Fabrication and Characterization of Brain Tissue Phantoms Using Agarose Gels for Ultraviolet Vision Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Transmission and Reflection Tests with Agarose Films
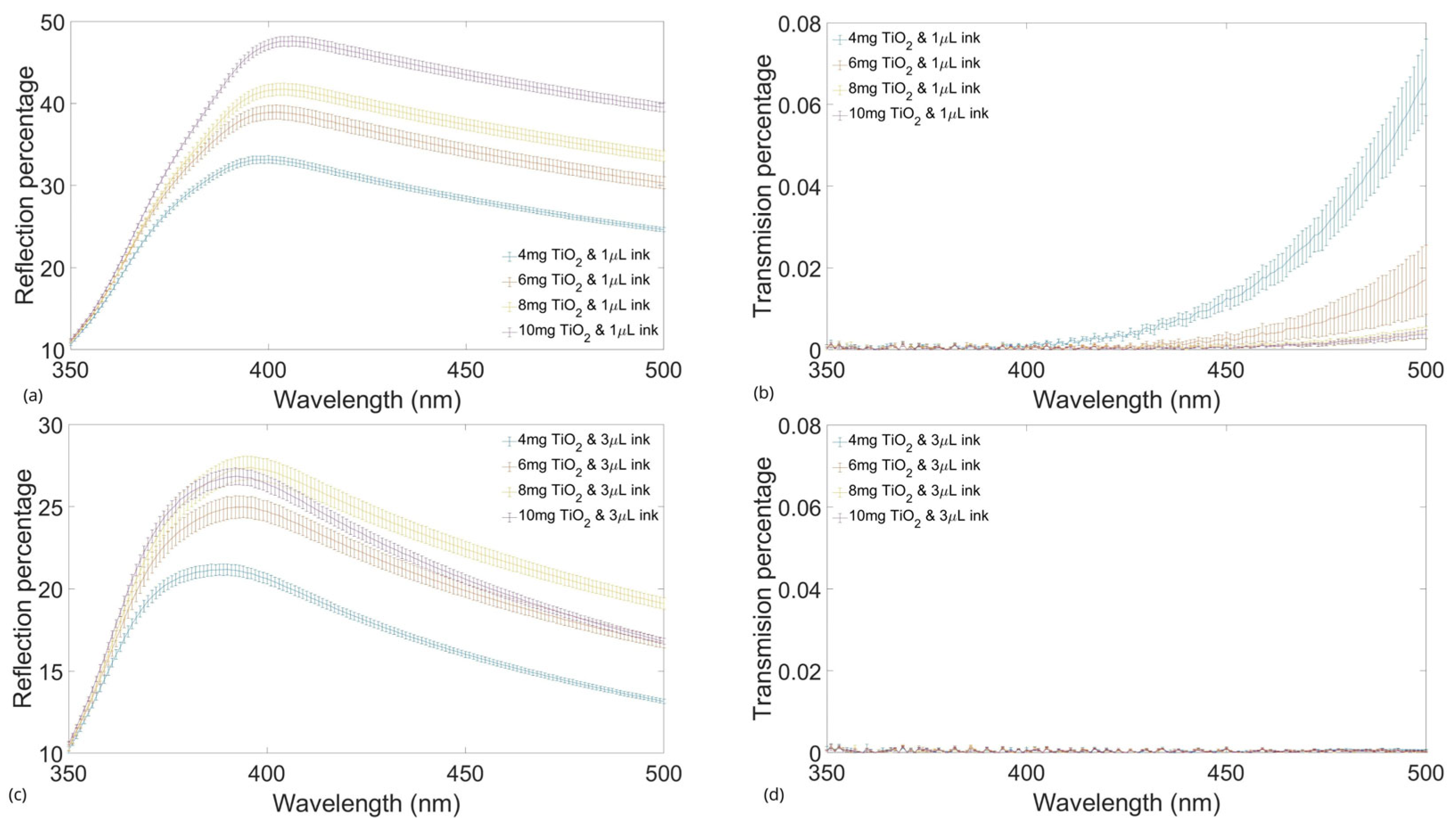
2.2. Transmission and Reflection Tests with Agents
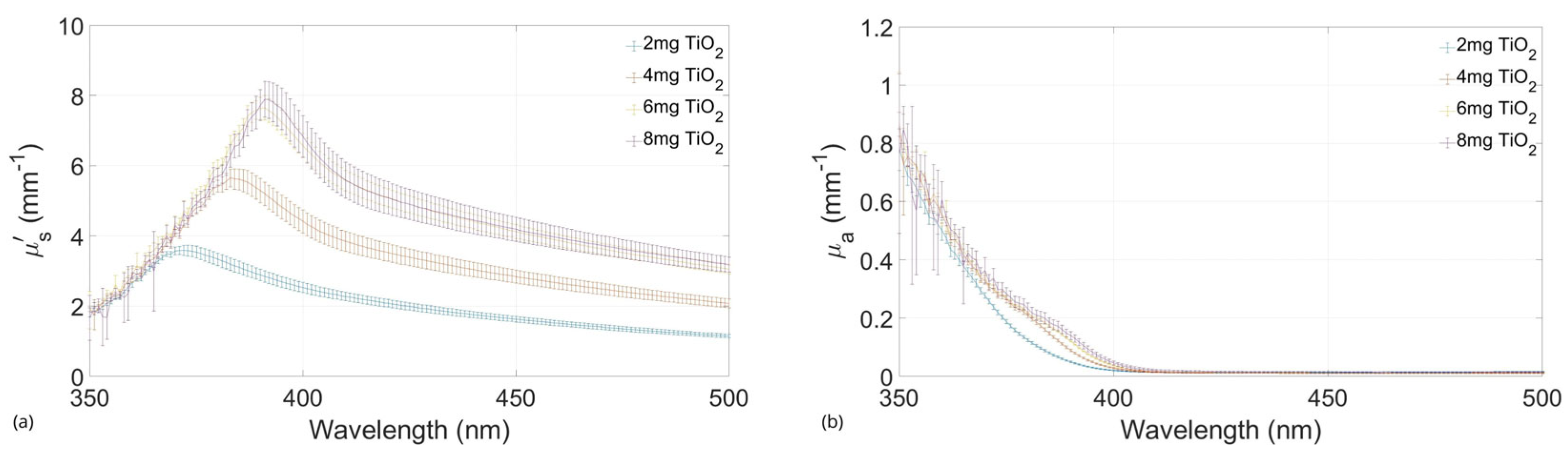
2.3. Absorbing and Scattering Agents Effects
2.4. Optical Coefficients between Phantom and Biological Samples
2.5. Discussions
3. Conclusions
4. Materials and Methods
4.1. Preparation of Agarose Tissue Simile
Sample Preparation
4.2. Experimental Setup
4.2.1. Objective of the Experiments
4.2.2. Samples
4.2.3. Optical Tests
4.2.4. Optical Coefficients Estimation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pogue, B.W.; Patterson, M.S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 2006, 11, 041102. [Google Scholar] [CrossRef]
- Sieryi, O.; Popov, A.; Kalchenko, V.; Bykov, A.; Meglinski, I. Tissue-mimicking phantoms for biomedical applications. In Proceedings of the Tissue Optics and Photonics, Online, 6–10 April 2020; Volume 11363, pp. 111–117. [Google Scholar]
- Cerussi, A.E.; Warren, R.; Hill, B.; Roblyer, D.; Leproux, A.; Durkin, A.F.; O’Sullivan, T.D.; Keene, S.; Haghany, H.; Quang, T.; et al. Tissue phantoms in multicenter clinical trials for diffuse optical technologies. Biomed. Opt. Express BOE 2012, 3, 966–971. [Google Scholar] [CrossRef]
- Lualdi, M.; Colombo, A.; Farina, B.; Tomatis, S.; Marchesini, R. A phantom with tissue-like optical properties in the visible and near infrared for use in photomedicine. Lasers Surg. Med. 2001, 28, 237–243. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, H.; Sun, Y.; Liu, J. The recent development of fluorescent probes for the detection of NADH and NADPH in living cells and in vivo. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118919. [Google Scholar] [CrossRef]
- Delgado, J.A.; Quesada, I.; Montaño, L.M.; Anasagasti, L. Safety of UV radiation for autofluorescence diagnosis of skin cancer. Rev. Mex. De Física E 2006, 52, 78–83. [Google Scholar]
- Carneiro, I.; Carvalho, S.; Henrique, R.; Selifonov, A.; Oliveira, L.; Tuchin, V.V. Enhanced Ultraviolet Spectroscopy by Optical Clearing for Biomedical Applications. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 7200108. [Google Scholar] [CrossRef]
