Patterned PVA Hydrogels with 3D Petri Dish® Micro-Molds of Varying Topography for Spheroid Formation of HeLa Cancer Cells: In Vitro Assessment
Abstract
1. Introduction
2. Results and Discussion
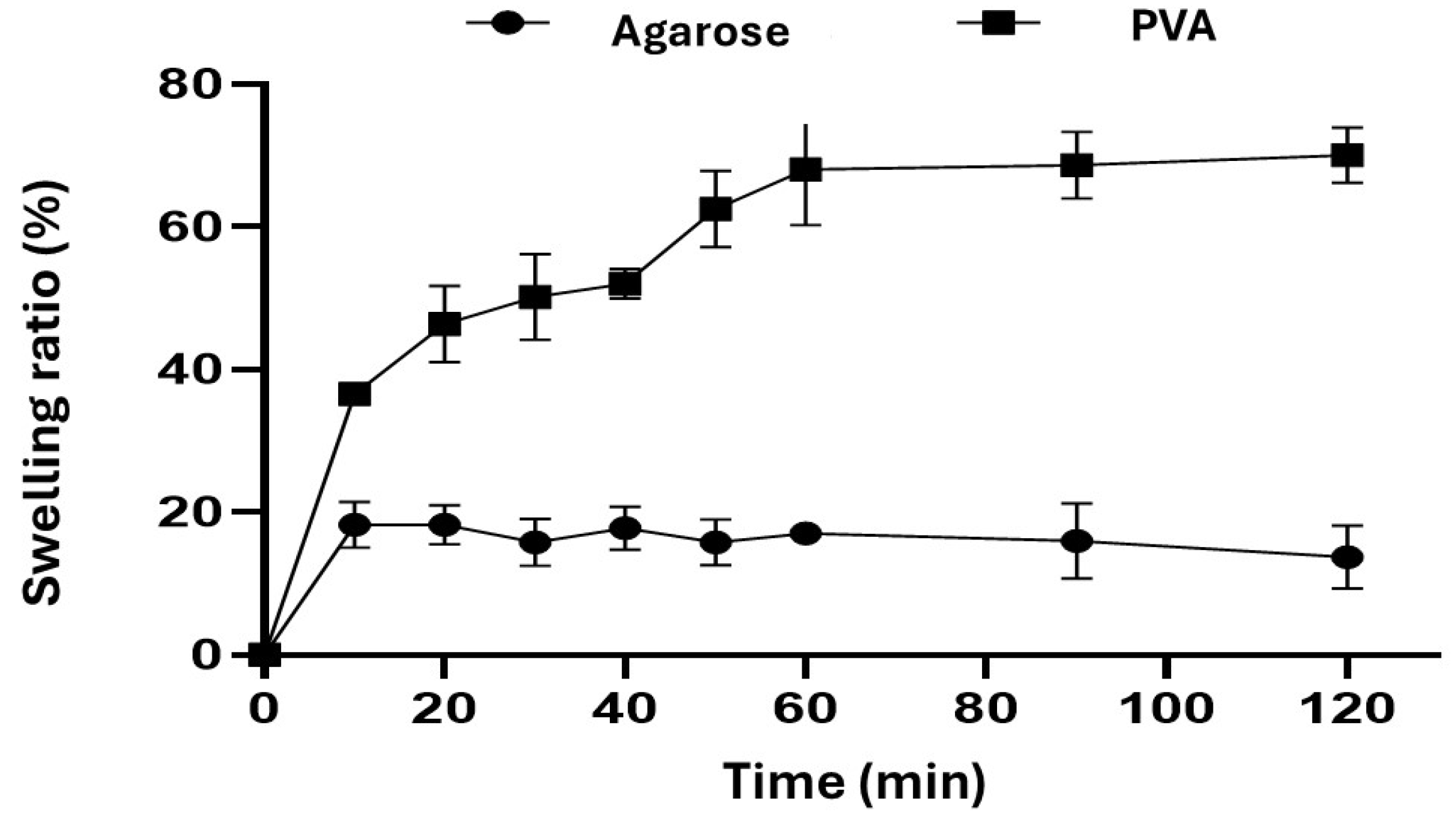
2.1. Swelling Behavior
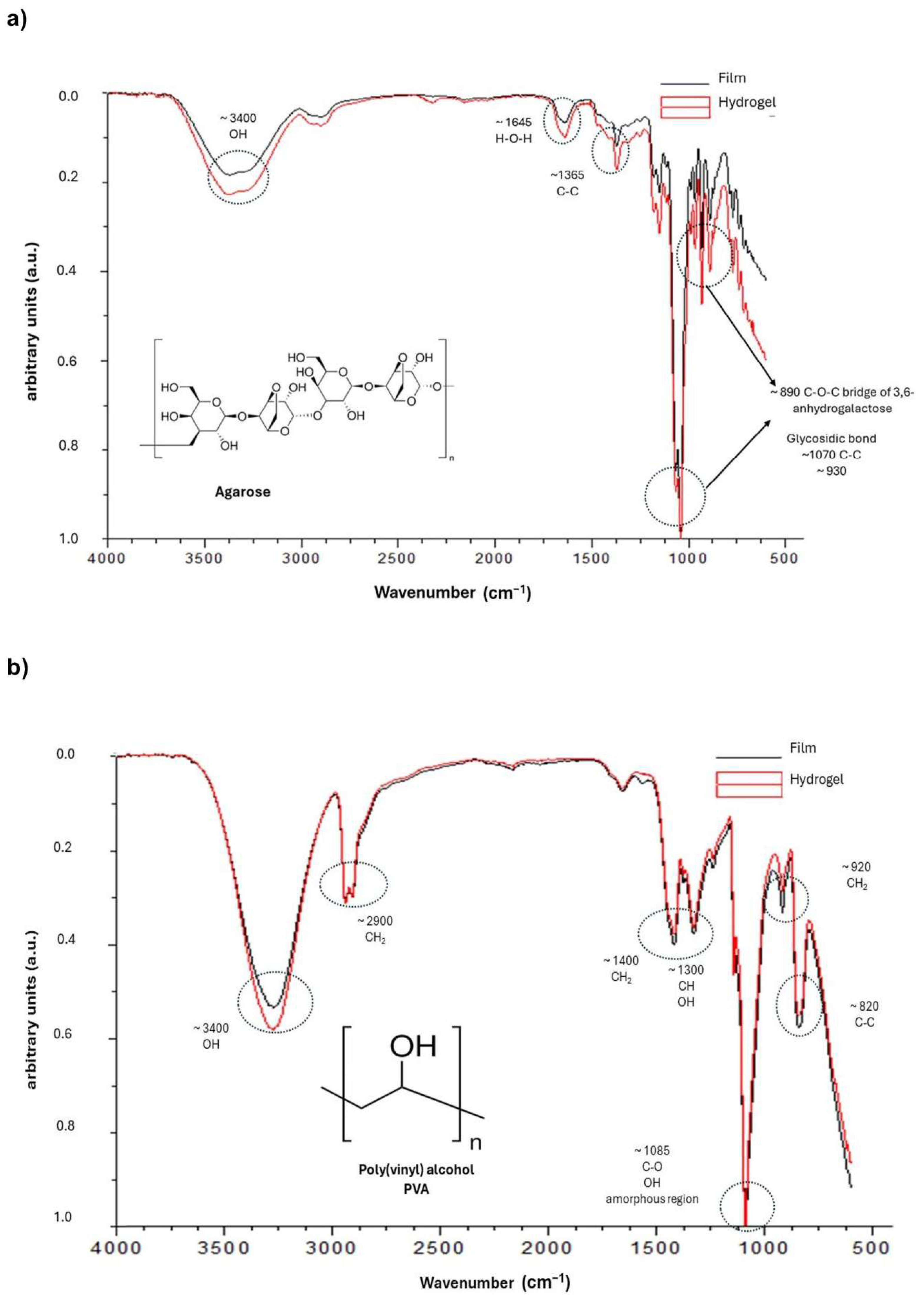
2.2. FTIR Analysis
2.3. Texture Profile Analysis
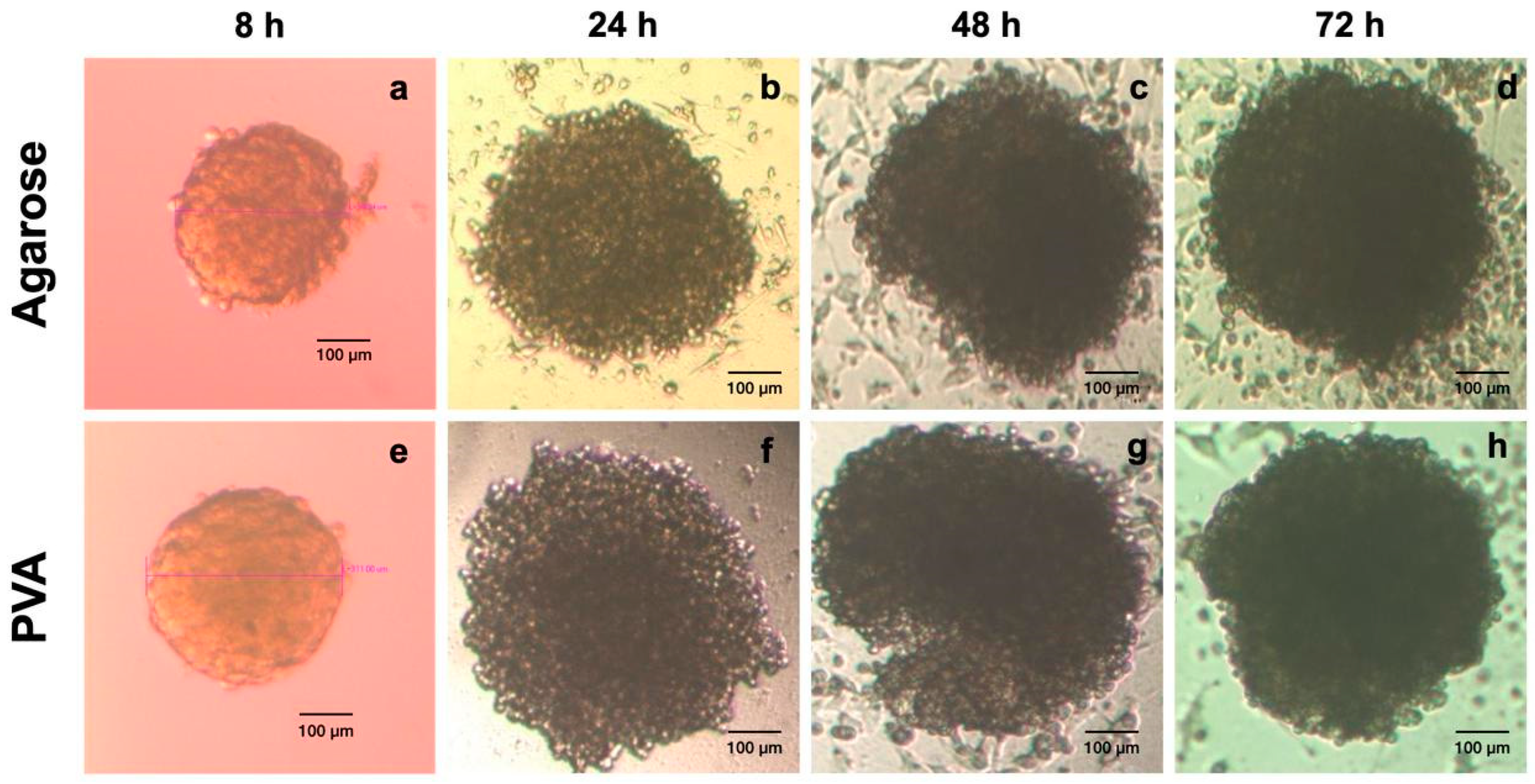
2.4. In Vitro Spheroid Assessment
Spheroid Formation
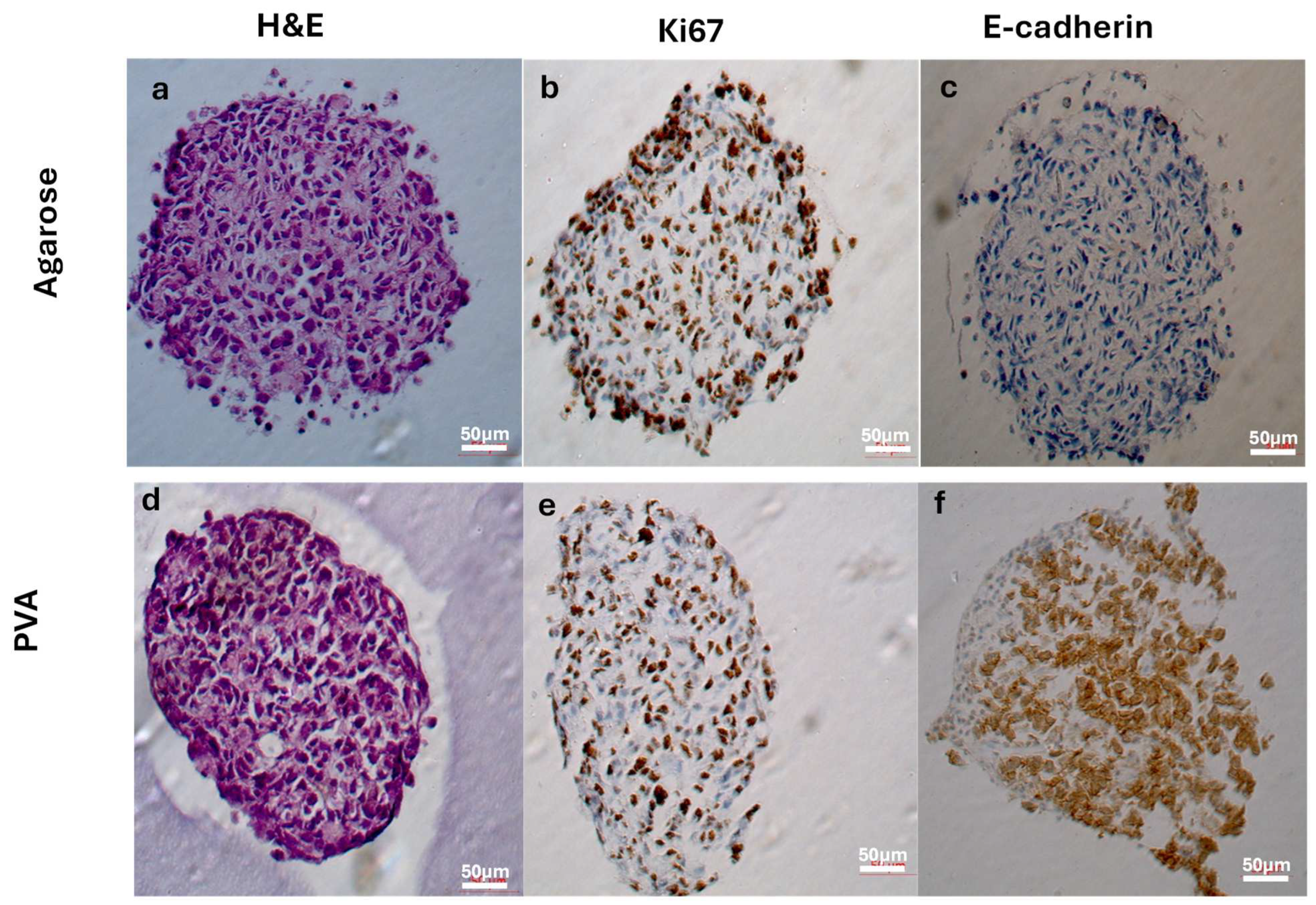
2.5. Histological and Immunohistochemical Analysis
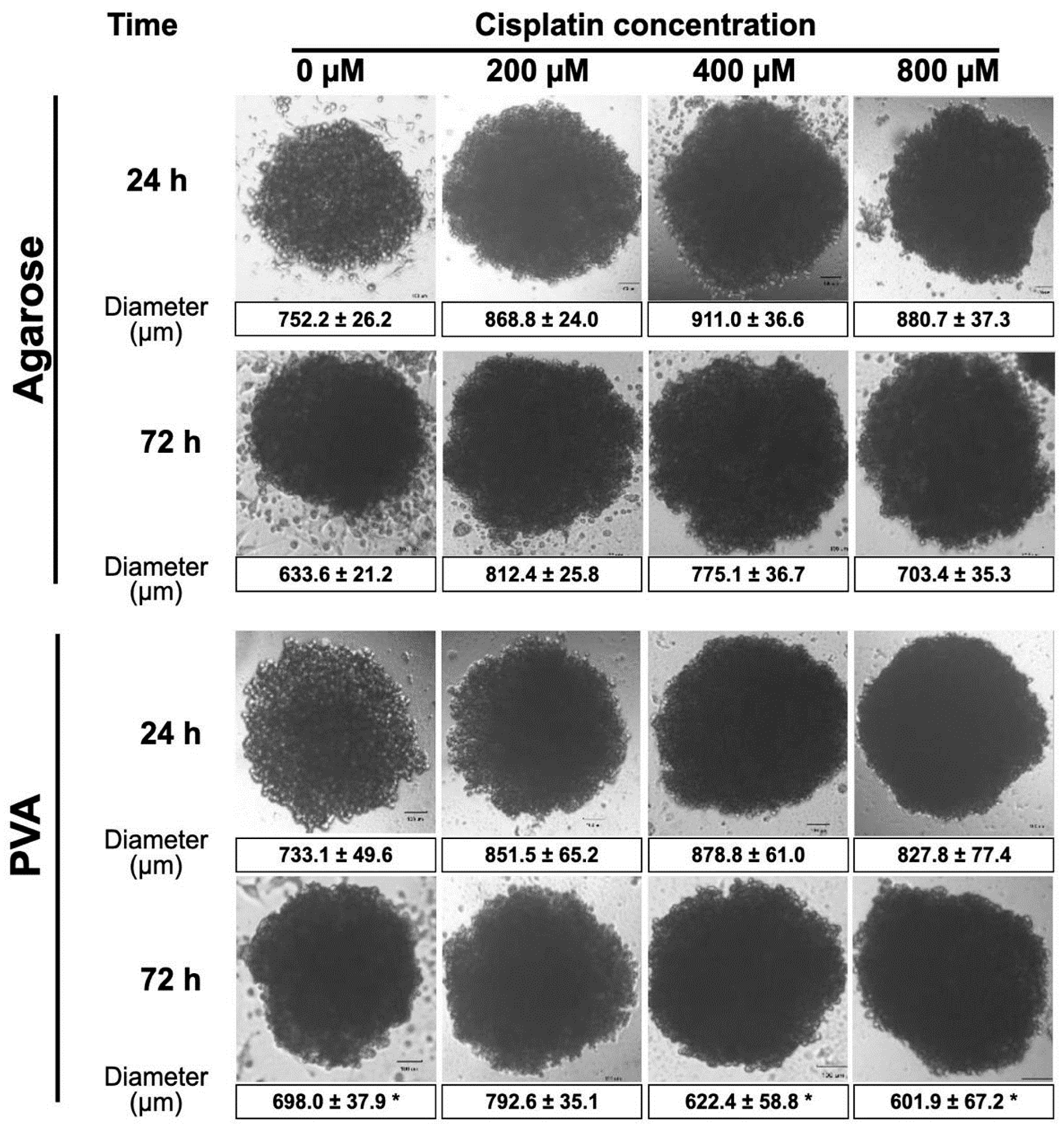
2.6. Anticancer Drug Sensitivity Tests
3. Conclusions
4. Materials and Methods
4.1. Hydrogel Preparation
4.1.1. Poly(vinyl alcohol) Hydrogels
4.1.2. Agarose Hydrogels
4.2. Hydrogel Characterization
4.2.1. Physical Appearance
4.2.2. Macroscopic Morphology
4.2.3. Fourier Transform Infrared Spectroscopy
4.2.4. Texture Analysis
4.2.5. Swelling Studies
4.3. Spheroid Formation
Cell Culture
4.4. In Vitro Spheroid Assessment
4.4.1. Measurement of Spheroid Size
4.4.2. Histochemistry and Immunohistochemistry
4.5. Anticancer Drug Sensitivity Tests
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Weth, F.R.; Hoggarth, G.B.; Weth, A.F.; Paterson, E.; White, M.P.J.; Tan, S.T.; Peng, L.; Gray, C. Unlocking hidden potential: Advancements, approaches, and obstacles in repurposing drugs for cancer therapy. Br. J. Cancer 2023, 130, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Tosca, E.M.; Ronchi, D.; Facciolo, D.; Magni, P. Replacement, Reduction, and Refinement of Animal Experiments in Anticancer Drug Development: The Contribution of 3D In Vitro Cancer Models in the Drug Efficacy Assessment. Biomedicines 2023, 11, 1058. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a type of three-dimensional cell cultures—Examples of methods of preparation and the most important application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.P.; Dean, D.M.; Man, A.J.; Youssef, J.; Ho, D.N.; Rago, A.P.; Lech, M.P.; Morgan, J.R. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. BioTechniques 2007, 43, 494–500. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef]
