Developments in Nanostructured MoS2-Decorated Reduced Graphene Oxide Composite Aerogel as an Electrocatalyst for the Hydrogen Evolution Reaction
Abstract
1. Introduction
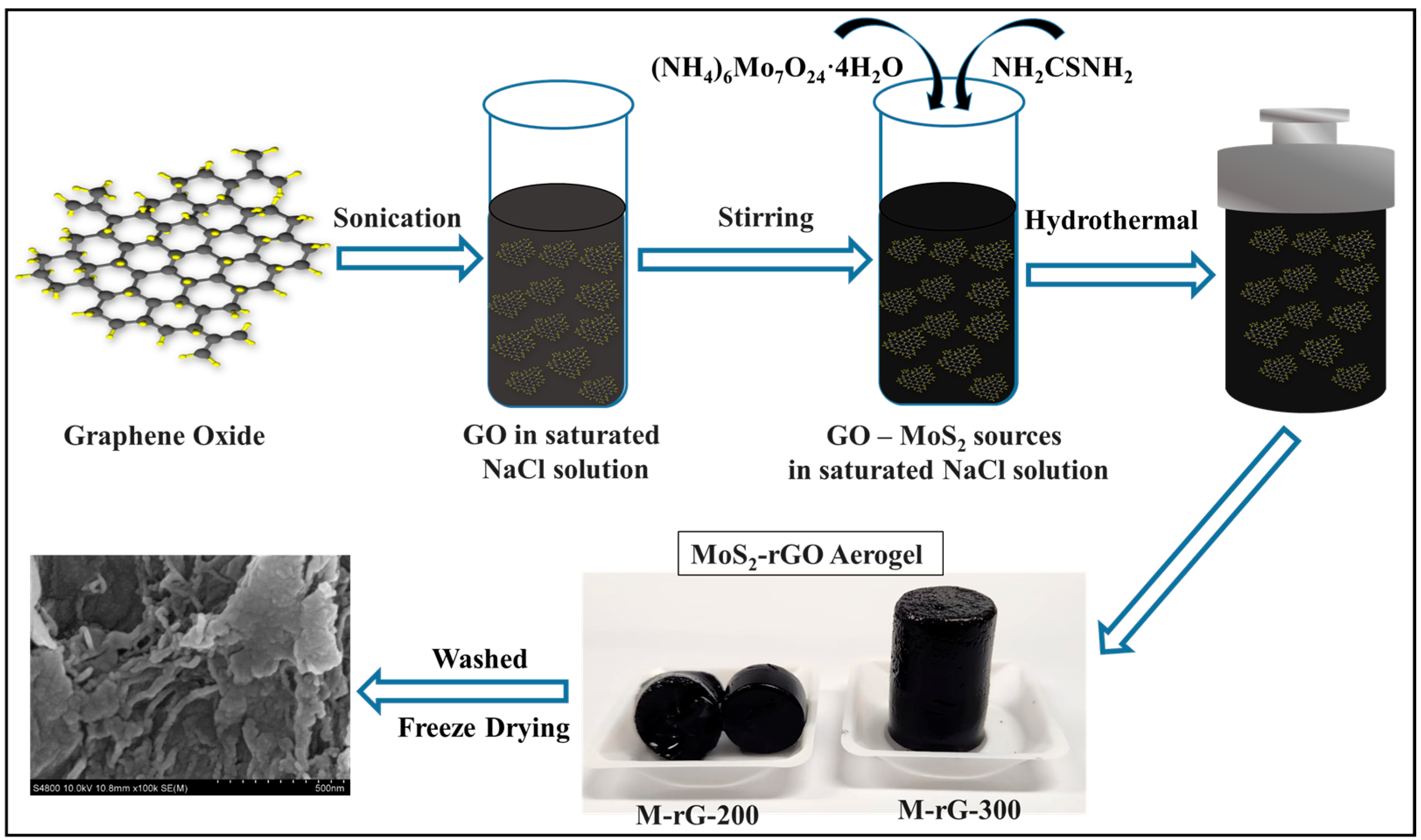
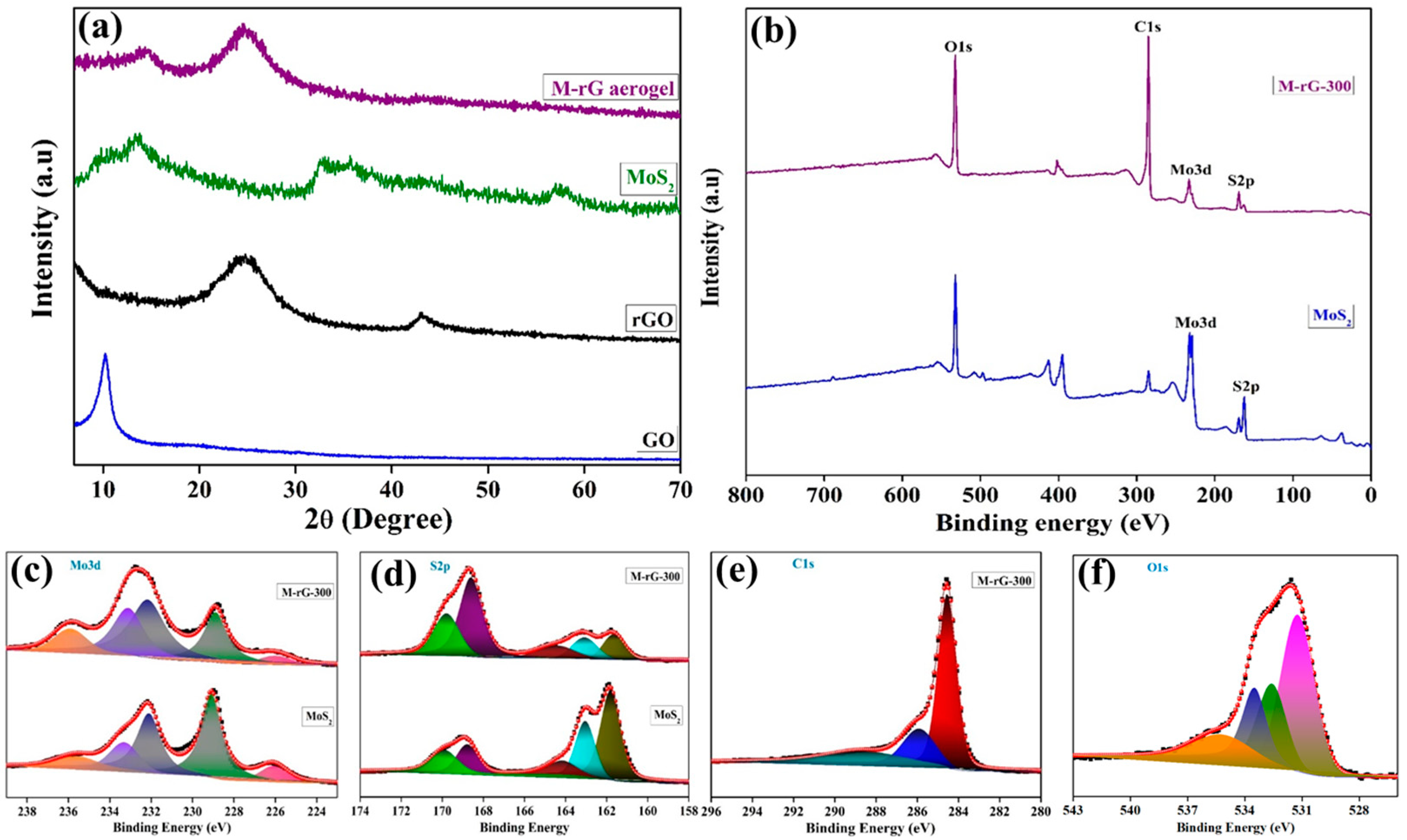
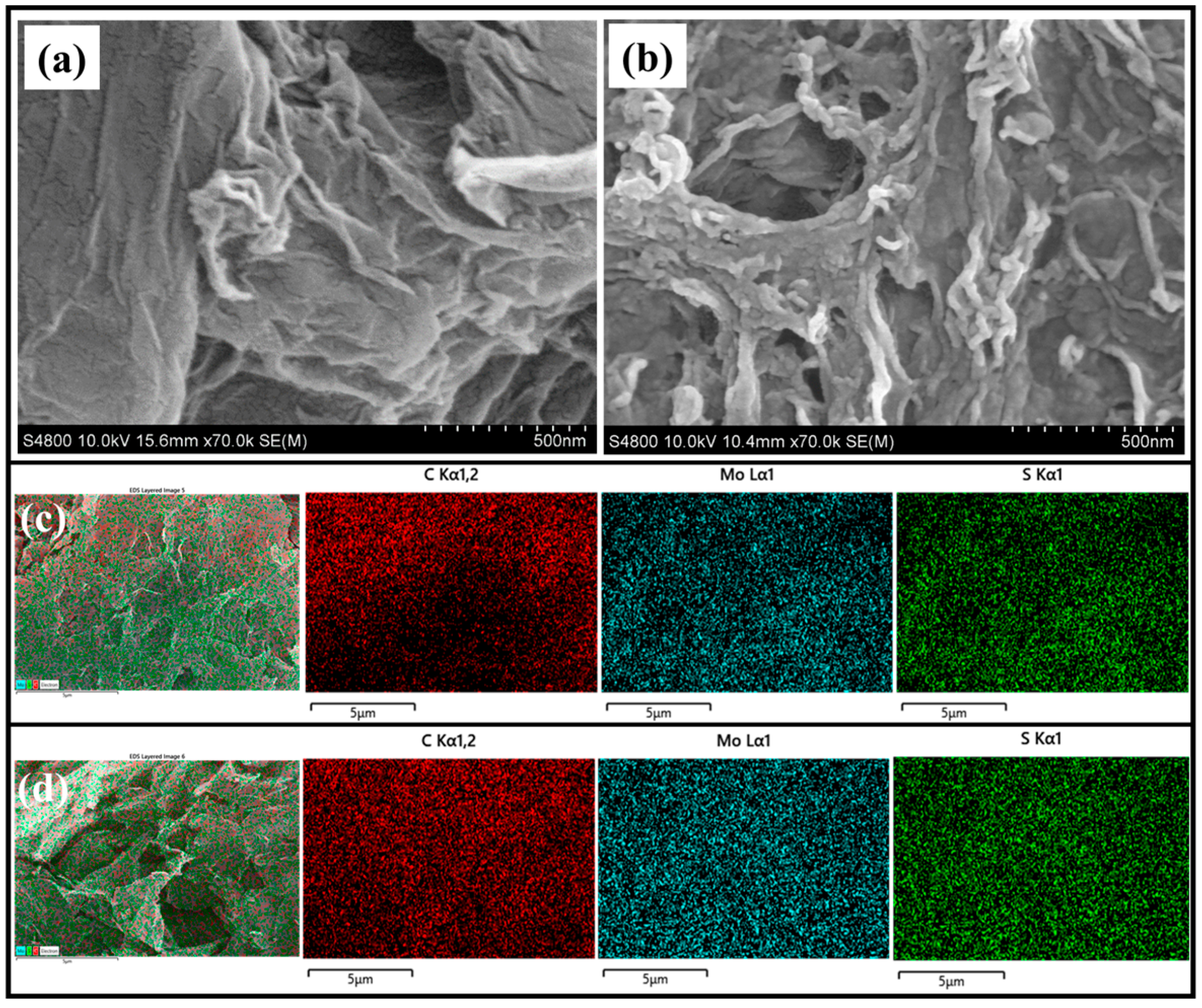
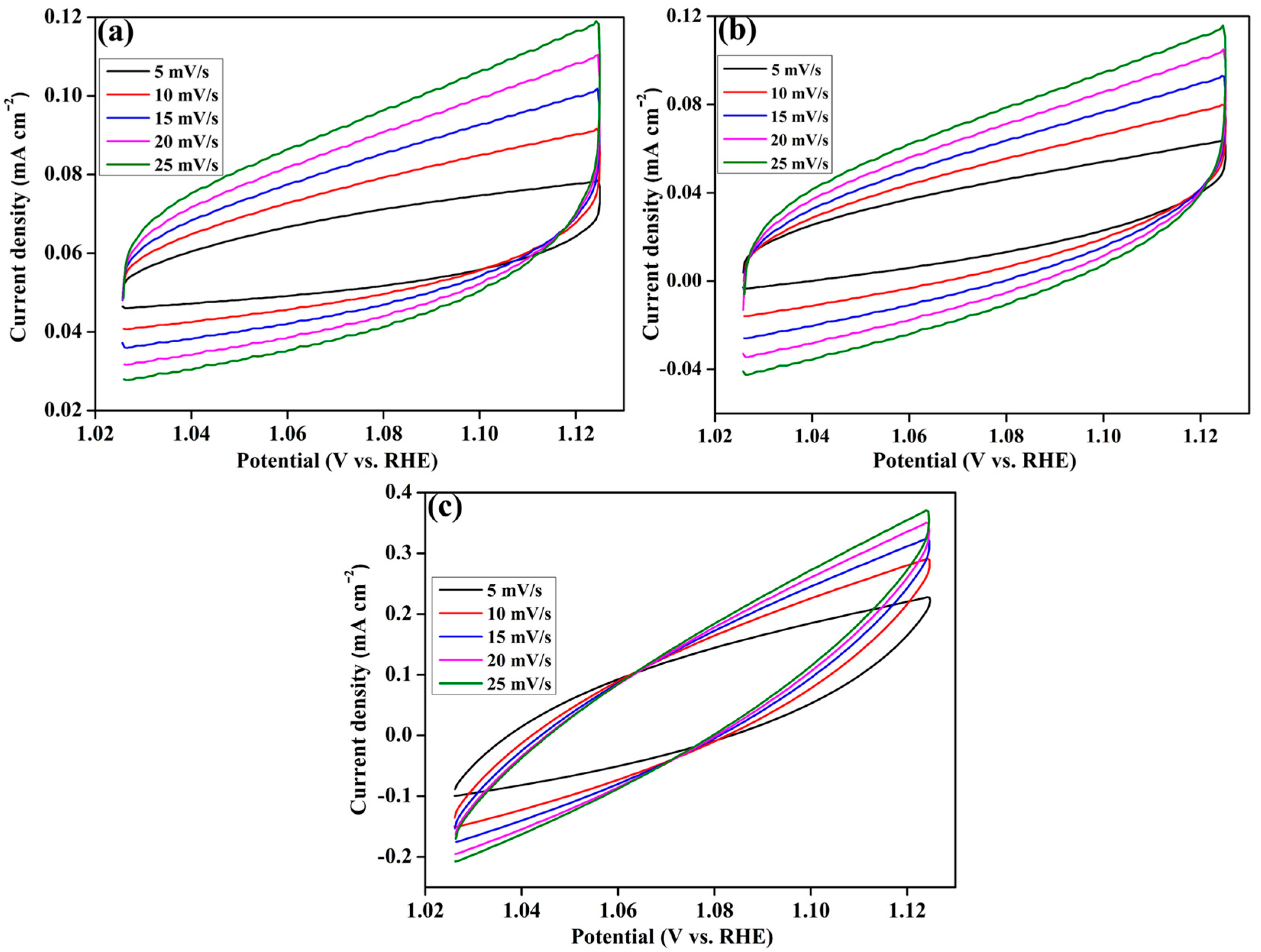
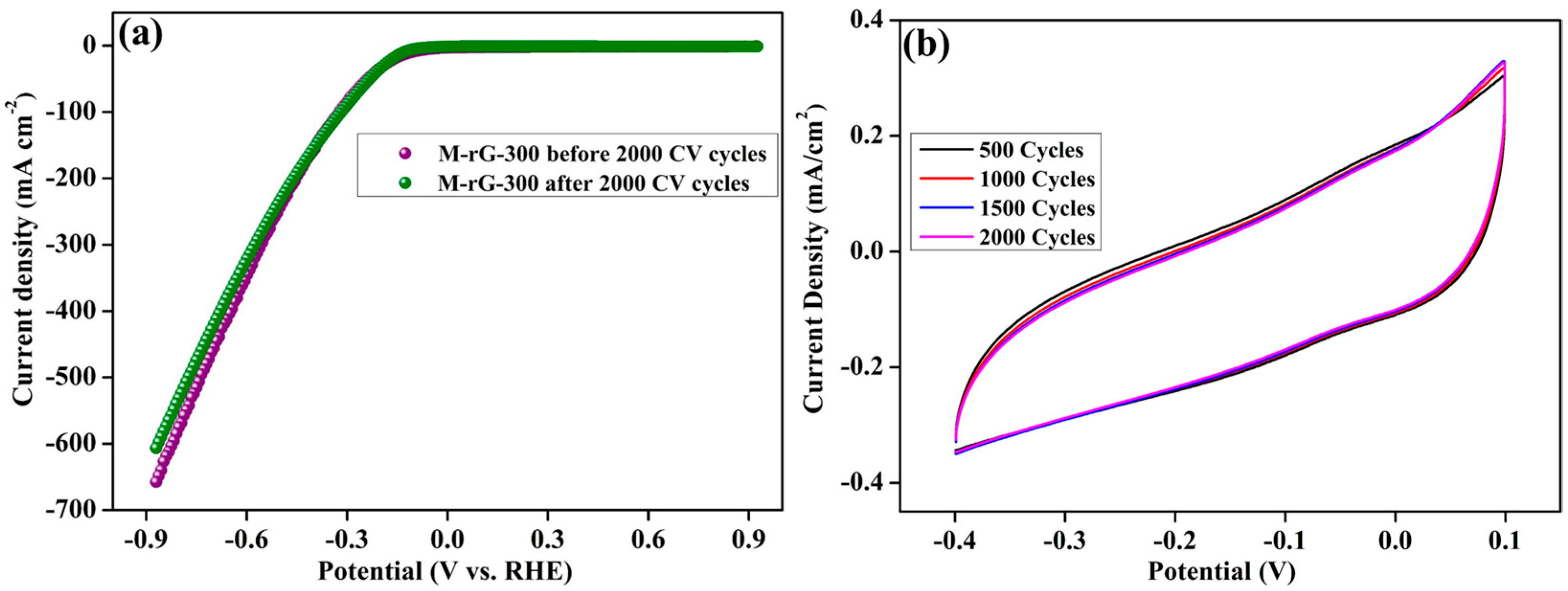
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Thangarasu, S.; Baby, N.; Bhosale, M.; Lee, J.; Jeong, C.; Oh, T.-H. Fe2O3/Ni Nanocomposite Electrocatalyst on Cellulose for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. Int. J. Mol. Sci. 2023, 24, 16282. [Google Scholar] [CrossRef]
- Razi, F.; Dincer, I. Renewable energy development and hydrogen economy in MENA region: A review. Renew. Sustain. Energy Rev. 2022, 168, 112763. [Google Scholar] [CrossRef]
- Lu, J.; Hao, W.; Wu, X.; Shen, X.; Cui, S.; Shi, W. Electronic Modulation of the 3D Architectured Ni/Fe Oxyhydroxide Anchored N-Doped Carbon Aerogel with Much Improved OER Activity. Gels 2023, 9, 190. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Muttaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrogen Energy 2022, 47, 17285–17312. [Google Scholar] [CrossRef]
- Khan, I.; Hou, F.; Irfan, M.; Zakari, A.; Le, H.P. Does energy trilemma a driver of economic growth? The roles of energy use, population growth, and financial development. Renew. Sustain. Energy Rev. 2021, 146, 111157. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion. Gels 2024, 10, 63. [Google Scholar] [CrossRef]