- Butte, P.V.; Mamelak, A.N.; Nuno, M.; Bannykh, S.I.; Black, K.L.; Marcu, L. Fluorescence lifetime spectroscopy for guided therapy of brain tumors. NeuroImage 2011, 54, S125–S135. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Garcia, A.; Bec, J.; Sridharan Weaver, S.; Hartl, B.; Unger, J.; Bobinski, M.; Lechpammer, M.; Girgis, F.; Boggan, J.; Marcu, L. Real-time augmented reality for delineation of surgical margins during neurosurgery using autofluorescence lifetime contrast. J. Biophotonics 2020, 13, e201900108. [Google Scholar] [CrossRef]
- Weyers, B.W.; Marsden, M.; Sun, T.; Bec, J.; Bewley, A.F.; Gandour-Edwards, R.F.; Moore, M.G.; Farwell, D.G.; Marcu, L. Fluorescence lifetime imaging for intraoperative cancer delineation in transoral robotic surgery. Transl. Biophotonics 2019, 1, e201900017. [Google Scholar] [CrossRef] [PubMed]
- Mattley, Y.D.; Mitrani-Gold, F.; Orton, S.; Bacon, C.P.; Leparc, G.F.; Bayona, M.; Potter, R.L.; Garcia-Rubio, L.H. Blood characterization using UV/vis spectroscopy. In Proceedings of the Advances in Fluorescence Sensing Technology II, San Jose, CA, USA, 6–8 February 1995; Volume 2388, pp. 462–470. [Google Scholar]
- Sun, A.; Guo, H.; Gan, Q.; Yang, L.; Liu, Q.; Xi, L. Evaluation of visible NIR-I and NIR-II light penetration for photoacoustic imaging in rat organs. Opt. Express OE 2020, 28, 9002–9013. [Google Scholar] [CrossRef] [PubMed]
- Fodor, L.; Sobec, R. Light Tissue Interactions. In Aesthetic Applications of Intense Pulsed Light; Fodor, L., Ullmann, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 13–23. ISBN 978-3-030-22829-3. [Google Scholar]
- Valdes, P.A.; Juvekar, P.; Agar, N.Y.R.; Gioux, S.; Golby, A.J. Quantitative Wide-Field Imaging Techniques for Fluorescence Guided Neurosurgery. Front. Surg. 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Valdés, P.A.; Fan, X.; Ji, S.; Harris, B.T.; Paulsen, K.D.; Roberts, D.W. Estimation of brain deformation for volumetric image updating in protoporphyrin IX fluorescence-guided resection. Ster. Funct. Neurosurg. 2010, 88, 1–10. [Google Scholar] [CrossRef]
- Sun, Y.; Hatami, N.; Yee, M.; Phipps, J.; Elson, D.S.; Gorin, F.; Schrot, R.J.; Marcu, L. Fluorescence lifetime imaging microscopy for brain tumor image-guided surgery. J. Biomed. Opt. 2010, 15, 056022. [Google Scholar] [CrossRef]
- Reichert, D.; Wadiura, L.I.; Erkkilae, M.T.; Gesperger, J.; Lang, A.; Roetzer-Pejrimovsky, T.; Makolli, J.; Woehrer, A.; Wilzbach, M.; Hauger, C.; et al. Flavin fluorescence lifetime and autofluorescence optical redox ratio for improved visualization and classification of brain tumors. Front. Oncol. 2023, 13, 1105648. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-T.; Lin, Y.-Y.; Lin, Y.-Y.; Lin, Y.-L.; Cheng, H.; Chang, Y.; Huang, M.-C. In Vivo Real-Time Discrimination Among Glioma, Infiltration Zone, and Normal Brain Tissue via Autofluorescence Technology. World Neurosurg. 2019, 122, e773–e782. [Google Scholar] [CrossRef] [PubMed]