- Microtissues®. Protocols-3D Petri Dish by Microtissues, Inc. Available online: https://www.microtissues.com/protocols (accessed on 16 April 2024).
- Leary, E.; Curran, S.; Susienka, M.; Manning, K.L.; Blakely, A.M.; Morgan, J.R. Micro-moulded non-adhesive hydrogels to form multicellular microtissues—The 3D Petri Dish®. In Technology Platforms for 3D Cell Culture; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Microtissues®.Harvesting-Multi-Cellular-Microtissues-from-3D-Petri-Dishes®.pdf—Google Drive. Available online: https://drive.google.com/file/d/1s99sKNiv78uQSpIduMYL0JPIdWcZxncq/view (accessed on 16 April 2024).
- Molyneaux, K.; Wnek, M.D.; Craig, S.E.; Vincent, J.; Rucker, I.; Wnek, G.E.; Brady-Kalnay, S.M. Physically-cross-linked poly(vinyl alcohol) cell culture plate coatings facilitate preservation of cell–cell interactions, spheroid formation, and stemness. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Roberts, A.I.; Shi, Y. Adhesion molecules. Cell Adhes. Migr. 2011, 5, 20–22. [Google Scholar] [CrossRef]
- Dou, X.; Li, P.; Schönherr, H. Three-Dimensional Microstructured Poly(vinyl alcohol) Hydrogel Platform for the Controlled Formation of Multicellular Cell Spheroids. Biomacromolecules 2017, 19, 158–166. [Google Scholar] [CrossRef]
- Wong, C.-W.; Han, H.-W.; Hsu, S.-H. Changes of cell membrane fluidity for mesenchymal stem cell spheroids on biomaterial surfaces. World J. Stem Cells 2022, 14, 616–632. [Google Scholar] [CrossRef]
- Vasanthan, K.S.; Subramaniam, A.; Krishnan, U.M.; Sethuraman, S. Influence of 3D porous galactose containing PVA/gelatin hydrogel scaffolds on three-dimensional spheroidal morphology of hepatocytes. J. Mater. Sci. Mater. Med. 2015, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, H.; Zaer, M.; Shojaosadati, S.A.; Hashemi-Najafabadi, S. Rapid generation of homogenous tumor spheroid microtissues in a scaffold-free platform for high-throughput screening of a novel combination nanomedicine. PLoS ONE 2023, 18, e0282064. [Google Scholar] [CrossRef]
