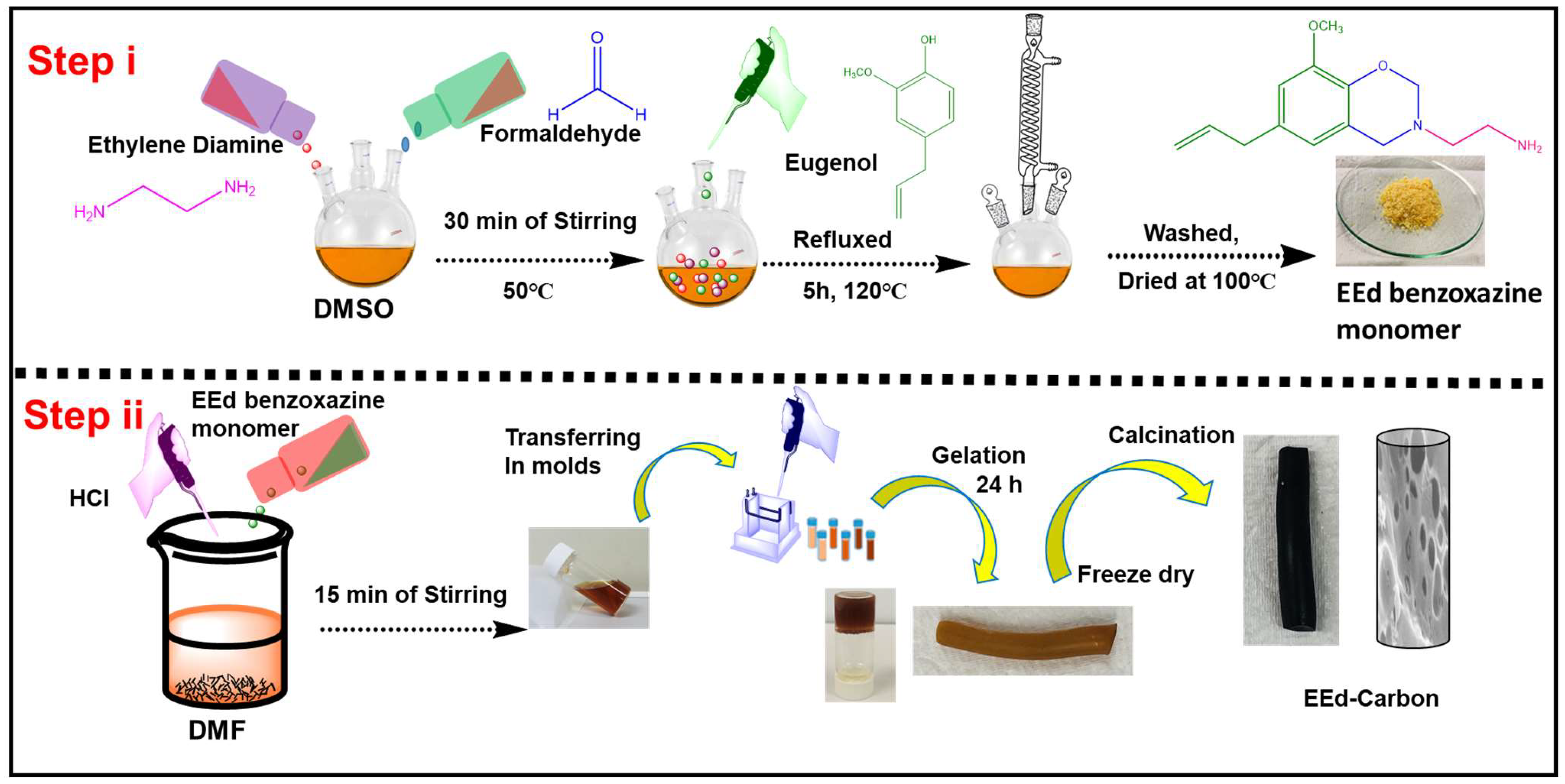
2. Efficient Synthesis of Ethylene Diamine Benzoxazine (EEd-Bzo) and Its Transformation into a Porous Carbon Material (PBZC)
2.1. Synthesis of EEd-Bzo Monomer
2.2. Conversion of EEd-Bzo Monomer to PBZC
2.3. Tailoring Porous Carbon: The Intricate Synthesis of Polybenzoxazine Aerogel Carbon (PBZGC)
3. Structural Analysis of EEd-Bzo
3.1. FT-IR Spectroscopy
3.2. NMR Spectroscopy
3.3. Unveiling a Lower-Temperature Polymerization Pathway for EEd-Bzo
4. Characterization of Carbon Structure and Graphitization
5. Porosity Characterization of Synthesized Carbon Materials
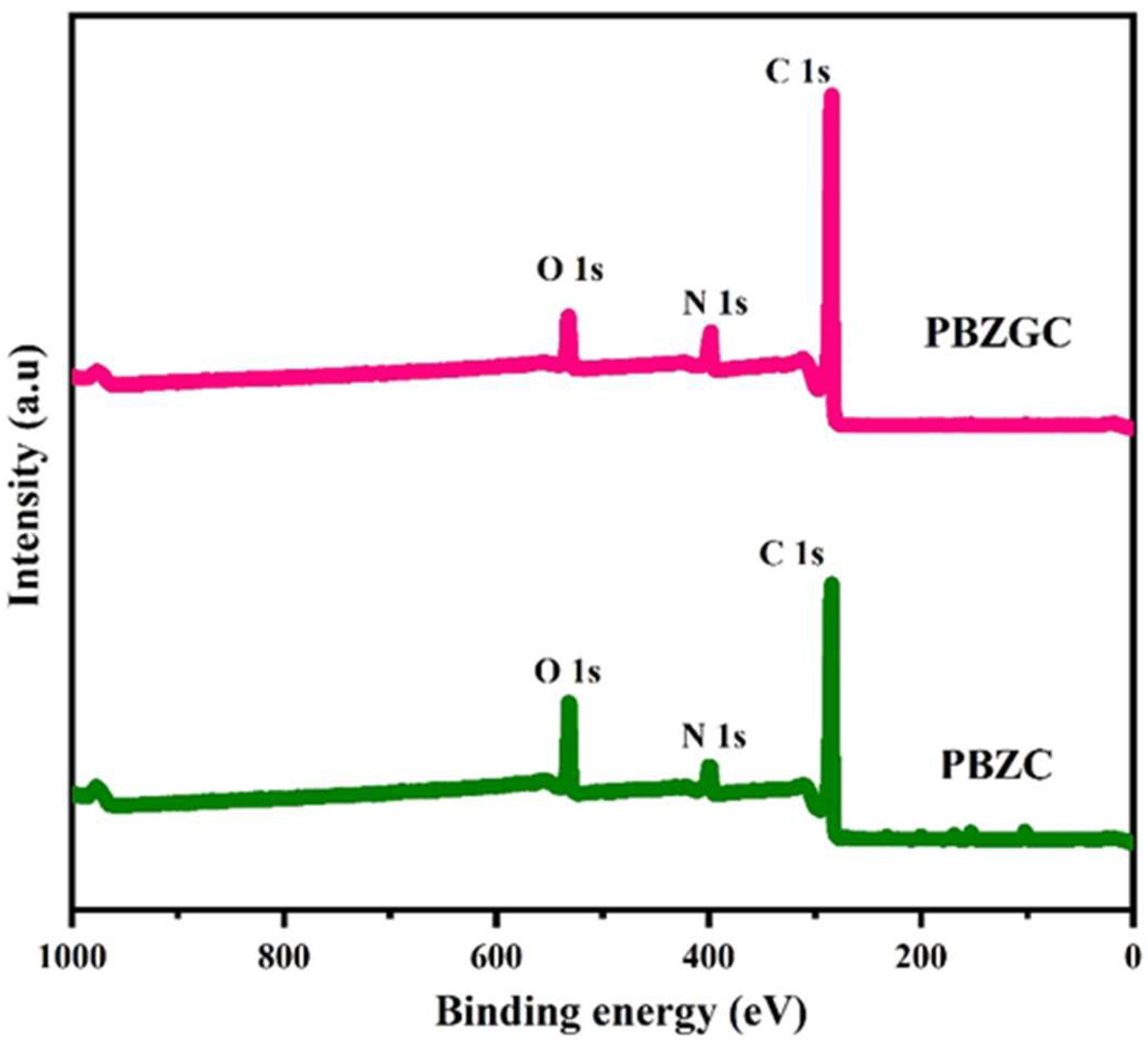
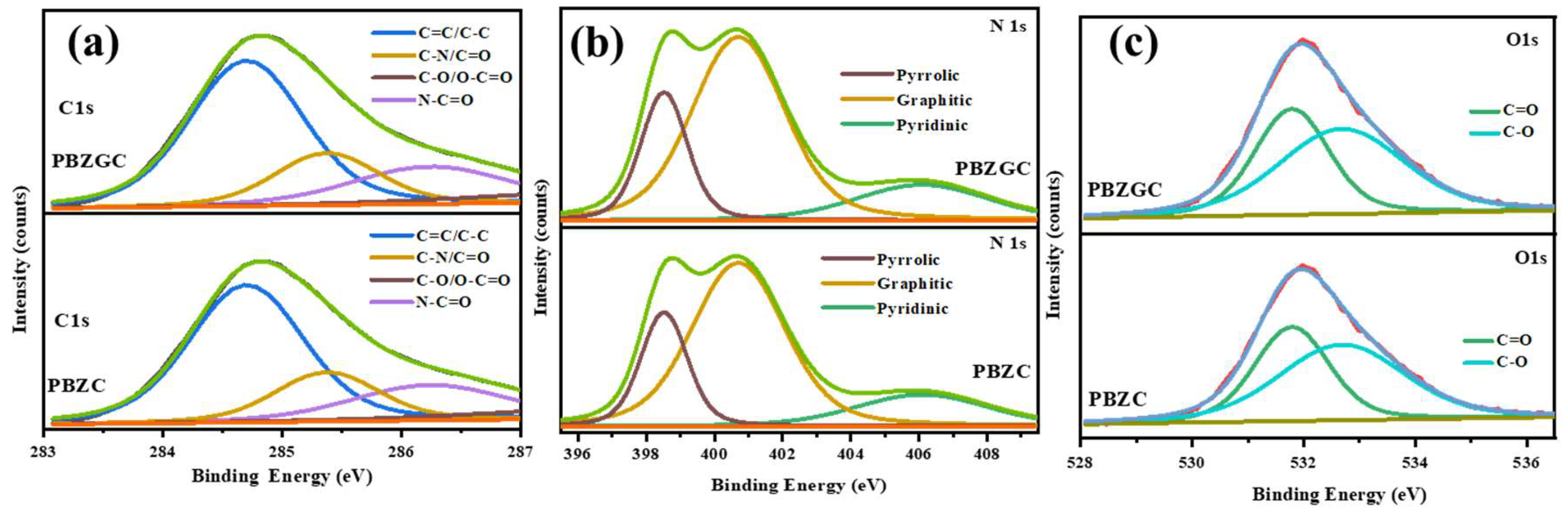
6. Unveiling the Chemical Landscape: XPS Analysis of Nitrogen-Doped Carbon Aerogels
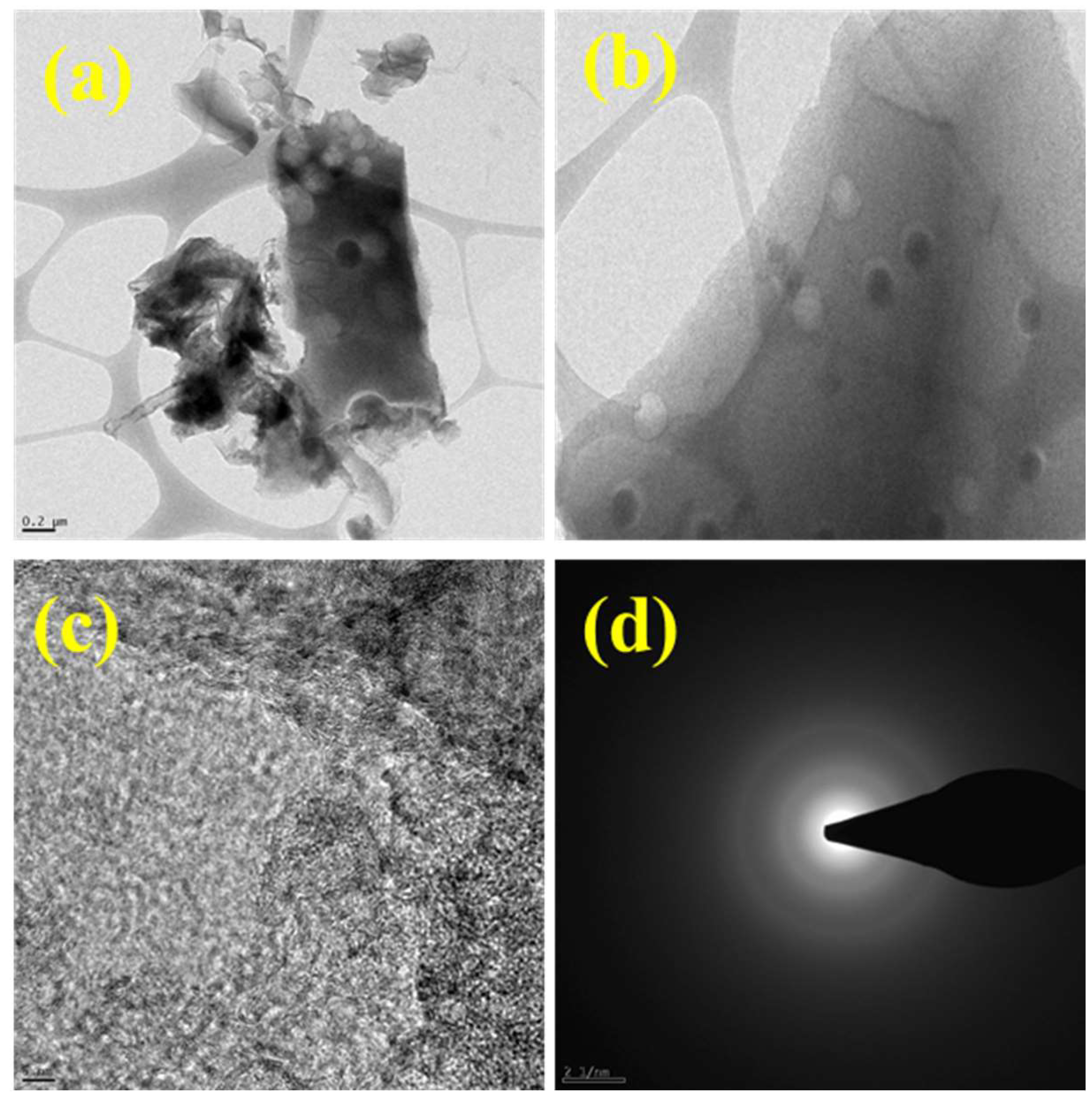
7. Contrasting Morphologies of the Carbon Materials
8. Electrochemical Studies
Nitrogen-Rich Porous Carbons for Supercapacitors: PBZC and PBZGC
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Rittidech, A.; Portia, L.; Bongkarn, T. The Relationship between Microstructure and Mechanical Properties of Al2O3-MgO Ceramics. Mater. Sci. Eng. A 2006, 438–440, 395–398. [Google Scholar] [CrossRef]
- Riu, D.H.; Kong, Y.M.; Kim, H.E. Effect of Cr2O3 Addition on Microstructural Evolution and Mechanical Properties of Al2O3. J. Eur. Ceram. Soc. 2000, 20, 1475–1481. [Google Scholar] [CrossRef]
- Ali, N.; Bashir, S.; Begum, N.; Rafique, M.S.; Husinsky, W. Effect of Liquid Environment on the Titanium Surface Modification by Laser Ablation. Appl. Surf. Sci. 2017, 405, 298–307. [Google Scholar] [CrossRef]
- Vlasova, M.; Aguilar, P.A.M.; Kakazey, M.; Reséndiz-González, M.C.; Bykov, A.; Ragulya, A.; Tomila, T. Modification of a SiC-Cr5Si3 Ceramic Surface by Laser Irradiation. Ceram. Int. 2007, 33, 433–437. [Google Scholar] [CrossRef]