- Jaime, R.A.O.; Basto, R.L.Q.; Lamien, B.; Orlande, H.R.B.; Eibner, S.; Fudym, O. Fabrication Methods of Phantoms Simulating Optical and Thermal Properties. Procedia Eng. 2013, 59, 30–36. [Google Scholar] [CrossRef]
- Diep, P.; Pannem, S.; Sweer, J.; Lo, J.; Snyder, M.; Stueber, G.; Zhao, Y.; Tabassum, S.; Istfan, R.; Wu, J.; et al. Three-dimensional printed optical phantoms with customized absorption and scattering properties. Biomed. Opt. Express 2015, 6, 4212. [Google Scholar] [CrossRef] [PubMed]
- Ntombela, L.; Adeleye, B.; Chetty, N. Low-cost fabrication of optical tissue phantoms for use in biomedical imaging. Heliyon 2020, 6, e03602. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Ishii, K.; Kajimoto, Y.; Kuroiwa, T.; Awazu, K. Determination of optical properties of human brain tumor tissues from 350 to 1000 nm to investigate the cause of false negatives in fluorescence-guided resection with 5-aminolevulinic acid. J. Biomed. Opt. 2018, 23, 075006. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavsky, A.N.; Schulze, P.C.; Yaroslavsky, I.V.; Schober, R.; Ulrich, F.; Schwarzmaier, H.-J. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 2002, 47, 2059. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, Q.; Feng, S.; Zhao, H.; Jin, W.; Qiu, H.; Gu, Y.; Chen, D. In situ detection of human glioma based on tissue optical properties using diffuse reflectance spectroscopy. J. Biophotonics 2023, 16, e202300195. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, S.C.; Lin, W.C.; Mahadevan-Jansen, A. In vitro determination of normal and neoplastic human brain tissue optical properties using inverse adding-doubling. Phys. Med. Biol. 2006, 51, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Shapey, J.; Xie, Y.; Nabavi, E.; Ebner, M.; Saeed, S.R.; Kitchen, N.; Dorward, N.; Grieve, J.; McEvoy, A.W.; Miserocchi, A.; et al. Optical properties of human brain and tumour tissue: An ex vivo study spanning the visible range to beyond the second near-infrared window. J. Biophotonics 2022, 15, e202100072. [Google Scholar] [CrossRef] [PubMed]
- Balafar, M.A.; Ramli, A.R.; Saripan, M.I.; Mashohor, S. Review of brain MRI image segmentation methods. Artif. Intell. Rev. 2010, 33, 261–274. [Google Scholar] [CrossRef]
- Setsompop, K.; Feinberg, D.A.; Polimeni, J.R. Rapid brain MRI acquisition techniques at ultra-high fields. NMR Biomed. 2016, 29, 1198–1221. [Google Scholar] [CrossRef]