- Masters, J.R. HeLa cells 50 years on: The good, the bad and the ugly. Nat. Rev. Cancer 2002, 2, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2000; pp. 37–65. [Google Scholar] [CrossRef]
- Sekar, M.P.; Budharaju, H.; Sethuraman, S.; Sundaramurthi, D. Carboxymethyl cellulose-agarose-gelatin: A thermoresponsive triad bioink composition to fabricate volumetric soft tissue constructs. JALA J. Assoc. Lab. Autom. 2023, 28, 183–198. [Google Scholar] [CrossRef]
- Sacco, P.; Piazza, F.; Marsich, E.; Abrami, M.; Grassi, M.; Donati, I. Ionic Strength Impacts the Physical Properties of Agarose Hydrogels. Gels 2024, 10, 94. [Google Scholar] [CrossRef]
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of flexible ph-responsive agarose/succinoglycan hydrogels for controlled drug release. Polymers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Topuz, F.; Nadernezhad, A.; Caliskan, O.S.; Menceloglu, Y.Z.; Koc, B. Nanosilicate embedded agarose hydrogels with improved bioactivity. Carbohydr. Polym. 2018, 201, 105–112. [Google Scholar] [CrossRef]
- Boran, F. The influence of freeze-thawing conditions on swelling and long-term stability properties of poly(vinyl alcohol) hydrogels for controlled drug release. Polym. Bull. 2021, 78, 7369–7387. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Xiang, Y.; Yang, J. Enhanced Mechanical Properties of PVA Hydrogel by Low-Temperature Segment Self-Assembly vs. Freeze–Thaw Cycles. Polymers 2023, 15, 3782. [Google Scholar] [CrossRef] [PubMed]
- Fumio, U.; Hiroshi, Y.; Kumiko, N.; Sachihiko, N.; Kenji, S.; Yasunori, M. Swelling and mechanical properties of poly(vinyl alcohol) hydrogels. Int. J. Pharm. 1990, 58, 135–142. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, e2303326. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Y.; Narayanan, J.; Liu, X.-Y.; Chong, T.K.; Chen, S.B.; Chung, T.-S. Topology evolution and gelation mechanism of agarose gel. J. Phys. Chem. B 2005, 109, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- Felfel, R.M.; Gideon-Adeniyi, M.J.; Hossain, K.M.Z.; Roberts, G.A.; Grant, D.M. Structural, mechanical and swelling characteristics of 3D scaffolds from chitosan-agarose blends. Carbohydr. Polym. 