- Sartinska, L.L.; Barchikovski, S.; Wagenda, N.; Rud’, B.M.; Timofeeva, I.I. Laser Induced Modification of Surface Structures. Appl. Surf. Sci. 2007, 253, 4295–4299. [Google Scholar] [CrossRef]
- Stolz, B.; Backes, G.; Gillner, A.; Kreutz, E.W. Selective Surface Modification of Ceramics with Laser Radiation. Appl. Surf. Sci. 1997, 109–110, 242–248. [Google Scholar] [CrossRef]
- Ouraipryvan, P.; Sreethawong, T.; Chavadej, S. Synthesis of Crystalline MgO Nanoparticle with Mesoporous-Assembled Structure via a Surfactant-Modified Sol-Gel Process. Mater. Lett. 2009, 63, 1862–1865. [Google Scholar] [CrossRef]
- Xu, X.; Hu, S.; Pan, Q.; Huang, Y.; Zhang, J.; Chen, Y.; Wang, H.; Zheng, F.; Li, Q. Enhancing Structure Stability by Mg/Cr Co-Doped for High-Voltage Sodium-Ion Batteries. Small 2024, 20, 2307377. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Jia, Q.; Zhang, Z.; Lei, L.; Chen, L. Corrosion Mechanism of Reactive MgO-Bonded Cr2O3-Bearing Castables in CaO–Al2O3–Fe2O3–SiO2-Based Steel-Making Slag. J. Am. Ceram. Soc. 2024, 107, 1232–1248. [Google Scholar] [CrossRef]
- Pourdelan, H.; Alavi, S.M.; Rezaei, M.; Akbari, E.; Klyamkin, S. Production of Pure Hydrogen through Thermocatalytic Methane Decomposition Using NiO-MgO Catalysts Promoted by Chromium and Copper Prepared via Mechanochemical Method. Int. J. Energy Res. 2023, 2023, 5132640. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, Y.; Sun, J.; Li, H.; Liu, W. Progress in MgO Sorbents for Cyclic CO2 Capture: A Comprehensive Review. J. Mater. Chem. A 2019, 7, 20103–20120. [Google Scholar] [CrossRef]