- Nomoni, M.; May, J.M.; Kyriacou, P.A. Novel Polydimethylsiloxane (PDMS) Pulsatile Vascular Tissue Phantoms for the In-Vitro Investigation of Light Tissue Interaction in Photoplethysmography. Sensors 2020, 20, 4246. [Google Scholar] [CrossRef]
- Zell, K.; Sperl, J.I.; Vogel, M.W.; Niessner, R.; Haisch, C. Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys. Med. Biol. 2007, 52, N475–N484. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wear, K.A.; Pfefer, T.J.; Vogt, W.C. Tissue-mimicking phantoms for performance evaluation of photoacoustic microscopy systems. Biomed. Opt. Express BOE 2022, 13, 1357–1373. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Edwards, S.A.; Iorizzo, T.W.; Longo, B.N.; Yaroslavsky, A.N.; Kaplan, D.L.; Mallidi, S. Investigation of silk as a phantom material for ultrasound and photoacoustic imaging. Photoacoustics 2022, 28, 100416. [Google Scholar] [CrossRef]
- Amado Rey, A.B.; Mittnacht, A.; Stieglitz, T. Experimental characterization of optoacoustic phantoms in gel wax and polyvinyl alcohol for blood pressure measurements. In Proceedings of the Biomedical Engineering Ranging from Wellness to Intensive care: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC): 41st EMB Conference, Berlin, Germany, 23–27 July 2019; pp. 5820–5823. [Google Scholar] [CrossRef]
- Rezende, T.K.L.; Barbosa, H.P.; dos Santos, L.F.; de Lima, O.K.; Alves de Matos, P.; Tsubone, T.M.; Gonçalves, R.R.; Ferrari, J.L. Upconversion rare Earths nanomaterials applied to photodynamic therapy and bioimaging. Front. Chem. 2022, 10, 1035449. [Google Scholar] [CrossRef]
- Tsang, C.Y.; Zhang, Y. Nanomaterials for light-mediated therapeutics in deep tissue. Chem. Soc. Rev. 2024, 53, 2898–2931. [Google Scholar] [CrossRef] [PubMed]
- Wales, D.J.; Keshavarz, M.; Howe, C.; Yeatman, E. 3D Printability Assessment of Poly(octamethylene maleate (anhydride) citrate) and Poly(ethylene glycol) Diacrylate Copolymers for Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4, 5457–5470. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, M.; Miyazono, Y.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 1997, 44, 1299–1305. [Google Scholar] [CrossRef]
- Saturated Solubility and Thermodynamic Evaluation of l-Tryptophan in Eight Pure Solvents and Three Groups of Binary Mixed Solvents by the Gravimetric Method at T = 278.15–333.15 K|Journal of Chemical & Engineering Data. Available online: https://pubs.acs.org/doi/full/10.1021/acs.jced.9b00562 (accessed on 23 February 2024).