- Thamer, B.M.; Abdul Hameed, M.M.; El-Newehy, M.H. Molten Salts Approach of Poly(vinyl alcohol)-Derived Bimetallic Nickel–Iron Sheets Supported on Porous Carbon Nanosheet as an Effective and Durable Electrocatalyst for Methanol Oxidation. Gels 2023, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Altaleb, H.A.; Salah, A.; Thamer, B.M. Hydrogel Nanocomposite-Derived Nickel Nanoparticles/Porous Carbon Frameworks as Non-Precious and Effective Electrocatalysts for Methanol Oxidation. Gels 2022, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Mandić, V.; Bafti, A.; Panžić, I.; Radovanović-Perić, F. Bio-Based Aerogels in Energy Storage Systems. Gels 2024, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Thangarasu, S.; Shalu; Palanisamy, G.; Sadhasivam, S.; Selvakumar, K.; Eswar Neerugatti, K.R.; Oh, T.H. Deciphering the role of 2D graphene oxide nanofillers in polymer membranes for vanadium redox flow batteries. J. Mater. Chem. A 2024, 12, 11176–11234. [Google Scholar] [CrossRef]
- Kalnaus, S.; Dudney, N.J.; Westover, A.S.; Herbert, E.; Hackney, S. Solid-state batteries: The critical role of mechanics. Science 2023, 381, eabg5998. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, Y. Designing modern aqueous batteries. Nat. Rev. Mater. 2023, 8, 109–122. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 636–678. [Google Scholar] [CrossRef]
- Pan, J.; Yu, S.; Jing, Z.; Zhou, Q.; Dong, Y.; Lou, X.; Xia, F. Electrocatalytic Hydrogen Evolution Reaction Related to Nanochannel Materials. Small Struct. 2021, 2, 2100076. [Google Scholar] [CrossRef]
- Ni, C.; Huang, S.; Koudama, T.D.; Wu, X.; Cui, S.; Shen, X.; Chen, X. Tuning the Electronic Structure of a Novel 3D Architectured Co-N-C Aerogel to Enhance Oxygen Evolution Reaction Activity. Gels 2023, 9, 313. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The future of hydrogen energy: Bio-hydrogen production technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Li, L.; Lu, S.; Fang, L.; Wei, Y.; Yang, S. Review on Biomass-Derived Carbon-Based Materials for Electrocatalytic Hydrogen Production: State of the Art and Outlook. Energy Fuels 2023, 37, 18485–18501. [Google Scholar] [CrossRef]