- Choi, D.; Park, Y. Structural Modification of Salt-Promoted MgO Sorbents for Intermediate Temperature CO2 Capture. Nanoscale Adv. 2022, 4, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhan, S.; Sun, J.; Yao, L.; Zhu, J.; Feng, J.; Xiong, Y.; Tian, S. Enhanced Ozonation of Pollutants by MgO Nanoclusters/Sewage Sludge-Derived Hierarchical Porous Carbon: Experimental and Theoretical Study. Environ. Sci. Nano 2021, 8, 2569–2583. [Google Scholar] [CrossRef]
- Mori, K.; Fujita, T.; Yamashita, H. Boosting the Activity of PdAg Alloy Nanoparticles during H2 Production from Formic Acid Induced by CrOx as an Inorganic Interface Modifier. EES Catal. 2023, 1, 84–93. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, Y.; Liu, S.; Li, Z.; Liu, X.; Zhang, S.; Li, Z. Boric Acid-Regulated Gelation and Ethanol-Assisted Preparation of Polybenzoxazine Aerogels. Chem. Eng. J. 2024, 486, 150228. [Google Scholar] [CrossRef]
- Mansoor, M.A.; Munawar, K.; Naeem, R.; Sarih, N.M.; Asghar, M.A.; Haider, A.; Zubir, M.N.M.; Zaharinie, T. Aerosol-Assisted Facile Fabrication of Bimetallic Cr2O3-Mn2O3 Thin Films for Photoelectrochemical Water Splitting. New J. Chem. 2023, 47, 8347–8354. [Google Scholar] [CrossRef]
- Bhateja, Y.; Ghosh, R.; Sponer, J.; Majumdar, S.; Cassone, G. A Cr2O3-Doped Graphene Sensor for Early Diagnosis of Liver Cirrhosis: A First-Principles Study. Phys. Chem. Chem. Phys. 2022, 24, 21372–21380. [Google Scholar] [CrossRef] [PubMed]
- Jan, F.; Zhi, S.; Sun, X.Y.; Li, B. Enhancing Catalytic Activity of Cr2O3 in CO2-Assisted Propane Dehydrogenation with Effective Dopant Engineering: A DFT-Based Microkinetic Simulation. Phys. Chem. Chem. Phys. 2024, 26, 9708–9721. [Google Scholar] [CrossRef]
- Ding, J.; Ming, J.; Lu, D.; Wu, W.; Liu, M.; Zhao, X.; Li, C.; Yang, M.; Fang, P. Study of the Enhanced Visible-Light-Sensitive Photocatalytic Activity of Cr2O3-Loaded Titanate Nanosheets for Cr(VI) Degradation and H2 Generation. Catal. Sci. Technol. 2017, 7, 2283–2297. [Google Scholar] [CrossRef]