- Sandell, J.L.; Zhu, T.C. A review of in-vivo optical properties of human tissues and its impact on PDT. J. Biophotonics 2011, 4, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-J.; Gillies, G.T.; Broaddus, W.C.; Prabhu, S.S.; Fillmore, H.; Mitchell, R.M.; Corwin, F.D.; Fatouros, P.P. A realistic brain tissue phantom for intraparenchymal infusion studies. J. Neurosurg. 2004, 101, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Thulliez, M.; Bastin, O.; Nonclercq, A.; Delchambre, A.; Reniers, F. Gel models to assess distribution and diffusion of reactive species from cold atmospheric plasma: An overview for plasma medicine applications. J. Phys. D Appl. Phys. 2021, 54, 463001. [Google Scholar] [CrossRef]
- Monici, M. Cell and tissue autofluorescence research and diagnostic application. Biotechnol. Annu. Rev. 2005, 11, 227–256. [Google Scholar] [CrossRef]
- Campbell, J.M.; Habibalahi, A.; Handley, S.; Agha, A.; Mahbub, S.B.; Anwer, A.G.; Goldys, E.M. Emerging clinical applications in oncology for non-invasive multi- and hyperspectral imaging of cell and tissue autofluorescence. J. Biophotonics 2023, 16, e202300105. [Google Scholar] [CrossRef]
- Schaefer, P.M.; Kalinina, S.; Rueck, A.; von Arnim, C.A.F.; von Einem, B. NADH Autofluorescence-A Marker on its Way to Boost Bioenergetic Research. Cytom. A 2019, 95, 34–46. [Google Scholar] [CrossRef]
- Toms, S.A.; Konrad, P.E.; Lin, W.-C.; Weil, R.J. Neuro-Oncological Applications of Optical Spectroscopy. Technol. Cancer Res. Treat. 2006, 5, 231–238. [Google Scholar] [CrossRef]
- Duran-Padilla, M.; Serrano-Loyola, R.; Perez-Garcia, A.; Carrillo-Betancourt, R.; Campos-García Rojas, C.; Reyes-Alberto, M.; Franco, W.; Hernandez-Ruiz, J.; Gutierrez-Herrera, E. Tryptophan intrinsic fluorescence from wound healing correlates with re-epithelialization in a rabbit model. Ski. Res. Technol. 2024, 30, e13834. [Google Scholar] [CrossRef] [PubMed]
- Tejo-Otero, A.; Fenollosa-Artés, F.; Achaerandio, I.; Rey-Vinolas, S.; Buj-Corral, I.; Mateos-Timoneda, M.Á.; Engel, E. Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms. Gels 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Albor-Ramírez, E.; Reyes-Alberto, M.; Vidal-Flores, L.M.; Gutierrez-Herrera, E.; Padilla-Castañeda, M.A. Agarose Gel Characterization for the Fabrication of Brain Tissue Phantoms for Infrared Multispectral Vision Systems. Gels 2023, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Prahl, S. Everything I Think You Should Know about Inverse Adding-Doubling. Available online: https://omlc.org/software/iad/manual.pdf (accessed on 1 March 2024).