- Gao, T.; An, Q.; Tang, X.; Yue, Q.; Zhang, Y.; Li, B.; Li, P.; Jin, Z. Recent progress in energy-saving electrocatalytic hydrogen production via regulating the anodic oxidation reaction. Phys. Chem. Chem. Phys. 2024, 26, 19606–19624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Lu, X.F.; Wu, Z.-P.; Luan, D.; Lou, X.W. Engineering Platinum–Cobalt Nano-alloys in Porous Nitrogen-Doped Carbon Nanotubes for Highly Efficient Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2021, 60, 19068–19073. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Xu, T.; Liu, B.; Huang, K.; Qiao, L.; Liu, S.; Cao, J.; Jun, S.C.; Yamauchi, Y.; et al. Atomic-Level Platinum Filling into Ni-Vacancies of Dual-Deficient NiO for Boosting Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2022, 12, 2200434. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Bhosale, M.; Thangarasu, S.; Magdum, S.S.; Jeong, C.; Oh, T.-H. Enhancing the electrocatalytic performance of vanadium oxide by interface interaction with rGO and NiO nanostructures for electrochemical water oxidation. Int. J. Hydrogen Energy 2024, 54, 1449–1460. [Google Scholar] [CrossRef]
- Thamer, B.M.; Moydeen Abdulhameed, M.; El-Newehy, M.H. Tragacanth Gum Hydrogel-Derived Trimetallic Nanoparticles Supported on Porous Carbon Catalyst for Urea Electrooxidation. Gels 2022, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, S. Recent progress in electrode fabrication for electrocatalytic hydrogen evolution reaction: A mini review. Chem. Eng. J. 2020, 393, 124726. [Google Scholar] [CrossRef]