2019, 204, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Arnott, S.; Fulmer, A.; Scott, W.; Dea, I.; Moorhouse, R.; Rees, D. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974, 90, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Guenet, J.; Rochas, C. Agarose Sols and Gels Revisited. Macromol. Symp. 2006, 242, 65–70. [Google Scholar] [CrossRef]
- Ortega-Sánchez, C.; Melgarejo-Ramírez, Y.; Rodríguez-Rodríguez, R.; Jiménez-Ávalos, J.A.; Giraldo-Gomez, D.M.; Gutiérrez-Gómez, C.; Rodriguez-Campos, J.; Luna-Bárcenas, G.; Velasquillo, C.; Martínez-López, V.; et al. Hydrogel Based on Chitosan/Gelatin/Poly(Vinyl Alcohol) for In Vitro Human Auricular Chondrocyte Culture. Polymers 2024, 16, 479. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Han, X.; Li, M.; Fan, Z.; Zhang, Y.; Zhang, H.; Li, Q. PVA/Agar Interpenetrating Network Hydrogel with Fast Healing, High Strength, Antifreeze, and Water Retention. Macromol. Chem. Phys. 2020, 221, 2000237. [Google Scholar] [CrossRef]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze–thaw hydrogel fabrication method: Basic principles, synthesis parameters, properties, and biomedical applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Janarthanan, G.; Shin, H.S.; Kim, I.-G.; Ji, P.; Chung, E.-J.; Lee, C.; Noh, I. Self-crosslinking hyaluronic acid–carboxymethylcellulose hydrogel enhances multilayered 3D-printed construct shape integrity and mechanical stability for soft tissue engineering. Biofabrication 2020, 12, 045026. [Google Scholar] [CrossRef]
- Hu, X.; Liang, R.; Li, J.; Liu, Z.; Sun, G. Mechanically strong hydrogels achieved by designing homogeneous network structure. Mater. Des. 2018, 163, 107547. [Google Scholar] [CrossRef]
- Pangjantuk, A.; Kaokaen, P.; Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. 3D culture of alginate-hyaluronic acid hydrogel supports the stemness of human mesenchymal stem cells. Sci. Rep. 2024, 14, 4436. [Google Scholar] [CrossRef]