- Herdiech, M.W.; Zhu, X.; Morales-Acosta, M.D.; Walker, F.J.; Altman, E.I. The Modification of Ferroelectric LiNbO3(0001) Surfaces Using Chromium Oxide Thin Films. Phys. Chem. Chem. Phys. 2015, 17, 9488–9498. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y.R. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J. Taiwan Inst. Chem. Eng. 2016, 68, 489–495. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Lee, J. High-Performance Supercapacitor Electrodes from Fully Biomass-Based Polybenzoxazine Aerogels with Porous Carbon Structure. Gels 2024, 10, 462. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis and Copolymerization of Fully Biobased Benzoxazines from Renewable Resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Parveen, A.S.; Lee, Y.R.; Kim, S.-C. Polybenzoxazine originated N-doped mesoporous carbon ropes as an electrode material for high-performance supercapacitors. J. Alloys Compd. 2018, 750, 384–391. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Balasubramanian, R.; Parveen, A.S.; Kim, S.-C. Direct synthesis of nitrogen-rich carbon sheets via polybenzoxazine as highly active electrocatalyst for water splitting. Int. J. Hydrogen Energy 2018, 43, 13266–13275. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Muthusamy, S. New benzoxazines containing polyhedral oligomeric silsesquioxane from eugenol, guaiacol and vanillin. New J. Chem. 2014, 39, 1691–1702. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. Green synthesis of nitrogen-doped graphitic carbon sheets with use of Prunus persica for supercapacitor applications. Appl. Surf. Sci. 2017, 393, 276–286. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Karthikeyan, D.; Pandurangan, A.; Lee, Y.R. Synthesis and characterization of graphitic mesoporous carbon using metal–metal oxide by chemical vapor deposition method. Microporous Mesoporous Mater. 2015, 215, 123–132. [Google Scholar] [CrossRef]