- Sim, Y.; Chae, Y.; Kwon, S.-Y. Recent advances in metallic transition metal dichalcogenides as electrocatalysts for hydrogen evolution reaction. iScience 2022, 25, 105098. [Google Scholar] [CrossRef]
- Mondal, A.; Vomiero, A. 2D Transition Metal Dichalcogenides-Based Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2208994. [Google Scholar] [CrossRef]
- Kwon, H.R.; Park, H.; Jun, S.E.; Choi, S.; Jang, H.W. High performance transition metal-based electrocatalysts for green hydrogen production. Chem. Commun. 2022, 58, 7874–7889. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, F.; Song, S. Recent advances in structural engineering of molybdenum disulfide for electrocatalytic hydrogen evolution reaction. Chem. Eng. J. 2021, 405, 127013. [Google Scholar] [CrossRef]
- Cao, Y. Roadmap and Direction toward High-Performance MoS2 Hydrogen Evolution Catalysts. ACS Nano 2021, 15, 11014–11039. [Google Scholar] [CrossRef]
- Sundara Venkatesh, P.; Kannan, N.; Ganesh Babu, M.; Paulraj, G.; Jeganathan, K. Transition metal doped MoS2 nanosheets for electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 37256–37263. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, H.; Wu, H.; Zhang, P.; Tang, Z.; Lu, S. Defects Enhance the Electrocatalytic Hydrogen Evolution Properties of MoS2-based Materials. Chem. Asian J. 2020, 15, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhu, J.; Wang, H.; Yang, K.; Shu, Y.; He, J. Facile synthesis of Fe-MoS2/NRGO composite material as effective electrocatalyst for high-efficiency hydrogen evolution reaction. Appl. Surf. Sci. 2022, 587, 152842. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.; Zhang, L.; Sun, Y.; Xu, K.; Dai, Z.; Ma, F. Mace-like hierarchical MoS2/NiCo2S4 composites supported by carbon fiber paper: An efficient electrocatalyst for the hydrogen evolution reaction. J. Power Sources 2018, 377, 142–150. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, J.; Bai, L.; Zhang, W.; Wang, W.; Ma, X.; Wu, L. Dense MoS2/CoS2 Heterointerfaces with Optimized Electronic Structure for Efficient Alkaline Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 2479–2488. [Google Scholar] [CrossRef]
- He, Z.; Guo, Z.; Wa, Q.; Zhong, X.; Wang, X.; Chen, Y. NiSx@MoS2 heterostructure prepared by atomic layer deposition as high-performance hydrogen evolution reaction electrocatalysts in alkaline media. J. Mater. Res. 2020, 35, 822–830. [Google Scholar] [CrossRef]
- Bolar, S.; Shit, S.; Samanta, P.; Chandra Murmu, N.; Kolya, H.; Kang, C.-W.; Kuila, T. Conducting scaffold supported defect rich 3D rGO-CNT/MoS2 nanostructure for efficient HER electrocatalyst at variable pH. Compos. Part B Eng. 2022, 230, 109489. [Google Scholar] [CrossRef]
- Xu, W.; Dong, X.; Wang, Y.; Zheng, N.; Zheng, B.; Lin, Q.; Zhao, Y. Controllable Synthesis of MoS2/Carbon Nanotube Hybrids with Enlarged Interlayer Spacings for Efficient Electrocatalytic Hydrogen Evolution. ChemistrySelect 2020, 5, 13603–13608. [Google Scholar] [CrossRef]
- Khan, M.S.; Noor, T.; Pervaiz, E.; Iqbal, N.; Zaman, N. Fabrication of MoS2/rGO hybrids as electrocatalyst for water splitting applications. RSC Adv. 2024, 14, 12742–12753. [Google Scholar] [CrossRef]
- Fioravanti, F.; Martínez, S.; Delgado, S.; García, G.; Rodriguez, J.L.; Tejera, E.P.; Lacconi, G.I. Effect of MoS2 in doped-reduced graphene oxide composites. Enhanced electrocatalysis for HER. Electrochim. Acta 2023, 441, 141781. [Google Scholar] [CrossRef]