- Preda, P.; Enciu, A.-M.; Tanase, C.; Dudau, M.; Albulescu, L.; Maxim, M.-E.; Darie-Niță, R.N.; Brincoveanu, O.; Avram, M. Assessing Polysaccharides/Aloe Vera–Based Hydrogels for Tumor Spheroid Formation. Gels 2023, 9, 51. [Google Scholar] [CrossRef]
- Kusjuriansah, K.; Rodhiyah, M.; Syifa, N.A.; Luthfianti, H.R.; Waresindo, W.X.; Hapidin, D.A.; Suciati, T.; Edikresnha, D.; Khairurrijal, K. Composite Hydrogel of Poly(vinyl alcohol) Loaded by Citrus hystrix Leaf Extract, Chitosan, and Sodium Alginate with In Vitro Antibacterial and Release Test. ACS Omega 2024, 9, 13306–13322. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Sun, Q.; Li, Q.; Li, S.; Li, X. Dynamic Hydrogels with Viscoelasticity and Tunable Stiffness for the Regulation of Cell Behavior and Fate. Materials 2023, 16, 5161. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A Review on the Design of Hydrogels with Different Stiffness and Their Effects on Tissue Repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef]
- Kalli, M.; Stylianopoulos, T. Defining the role of solid stress and matrix stiffness in cancer cell proliferation and metastasis. Front. Oncol. 2018, 8, 55. [Google Scholar] [CrossRef]
- Patlolla, V.G.R.; Holbrook, W.P.; Gizurarson, S.; Kristmundsdottir, P. Evaluation of in vitro mucoadhesiveness and texture profile analysis of doxycycline in situ hydrogels. Pharmazie 2020, 75, 7–12. [Google Scholar] [CrossRef]
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T.A. Physics of agarose fluid gels: Rheological properties and microstructure. Curr. Res. Food Sci. 2021, 4, 436–448. [Google Scholar] [CrossRef]
- Öztürk, S.; Demir, M.; Koçkaya, E.A.; Karaaslan, C.; Süloğlu, A.K. Establishment of a 3D multicellular placental microtissues for investigating the effect of antidepressant vortioxetine. Reprod. Toxicol. 2024, 123, 108519. [Google Scholar] [CrossRef]
- Gomi, F.; Sasaki, N.; Shichi, Y.; Minami, F.; Shinji, S.; Toyoda, M.; Ishiwata, T. Polyvinyl alcohol increased growth, migration, invasion, and sphere size in the PK-8 pancreatic ductal adenocarcinoma cell line. Heliyon 2021, 7, e06182. [Google Scholar] [CrossRef]