- Lamberti, C.; Zecchina, A.; Groppo, E.; Bordiga, S. Probing the Surfaces of Heterogeneous Catalysts by in Situ IR Spectroscopy. Chem. Soc. Rev. 2010, 39, 4951–5001. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Sui, C.; Liu, Z.; Liu, Y.; Li, Y.; Bai, J.; Liu, F.; Lu, G. Highly Selective and Humidity-Resistant Triethylamine Sensors Based on Pt and Cr2O3Nanoparticles. ACS Appl. Nano Mater. 2022, 5, 15053–15061. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Huang, X.; Ding, L.; Ren, Y.; Xu, M.; Zhu, J.; Miao, Z.; Liu, H. Enabling Wasted A4 Papers as a Promising Carbon Source to Construct Partially Graphitic Hierarchical Porous carbon for High-Performance Aqueous Zn-Ion Storage. ACS Appl. Mater. Interfaces 2024, 16, 10126–10137. [Google Scholar] [CrossRef] [PubMed]
- Song, B.Y.; Zhang, X.F.; Huang, J.; Cheng, X.L.; Deng, Z.P.; Xu, Y.M.; Huo, L.H.; Gao, S. Porous Cr2O3 Architecture Assembled by Nano-Sized Cylinders/ Ellipsoids for Enhanced Sensing to Trace H2S Gas. ACS Appl. Mater. Interfaces 2022, 14, 22302–22312. [Google Scholar] [CrossRef]
- Simeonidis, K.; Kalaitzidou, K.; Asimakidou, T.; Martinez-Boubeta, C.; Makridis, A.; Haeussler, A.; Vourlias, G.; Balcells, L. Tin Oxide Nanoparticles via Solar Vapor Deposition for Hexavalent Chromium Remediation. ACS Appl. Nano Mater. 2023, 6, 13902–13911. [Google Scholar] [CrossRef] [PubMed]
- Lodesani, A.; Picone, A.; Brambilla, A.; Giannotti, D.; Jagadeesh, M.S.; Calloni, A.; Bussetti, G.; Berti, G.; Zani, M.; Finazzi, M.; et al. Graphene as an Ideal Buffer Layer for the Growth of High-Quality Ultrathin Cr2O3 Layers on Ni(111). ACS Nano 2019, 13, 4361–4367. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, W.; Kumazawa, R.; Ishii, D.; Hayashi, R.; Niihori, Y.; Hossain, S.; Nair, L.V.; Takayama, T.; Iwase, A.; Yamazoe, S.; et al. Au25-Loaded BaLa4Ti4O15 Water-Splitting Photocatalyst with Enhanced Activity and Durability Produced Using New Chromium Oxide Shell Formation Method. J. Phys. Chem. C 2018, 122, 13669–13681. [Google Scholar] [CrossRef]
- Chanuta, N.; Stefaniuk, D.; Weaver, J.C.; Zhu, Y.; Yang, S.H.; Masic, A.; Ulm, F.J. Carbon–cement supercapacitors as a scalable bulk energy storage solution. Proc. Natl. Acad. Sci. USA 2023, 120, e2304318120. [Google Scholar] [CrossRef] [PubMed]
- Aouani, H.; Wenger, J.; Gérard, D.; Rigneault, H.; Devaux, E.; Ebbesen, T.W.; Mahdavi, F.; Xu, T.; Blair, S. Crucial Role of the Adhesion Layer on the Plasmonic Fluorescence Enhancement. ACS Nano 2009, 3, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga-Ibarra, V.A.; Shaji, S.; Krishnan, B.; Johny, J.; Sharma Kanakkillam, S.; Avellaneda, D.A.; Martinez, J.A.A.; Roy, T.K.D.; Ramos-Delgado, N.A. Synthesis and Characterization of Black TiO2 Nanoparticles by Pulsed Laser Irradiation in Liquid. Appl. Surf. Sci. 2019, 483, 156–164. [Google Scholar] [CrossRef]
- García-Quiñonez, L.V.; Mendivil-Palma, M.I.; Roy, T.K.D.; Castillo-Rodríguez, G.A.; Gómez-Rodríguez, C.; Fernández-González, D.; Shaji, S. Effects of Irradiation Energy and Nanoparticle Concentrations on the Structure and Morphology of Laser Sintered Magnesia with Alumina and Iron Oxide Nanoparticles. Ceram. Int. 2020, 46, 7850–7860. [Google Scholar] [CrossRef]
- Guzmán, K.d.C.M.; Shaji, S.; Das Roy, T.K.; Krishnan, B.; Avellaneda, D.A.; Aguilar Martinez, J.A.; Valdes, J.J.R. Surface Modification of Sintered Magnesium Oxide (MgO) with Chromium Oxide (Cr2O3) by Pulsed Laser Irradiation in Air and Liquids. Ceram. Int. 2021, 47, 21625–21632. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.; Yan, X.; Cao, Y.; Gao, H.; Luo, H.; Gao, K.; Xue, S.; Jing, X. Recent Advances and Perspectives on Graphene-Based Gels for Superior Flexible All-Solid-State Supercapacitors. J. Power Sources 2023, 565, 232916. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Hamid, N.A.A.; Rahiman, W.; Mohamad, A.A. Electrode Polymer Binders for Supercapacitor Applications: A review. J. Mater. Res. Technol. 2023, 23, 3470–3491. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, G.; Jeong, S.M.; Rout, C.S. A review on supercapacitors based on plasma enhanced chemical vapor deposited vertical graphene arrays. J. Energy Storage 2022, 53, 105212. [Google Scholar] [CrossRef]
- Manickam, M.; Achini, S.; Jonathan, W.; Rob, A.; Pragati, A.S.; Katsuhiko, A.; Lok Kumar, S. Phosphorous—Containing Activated Carbon Derived From Natural Honeydew Peel Powers Aqueous Supercapacitors. Chem. Asian J. 2024, e202400622. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asrafali, S.P.; Periyasamy, T.; Lee, J. High-Performance Supercapacitors Using Compact Carbon Hydrogels Derived from Polybenzoxazine. Gels 2024, 10, 509. https://doi.org/10.3390/gels10080509
Asrafali SP, Periyasamy T, Lee J. High-Performance Supercapacitors Using Compact Carbon Hydrogels Derived from Polybenzoxazine. Gels. 2024; 10(8):509. https://doi.org/10.3390/gels10080509
Chicago/Turabian StyleAsrafali, Shakila Parveen, Thirukumaran Periyasamy, and Jaewoong Lee. 2024. "High-Performance Supercapacitors Using Compact Carbon Hydrogels Derived from Polybenzoxazine" Gels 10, no. 8: 509. https://doi.org/10.3390/gels10080509
APA StyleAsrafali, S. P., Periyasamy, T., & Lee, J. (2024). High-Performance Supercapacitors Using Compact Carbon Hydrogels Derived from Polybenzoxazine. Gels, 10(8), 509. https://doi.org/10.3390/gels10080509