- Moghaddam, M.S.; Bahari, A.; Litkohi, H.R. Using the synergistic effects of MoS2/rGO and bimetallic hybrids as a high-performance nanoelectrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2023, 48, 33139–33154. [Google Scholar] [CrossRef]
- Murthy, A.P. Aerogels for Electrocatalytic Hydrogen Production. In Aerogels for Energy Saving and Storage; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024; pp. 386–406. [Google Scholar] [CrossRef]
- Kuang, P.; Sayed, M.; Fan, J.; Cheng, B.; Yu, J. 3D Graphene-Based H2-Production Photocatalyst and Electrocatalyst. Adv. Energy Mater. 2020, 10, 1903802. [Google Scholar] [CrossRef]
- Obeid, E.; Younes, K. Uncovering Key Factors in Graphene Aerogel-Based Electrocatalysts for Sustainable Hydrogen Production: An Unsupervised Machine Learning Approach. Gels 2024, 10, 57. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, Y.; Chen, B.; Hu, X.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. One-step hydrothermal synthesis of hierarchically structured MoS2 nanorods via reaction intermediates as self-templates for chemoselective hydrogenation. Chem. Eng. J. 2023, 454, 140330. [Google Scholar] [CrossRef]
- Amir Faiz, M.S.; Che Azurahanim, C.A.; Raba’ah, S.A.; Ruzniza, M.Z. Low cost and green approach in the reduction of graphene oxide (GO) using palm oil leaves extract for potential in industrial applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Strimaitis, J.; Danquah, S.A.; Denize, C.F.; Pradhan, S.K.; Bahoura, M. The Effects of Graphene Oxide and Reduced Graphene Oxide Conductive Additives on Activated Carbon Supercapacitors. Processes 2022, 10, 2190. [Google Scholar] [CrossRef]