- Valdoz, J.C.; Jacobs, D.J.; Cribbs, C.G.; Johnson, B.C.; Hemeyer, B.M.; Dodson, E.L.; Saunooke, J.A.; Franks, N.A.; Poulson, P.D.; Garfield, S.R.; et al. An improved scalable hydrogel dish for spheroid culture. Life 2021, 11, 517. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Gaihre, B.; Park, S.; Li, Y.; Terzic, A.; Elder, B.D.; Lu, L. Scaffold-Free Spheroids with Two-Dimensional Heteronano-Layers (2DHNL) Enabling Stem Cell and Osteogenic Factor Codelivery for Bone Repair. ACS Nano 2022, 16, 2741–2755. [Google Scholar] [CrossRef]
- Dissanayaka, W.; Zhu, L.; Hargreaves, K.; Jin, L.; Zhang, C. Scaffold-free prevascularized microtissue spheroids for pulp regeneration. J. Dent. Res. 2014, 93, 1296–1303. [Google Scholar] [CrossRef]
- Ahearne, M. Introduction to cell–hydrogel mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef]
- Mansouri, M.; Leipzig, N.D. Advances in removing mass transport limitations for more physiologically relevant in vitro 3D cell constructs. Biophys. Rev. 2021, 2, 021305. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Gabriel, C.; Walter, I. Morphological and Immunohistochemical Characterization of Canine Osteosarcoma Spheroid Cell Cultures. Anat. Histol. Embryol. 2015, 45, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef] [PubMed]
- Daum, A.-K.; Dittmann, J.; Jansen, L.; Peters, S.; Dahmen, U.; Heger, J.; Hoppe-Seyler, F.; Gille, A.; Clement, J.H.; Runnebaum, I.B.; et al. ITIH5 shows tumor suppressive properties in cervical cancer cells grown as multicellular tumor spheroids. Am. J. Transl. Res. 2021, 13, 10298–10314. [Google Scholar] [PubMed]
- Mège, R.M.; Ishiyama, N. Integration of cadherin adhesion and cytoskeleton at adherens junctions. Cold Spring Harb. Perspect. Biol. 2017, 9, a028738. [Google Scholar] [CrossRef] [PubMed]
- Powan, P.; Luanpitpong, S.; He, X.; Rojanasakul, Y.; Chanvorachote, P. Detachment-induced E-cadherin expression promotes 3D tumor spheroid formation but inhibits tumor formation and metastasis of lung cancer cells. Am. J. Physiol. Physiol. 2017, 313, C556–C566. [Google Scholar] [CrossRef]