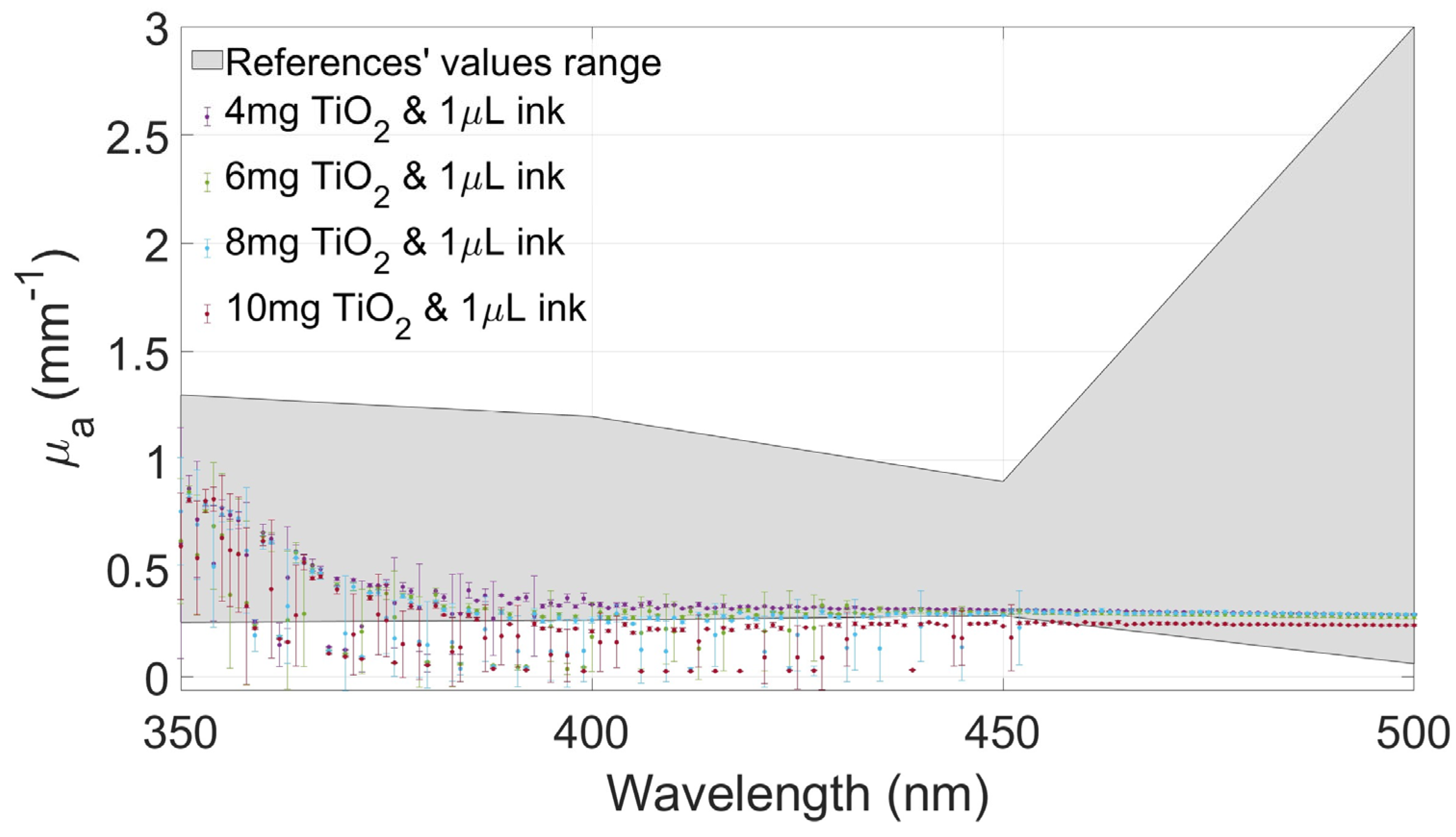



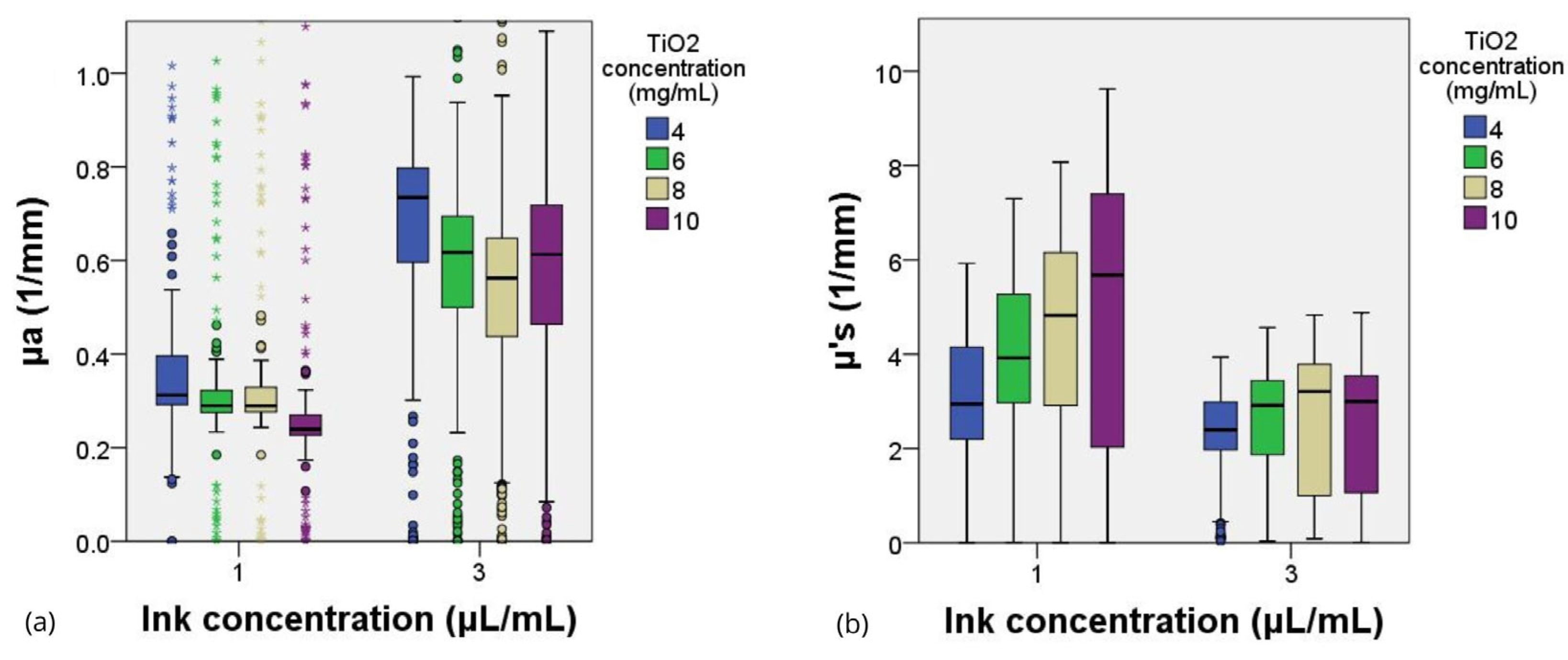
| References | µa (mm−1) @ 350 nm | µa (mm−1) @ 400 nm | µa (mm−1) @ 450 nm | µa (mm−1) @ 500 nm | ||||
|---|---|---|---|---|---|---|---|---|
| Min. Value | Max. Value | Min. Value | Max. Value | Min. Value | Max. Value | Min. Value | Max. Value | |
| This work’s gels with 1 µL/mL | 0.93 | 1.02 | 0.208 | 0.33 | 0.23 | 0.31 | 0.24 | 0.29 |
| This work’s gels with 3 µL/mL | 0.98 | 1.03 | 0.45 | 0.54 | 0.54 | 0.66 | 0.66 | 0.8 |
| Biotissue White brain matter | ||||||||
| Honda et al. [22] | 1 | 0.9 | 0.8 | 0.4 | ||||
| Li et al. [24] | 2.2 | |||||||
| Gebhart et al. [25] | 1 | 0.5 | 0.2 | |||||
| Shapey et al. [26] | 0.3 | 0.28 | 0.22 | |||||
| Yaroslavsky et al. [23] | 0.25 | 0.3 | 0.14 | 0.1 | ||||
| Biotissue Gray brain matter | ||||||||
| Honda et al. [22] | 1.3 | 1.2 | 0.85 | 0.4 | ||||
| Li et al. [24] | 3 | |||||||
| Gebhart et al. [25] | 1 | 0.5 | 0.25 | |||||
| Shapey et al. [26] | 0.95 | 0.9 | 0.38 | |||||