- He, B.; Chen, L.; Jing, M.; Zhou, M.; Hou, Z.; Chen, X. 3D MoS2-rGO@Mo nanohybrids for enhanced hydrogen evolution: The importance of the synergy on the Volmer reaction. Electrochim. Acta 2018, 283, 357–365. [Google Scholar] [CrossRef]
- Dong, W.; Liu, H.; Liu, X.; Wang, H.; Li, X.; Tian, L. Defective-MoS2/rGO heterostructures with conductive 1T phase MoS2 for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 9360–9370. [Google Scholar] [CrossRef]
- Badiger, J.G.; Arunachalam, M.; Kanase, R.S.; Sayed, S.A.; Ahn, K.-S.; Ha, J.-S.; Kang, S.H. Highly stable MoS2/MnMoO4@Ti nanocomposite electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 51, 156–168. [Google Scholar] [CrossRef]
- Sultan, M.S.; Uddin, W.; Hamza, S.; Alanazi, A.K. Synthesis of Ni, Co-doped MoS2 as Electrocatalyst for Oxygen Evolution Reaction. Int. J. Electrochem. Sci. 2022, 17, 221280. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, L.; Lian, J. Arrays of hierarchical nickel sulfides/MoS2 nanosheets supported on carbon nanotubes backbone as advanced anode materials for asymmetric supercapacitor. J. Power Sources 2017, 343, 373–382. [Google Scholar] [CrossRef]
- Wei, G.; Yu, J.; Gu, M.; Tang, T.B. Dielectric relaxation and hopping conduction in reduced graphite oxide. J. Appl. Phys. 2016, 119, 224102. [Google Scholar] [CrossRef]
- Wu, N.; She, X.; Yang, D.; Wu, X.; Su, F.; Chen, Y. Synthesis of network reduced graphene oxide in polystyrene matrix by a two-step reduction method for superior conductivity of the composite. J. Mater. Chem. 2012, 22, 17254–17261. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Ju, L.; Yang, G.; Yuan, D.; Feng, Z.; Wang, W. The charge effects on the hydrogen evolution reaction activity of the defected monolayer MoS2. Phys. Chem. Chem. Phys. 2023, 25, 10956–10965. [Google Scholar] [CrossRef] [PubMed]