- Becconi, M.; De Zio, S.; Falciani, F.; Santamaria, M.; Malferrari, M.; Rapino, S. Nano-Electrochemical Characterization of a 3D Bioprinted Cervical Tumor Model. Cancers 2023, 15, 1327. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Liu, S.; Ip, S.-M.; Wong, L.; Ngan, H.; Ng, T. E-cadherin expression is silenced by DNA methylation in cervical cancer cell lines and tumours. Eur. J. Cancer 2003, 39, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Vessey, C.J.; Wilding, J.; Folarin, N.; Hirano, S.; Takeichi, M.; Soutter, P.; Stamp, G.W.H.; Pignatelli, M. Altered expression and function of E-cadherin in cervical intraepithelial neoplasia and invasive squamous cell carcinoma. J. Pathol. 1995, 176, 151–159. [Google Scholar] [CrossRef]
- Hollestelle, A.; Peeters, J.K.; Smid, M.; Timmermans, M.; Verhoog, L.C.; Westenend, P.J.; Heine, A.A.J.; Chan, A.; Sieuwerts, A.M.; Wiemer, E.A.C.; et al. Loss of E-cadherin is not a necessity for epithelial to mesenchymal transition in human breast cancer. Breast Cancer Res. Treat. 2013, 138, 47–57. [Google Scholar] [CrossRef]
- Rubtsova, S.N.; Zhitnyak, I.Y.; Gloushankova, N.A. Dual role of E-cadherin in cancer cells. Tissue Barriers 2021, 10, 2005420. [Google Scholar] [CrossRef]
- Cho, A.Y.; Lee, H.J. Investigating the Impact of Mechanical Properties and Cell-Collagen Interaction on NIH3T3 Function: A Comparative Study on Different Substrates and Culture Environments. Gels 2023, 9, 922. [Google Scholar] [CrossRef]
- An, S.S.A.; Seo, O.W.; Lee, J.; Hulme, J.; Baek, N. Real-time monitoring of cisplatin cytotoxicity on three-dimensional spheroid tumor cells. Drug Des. Dev. Ther. 2016, 10, 2155–2165. [Google Scholar] [CrossRef]
- Sirenko, O.; Mitlo, T.; Hesley, J.; Luke, S.; Owens, W.; Cromwell, E.F. High-Content Assays for Characterizing the Viability and Morphology of 3D Cancer Spheroid Cultures. ASSAY Drug Dev. Technol. 2015, 13, 402–414. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, srep19103. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Martínez-Colin, E.J.; González-Torres, M.; Ortega-Sánchez, C.; Sánchez-Sánchez, R.; Delgado-Meza, J.; Machado-Bistraín, F.; Martínez-López, V.; Giraldo, D.; Márquez-Gutiérrez, A.; et al. Chondrogenic Potential of Human Adipose-Derived Mesenchymal Stromal Cells in Steam Sterilized Gelatin/Chitosan/Polyvinyl Alcohol Hydrogels. Polymers 2023, 15, 3938. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno Valtierra, M.; Urue Corral, A.; Jiménez-Avalos, J.A.; Barbosa Avalos, E.; Dávila-Rodríguez, J.; Morales Hernández, N.; Comas-García, M.; Toriz González, G.; Oceguera-Villanueva, A.; Cruz-Ramos, J.A.; et al. Patterned PVA Hydrogels with 3D Petri Dish® Micro-Molds of Varying Topography for Spheroid Formation of HeLa Cancer Cells: In Vitro Assessment. Gels 2024, 10, 518. https://doi.org/10.3390/gels10080518
Moreno Valtierra M, Urue Corral A, Jiménez-Avalos JA, Barbosa Avalos E, Dávila-Rodríguez J, Morales Hernández N, Comas-García M, Toriz González G, Oceguera-Villanueva A, Cruz-Ramos JA, et al. Patterned PVA Hydrogels with 3D Petri Dish® Micro-Molds of Varying Topography for Spheroid Formation of HeLa Cancer Cells: In Vitro Assessment. Gels. 2024; 10(8):518. https://doi.org/10.3390/gels10080518
Chicago/Turabian StyleMoreno Valtierra, Maira, Adriana Urue Corral, Jorge Armando Jiménez-Avalos, Erika Barbosa Avalos, Judith Dávila-Rodríguez, Norma Morales Hernández, Mauricio Comas-García, Guillermo Toriz González, Antonio Oceguera-Villanueva, José Alfonso Cruz-Ramos, and et al. 2024. "Patterned PVA Hydrogels with 3D Petri Dish® Micro-Molds of Varying Topography for Spheroid Formation of HeLa Cancer Cells: In Vitro Assessment" Gels 10, no. 8: 518. https://doi.org/10.3390/gels10080518
APA StyleMoreno Valtierra, M., Urue Corral, A., Jiménez-Avalos, J. A., Barbosa Avalos, E., Dávila-Rodríguez, J., Morales Hernández, N., Comas-García, M., Toriz González, G., Oceguera-Villanueva, A., Cruz-Ramos, J. A., Hernández Gutiérrez, R., Martínez Velázquez, M., & García Carvajal, Z. Y. (2024). Patterned PVA Hydrogels with 3D Petri Dish® Micro-Molds of Varying Topography for Spheroid Formation of HeLa Cancer Cells: In Vitro Assessment. Gels, 10(8), 518. https://doi.org/10.3390/gels10080518