| Yaroslavsky et al. [23] | 0.35 | 0.26 | 0.07 | 0.05 | ||||
| References | (mm−1) @ 350 nm | (mm−1) @ 400 nm | (mm−1) @ 450 nm | (mm−1) @ 500 nm | ||||
|---|---|---|---|---|---|---|---|---|
| Min. Value | Max. Value | Min. Value | Max. Value | Min. Value | Max. Value | Min. Value | Max. Value | |
| This work’s gels with 1 µL/mL | 2 | 2.2 | 5.17 | 8.35 | 3.37 | 7.47 | 2.4 | 5.6 |
| This work’s gels with 3 µL/mL | 1.86 | 2 | 3.24 | 4.6 | 2.7 | 3.7 | 2.27 | 3.5 |
| Biotissue White brain matter | ||||||||
| Honda et al. [22] | 10 | 10 | 9.7 | 10 | ||||
| Li et al. [24] | 2.8 | |||||||
| Gebhart et al. [25] | 9 | 8.8 | 7 | |||||
| Shapey et al. [26] | 10 | 11 | 11 | |||||
| Yaroslavsky et al. [23] | 12 | 10.5 | 9.24 | 8.4 | ||||
| Biotissue Gray brain matter | ||||||||
| Honda et al. [22] | 4 | 4 | 3.7 | 3.5 | ||||
| Li et al. [24] | 1.9 | |||||||
| Gebhart et al. [25] | 2.8 | 2 | 1.9 | |||||
| Shapey et al. [26] | 6 | 5 | 4.8 | |||||