- Prats, H.; Chan, K. The determination of the HOR/HER reaction mechanism from experimental kinetic data. Phys. Chem. Chem. Phys. 2021, 23, 27150–27158. [Google Scholar] [CrossRef]
- Yao, S.; Wu, C.; Li, D.; Gao, B.; Wen, X.; Liu, Z.; Li, W. Coupling SnS2 and rGO aerogel to CuS for enhanced light-assisted OER electrocatalysis. Dalton Trans. 2021, 50, 5530–5539. [Google Scholar] [CrossRef]
- Kale, S.B.; Babar, P.T.; Kim, J.-H.; Lokhande, C.D. Synthesis of one dimensional Cu2S nanorods using a self-grown sacrificial template for the electrocatalytic oxygen evolution reaction (OER). New J. Chem. 2020, 44, 8771–8777. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangarasu, S.; Bhosale, M.; Palanisamy, G.; Oh, T.H. Developments in Nanostructured MoS2-Decorated Reduced Graphene Oxide Composite Aerogel as an Electrocatalyst for the Hydrogen Evolution Reaction. Gels 2024, 10, 558. https://doi.org/10.3390/gels10090558
Thangarasu S, Bhosale M, Palanisamy G, Oh TH. Developments in Nanostructured MoS2-Decorated Reduced Graphene Oxide Composite Aerogel as an Electrocatalyst for the Hydrogen Evolution Reaction. Gels. 2024; 10(9):558. https://doi.org/10.3390/gels10090558
Chicago/Turabian StyleThangarasu, Sadhasivam, Mrunal Bhosale, Gowthami Palanisamy, and Tae Hwan Oh. 2024. "Developments in Nanostructured MoS2-Decorated Reduced Graphene Oxide Composite Aerogel as an Electrocatalyst for the Hydrogen Evolution Reaction" Gels 10, no. 9: 558. https://doi.org/10.3390/gels10090558
APA StyleThangarasu, S., Bhosale, M., Palanisamy, G., & Oh, T. H. (2024). Developments in Nanostructured MoS2-Decorated Reduced Graphene Oxide Composite Aerogel as an Electrocatalyst for the Hydrogen Evolution Reaction. Gels, 10(9), 558. https://doi.org/10.3390/gels10090558