| Yaroslavsky et al. [23] | 2.7 | 1.6 | 1.4 | 1.3 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Flores, L.M.; Reyes-Alberto, M.; Albor-Ramírez, E.; Domínguez-Velasco, C.F.; Gutierrez-Herrera, E.; Padilla-Castañeda, M.A. Fabrication and Characterization of Brain Tissue Phantoms Using Agarose Gels for Ultraviolet Vision Systems. Gels 2024, 10, 540. https://doi.org/10.3390/gels10080540
Vidal-Flores LM, Reyes-Alberto M, Albor-Ramírez E, Domínguez-Velasco CF, Gutierrez-Herrera E, Padilla-Castañeda MA. Fabrication and Characterization of Brain Tissue Phantoms Using Agarose Gels for Ultraviolet Vision Systems. Gels. 2024; 10(8):540. https://doi.org/10.3390/gels10080540
Chicago/Turabian StyleVidal-Flores, Luis M., Miguel Reyes-Alberto, Efraín Albor-Ramírez, César F. Domínguez-Velasco, Enoch Gutierrez-Herrera, and Miguel A. Padilla-Castañeda. 2024. "Fabrication and Characterization of Brain Tissue Phantoms Using Agarose Gels for Ultraviolet Vision Systems" Gels 10, no. 8: 540. https://doi.org/10.3390/gels10080540






